Phylogeographic Investigation of an Endangered Longhorn Beetle, Callipogon relictus (Coleoptera: Cerambycidae), in Northeast Asia: Implications for Future Restoration in Korea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Specimens
2.2. DNA Extraction, Polymerase Chain Reaction (PCR) Amplification, and Sequencing
2.3. Phylogenetic Analysis, Genetic Diversity, and Haplotype Network
2.4. Geographic Population Structure
3. Results
3.1. Genetic Diversity and Phylogenetic Analyses
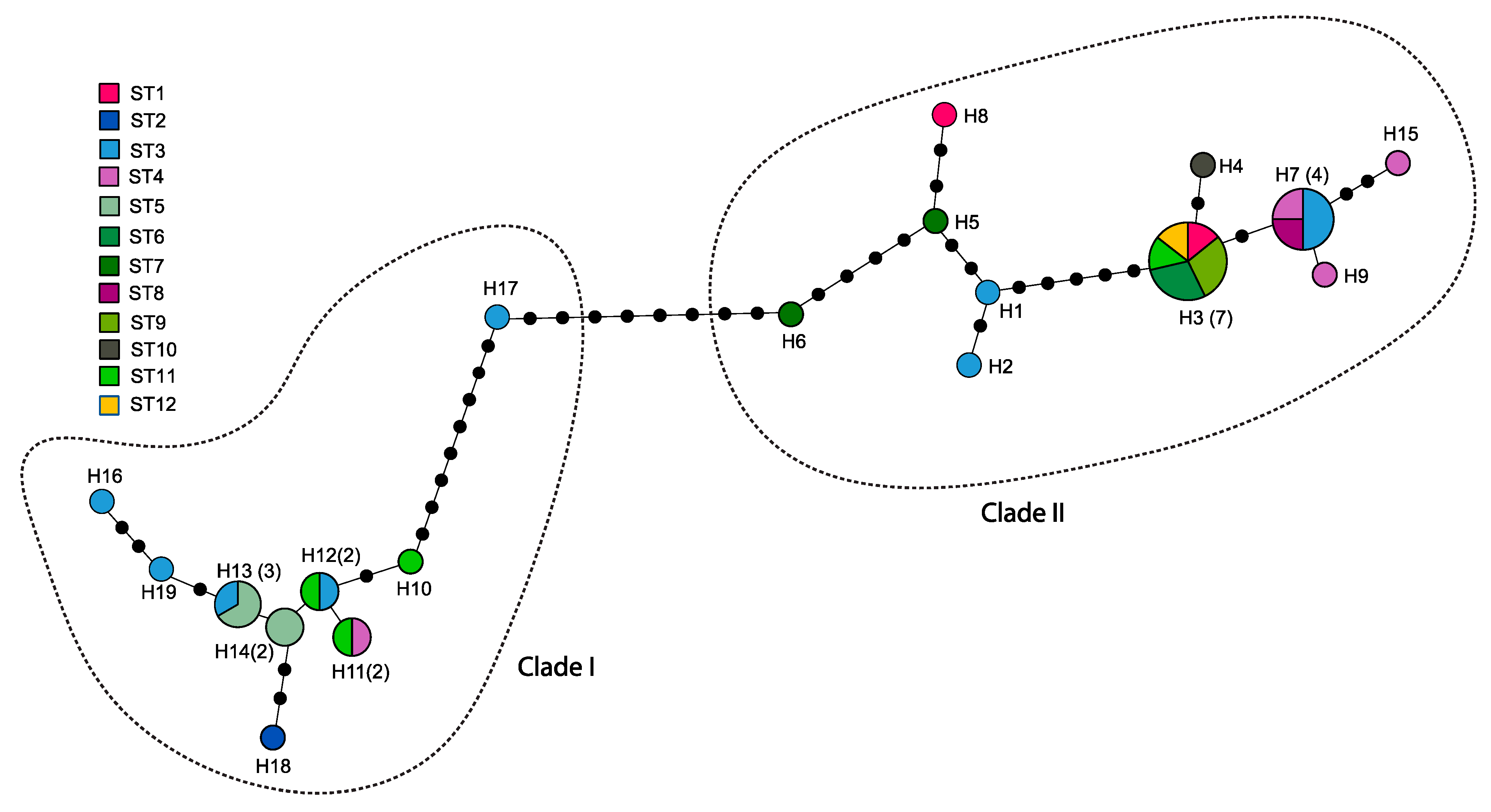
3.2. Haplotype Analysis
3.3. Geographical Population Structure and Demographic History
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semenov, A.P. Callipogon (Eoxenus) relictus sp. n. new species of Cerambycidae in fauna of Russia. Russ. Entomol. J. 1899, 32, 562–580. [Google Scholar]
- Semenov, A.P. Another undescribed male Callipogon (Eoxenus) relictus Sem. (Coleoptera, Cerambycidae). Rus. Entomol. Obozr. 1903, 6, 372–373. [Google Scholar]
- Monné, M. Catalogue of the Cerambycidae (Coleoptera) of the Neotropical Region. Part III. Subfamilies Lepturinae, Necydalinae, Parandrinae, Prioninae, Spondylidinae and Families Oxypeltidae, Vesperidae and Disteniidae. Zootaxa 2018, 1212, 1. [Google Scholar] [CrossRef]
- Kim, S.I.; De Medeiros, B.A.S.; Byun, B.K.; Lee, S.H.; Kang, J.H.; Lee, B.W.; Farrell, B.D. West meets east: How do rainforest beetles become circum-Pacific? Evolutionary origin of Callipogon relictus and allied species (Cerambycidae: Prioninae) in the New and Old Worlds. Mol. Phylogenet. Evol. 2018, 125, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S. On several species of long-horned beetle from Korea peninsular. Mag. Nat. Hist. 1934, 17, 41. [Google Scholar]
- Kuprin, A.V.; Bezborodov, V.G. Geographic range of Callipogon relictus Semenov, 1899 (Coleoptera, Cerambycidae) in the Russian Far East. Izv. RAN. Ser. Biol. 2012, 39, 459–463. [Google Scholar]
- Kuprin, A.V.; Bezborodov, V.G. Areal of Callipogon relictus Semenov, 1899 (Coleoptera, Cerambycidae) in the Russian Far East. Biol. Bull. 2012, 39, 387–391. [Google Scholar] [CrossRef]
- Yi, D.A.; Kuprin, A.V.; Bae, Y.J. Distribution of the longhorned beetle Callipogon relictus (Coleoptera: Cerambycidae) in Northeast Asia. Zootaxa 2018, 4369, 101–108. [Google Scholar] [CrossRef]
- Kuprin, A.V.; Bezborodov, V.G.; Yi, D.A.; Kotlyar, A.K. Developmental Biology and Ecological Peculiarities of the Relict Longhorn Beetle Callipogon relictus Semenov, 1899 (Coleoptera, Cerambycidae). Entomol. Rev. 2014, 94, 1251–1256. [Google Scholar] [CrossRef]
- Yi, D.A. Breeding and Restoration of Korean Relic Long-Horned Beetle: Callipogon Relictus; National Institute of Biological Resources: Incheon, Korea, 2014.
- Google. Gwangneung, South Korea. Available online: https://www.google.co.kr/maps/search/Gwangneung/@40.9094805,119.9757406,6z?hl=en (accessed on 22 May 2021).
- Murayama, J. On the larva and food plant of Callipogon relictus Semenov. Jpn. J. Entomol. 1936, 10, 280–290. [Google Scholar]
- Kim, C.W.; Yoon, I.B.; Nam, S.H. On the habitats and habits of Callipogon relictus. (Coleoptera: Cerambycidae). Nat. Conserv. 1976, 11, 5–16. [Google Scholar]
- Byun, B.K.; Kwon, T.S.; Weon, G.J.; Jo, D.G.; Lee, B.W.; Lee, Y.M.; Choi, H.J.; Kim, C.H.; Lee, S.H.; Bae, Y.S.; et al. Occurrence of Callipogon relictus Semenov (Coleoptera: Cerambycidae) in the Gwangneung Forest, Korea with suggestion for the conservation. Korean J. Appl. Entomol. 2007, 46, 19–25. [Google Scholar] [CrossRef]
- Li, J.; Drumont, A.; Xueping, G.; Wei, G. The checklist of Northeast China’s subfamily Prioninae and biological observations of Callipogon (Eoxenus) relictus Semenov-Tian-Shanskij 1899 (Coleoptera, Cerambycidae, Prioninae). Les Cah. Magellanes 2012, 9, 50–56. [Google Scholar]
- Lim, J.; Jung, S.Y.; Lim, J.S.; Jang, J.; Kim, K.M.; Lee, Y.M.; Lee, B.W. A review of host plants of Cerambycidae (Coleoptera: Chrysomeloidea) with new host records for fourteen Cerambycids, including the Asian Longhorn Beetle (Anoplophora glabripennis Motschulsky), in Korea. Korean J. Appl. Entomol. 2014, 53, 111–133. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.G.; Kim, C.; Choi, I.J.; Kuprin, A.V.; Lim, J. A review of host plants of Callipogon (Eoxenus) relictus Semenov (Coleoptera: Cerambycidae: Prioninae), a Korea natural monument, with a new host, Quercus aliena Blume. J. Asia Pac. Entomol. 2019, 22, 353–358. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, C.; Kuprin, A.V.; Kang, J.H.; Lee, B.W.; Oh, S.H.; Lim, J. Survey research on the habitation and biological information of Callipogon relictus Semenov in Gwangneung forest, Korea and Ussurisky nature reserve, Russia (Coleoptera, Cerambycidae, Prioninae). ZooKeys 2018, 792, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Seddon, P.J.; Armstrong, D.P.; Maloney, R. Developing the science of reintroduction biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef]
- Dempster, J.P.; Hall, M.L. An attempt at re-establishing the swallowtail butterfly at Wicken Fen. Ecol. Entomol. 1980, 5, 327–334. [Google Scholar] [CrossRef]
- Drag, L.; Cizek, L. Successful reintroduction of an endangered veteran tree specialist: Conservation and genetics of the Great Capricorn beetle (Cerambyx cerdo). Conserv. Genet. 2015, 16, 267–276. [Google Scholar] [CrossRef]
- Knisley, C.B.; Hill, J.M.; Scherer, A.M. Translocation of threatened tiger beetle Cicindela dorsalis dorsalis (Coleoptera: Cicindelidae) to Sandy Hook, New Jersey. Ann. Entomol. Soc. Am. 2005, 98, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Bajomi, B.; Pullin, A.S.; Stewart, G.B.; Takács-Sánta, A. Bias and dispersal in the animal reintroduction literature. Oryx 2010, 44, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, A.A.; Frank, J.M.; Croft, P.; Clarke, D.; Pearce-Kelly, P. Mortality of captive British wartbiter cricket: Implications for reintroduction programs. J. Wildl. Dis. 1997, 33, 673–676. [Google Scholar] [CrossRef] [Green Version]
- Baur, B.; Thommen, G.H.; Coray, A. Dynamics of reintroduced populations of Oedipoda caerulescens (Orthoptera, Acrididae) over 21 years. J. Insect Sci. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardiner, T. Mottled grasshopper translocation to sand dunes in Essex, England. In Global Reintroduction Perspectives; IUCN/SSC Re-introduction Specialist Group & Environment Agency: Abu Dhabi, United Arab Emirates, 2011. [Google Scholar]
- Hannon, E.R.; Hafernik, J.E. Reintroduction of the rare damesfly Ischnura gemina (Odonata: Coenagrionidae) into an urban California park. J. Insect Conserv. 2007, 11, 141–149. [Google Scholar] [CrossRef]
- Englund, R.A. The impacts of introduced poeciliid fish and Odonata on endemic Megalagrion (Odonata) damselflies on O’ahu Island, Hawai’i. J. Insect Conserv. 1999, 3, 225–243. [Google Scholar] [CrossRef]
- Preston, D.J.; Englund, R.A.; McShane, M.K.K. Translocation and monitoring efforts to establish a second population of one of the rare Megalagrion xanthomelas (Selys-Longchamps) on O’ahu, Hawai’i (Zygoptera: Coenagrionidae). Bish. Mus. Bull. Cult. Environ. Stud. 2007, 3, 261–276. [Google Scholar]
- Andersen, A.; Simcox, D.; Thomas, J.A.; Nash, D.R. Assessing reintroduction schemes by comparing genetic diversity of reintroduced and source populations: A case study of the globally threatened large blue butterfly (Maculinea arion). Biol. Conserv. 2014, 175, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Wynhoff, I. Lessons from the reintroduction of Maculinea teleius and M. nausithous in the Netherlands. J. Insect Conserv. 1998, 2, 47–57. [Google Scholar] [CrossRef]
- Duffey, E. The reestablishment of the large copper butterfly Lycaena dispar batavus Obth. on Woodwalton Fen National Nature Reserve, Cambridgeshire, England, 1969–1973. Biol. Conserv. 1977, 12, 143–158. [Google Scholar] [CrossRef]
- Chan, P.K.; Packer, L. Assessment of potential Karner Blue Butterfly (Lycaeides melissa samuelis) (Family: Lycanidae) reintroduction sites in Ontario, Canada. Restor. Ecol. 2006, 14, 645–652. [Google Scholar] [CrossRef]
- Babione, M. Bringing tiger beetles together. Endanger. Species Bull. 2003, 28, 28–29. [Google Scholar]
- Amaral, M.; Kozo, A.; French, T. Conservation status and reintroduction of the endangered American burying beetle. Northeast. Nat. 1997, 4, 121–132. [Google Scholar] [CrossRef]
- Mckenna-Foster, A.; Perrotti, L.; Blyth, J.; LoPresti, E.; Kennedy, R.S. Measuring success of a reintroduced population of the American burying beetle (Nicrophorus americanus Olivier) to Nantucket Island, MA. J. Insect Conserv. 2016, 20, 895–904. [Google Scholar] [CrossRef]
- Yoon, T.J.; Park, H.C.; Kang, J.H.; Bayartogtonkh, B.; Bae, Y.J. Genetic divergence between the South Korean and Mongolian populations of the dung beetle, Gymnopleurus mopsus (Coleoptera: Scarabaeidae) based on mitochondrial cytochrome c oxidase subunit I (COI) gene sequences. Entomol. Res. 2017, 47, 366–372. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Seddon, P.J. Directions in reintroduction biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, R.R. The Endangered Species Act of 1973: Preservation or Pandemonium. Environ. Law 1974, 5, 29. [Google Scholar]
- Pyle, R.M. The Eco-Geographic Basis for Lepidoptera Conservation. Ph.D. Thesis, Yale University, New Haven, CT, USA, 1976. [Google Scholar]
- Pyle, R. Insect Conservation. Annu. Rev. Entomol. 1981, 26, 233–258. [Google Scholar] [CrossRef]
- Anon. CITES Permits and Certificates; Office of Management Authority, U.S. Fish and Wildlife Service: Washington, DC, USA, 1999.
- IUCN. Guidelines for Reintroductions and Other Conservation Translocations; Version 1.0; IUCN/SSC Reintroduction Specialist Group: Grand, Switzerland, 2013. [Google Scholar]
- Jorgensen, D. Conservation implications of parasite co-reintroduction. Conserv. Biol. 2014, 29, 602–604. [Google Scholar] [CrossRef]
- Moritz, C.; Faith, D.P. Comparative phylogeography and the identification of genetically divergent areas for conservation. Mol. Ecol. 1998, 7, 419–429. [Google Scholar] [CrossRef]
- Godoy, J.A.; Negro, J.J.; Hiraldo, F.; Donázar, J.A. Phylogeography, genetic structure and diversity in the endangered bearded vulture (Gypaetus barbatus, L.) as revealed by mitochondrial DNA. Mol. Ecol. 2004, 13, 371–390. [Google Scholar] [CrossRef] [Green Version]
- Ryder, O.A. Species conservation and systematics: The dilemma of subspecies. Trends Ecol. Evol. 1986, 1, 9–10. [Google Scholar] [CrossRef]
- Green, D.M. Designatable units for status assessment of endangered species. Conserv. Biol. 2005, 19, 1813–1820. [Google Scholar] [CrossRef]
- Futuyma, D.J.; Peterson, S.C. Genetic variation in the use of resources by insects. Annu. Rev. Entomol. 1985, 30, 217–238. [Google Scholar] [CrossRef]
- Robinson, B.W.; Wilson, D.S. Genetic variation and phenotypic plasticity in a trophically polymorphic population of pumpkinseed sunfish (Lepomis gibbosus). Evol. Ecol. 1996, 10, 631–652. [Google Scholar] [CrossRef]
- Agashe, D. The stabilizing effect of intraspecific genetic variation on population dynamics in novel and ancestral habitats. Am. Nat. 2009, 174, 255–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geist, J. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.): A synthesis of conservation genetics and ecology. Hydrobiologia 2010, 644, 69–88. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Hong, Y.J.; Min, M.S.; Kim, K.S.; Kim, Y.J.; Voloshina, I.; Myslenkov, A.; Smith, G.J.D.; Cuong, N.D.; Tho, H.H.; et al. Genetic status of Asiatic black bear (Ursus thibetanus) reintroduced into South Korea based on DNA and microsatellite loci analysis. J. Hered. 2011, 102, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Kim, M.Y.; Kim, I.K.; Jung, S.H.; Lim, J.S.; Park, S.Y.; Kim, K.M.; Kim, C.H.; Byun, B.K.; Lee, B.W.; et al. Molecular identification and larval description of Callipogon relictus Semenov (Coleoptera: Cerambycidae), a natural monument of South Korea. J. Asia Pac. Entomol. 2013, 16, 223–227. [Google Scholar] [CrossRef]
- Lim, J.; Yi, D.K.; Kim, Y.H.; Lee, W.; Kim, S.; Kang, J.H.; Kim, I.K. Complete mitochondrial genome of Callipogon relictus Semenov (Coleoptera: Cerambycidae): A natural monument and endangered species in Korea. Mitochondrial DNA B 2017, 2, 629–631. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Teacher, A.G.F.; Griffiths, D.J. HapStar: Automated haplotype network layout and visualization. Mol. Ecol. Resour. 2011, 11, 151–153. [Google Scholar] [CrossRef]
- Drag, L.; Hauck, D.; Bérces, S.; Michalcewicz, J.; Šerić Jelaska, L.; Aurenhammer, S.; Cizek, L. Genetic differentiation of populations of the threatened saproxylic beetle Rosalia longicorn, Rosalia alpina (Coleoptera: Cerambycidae) in Central and South-east Europe. Biol. J. Linn. Soc. 2015, 116, 911–925. [Google Scholar] [CrossRef] [Green Version]
- Drag, L.; Hauck, D.; Rican, O.; Schmitt, T.; Shovkoon, D.F.; Godunko, R.J.; Curletti, G.; Cizek, L. Phylogeography of the endangered saproxylic beetle Rosalia longicorn, Rosalia alpina (Coleoptera, Cerambycidae), corresponds with its main host, the European beech (Fagus sylvatica, Fagaceae). J. Biogeogr. 2018, 45, 2631–2644. [Google Scholar] [CrossRef]
- Plewa, R.; Sikora, K.; Gutowski, J.M.; Jaworski, T.; Tarwacki, G.; Tkaczyk, M.; Rossa, R.; Hilszczański, J.; Magoga, G.; Kajtoch, Ł. Morphology, genetics and Wolbachia endosymbionts support distinctiveness of Monochamus sartor sartor and M. s. urussovii (Coleoptera: Cerambycidae). Arthropod Syst. Phylo. 2018, 76, 123–135. [Google Scholar]
- Cakmak, Y.E.; Soydabaş-Ayoub, H.K.; Uckan, F. A preliminary phylogenetic analysis of ribbed-pine-borer (Rhagium inquisitor) based on mitochondrial COI sequences. J. Asia Pac. Entomol. 2020, 23, 809–815. [Google Scholar] [CrossRef]
- Zhang, Y.; Manzoor, A.; Wang, X. Mitochondrial DNA analysis reveals spatial genetic structure and high genetic diversity of Massicus raddei (Blessig) (Coleoptera: Cerambycidae) in China. Ecol. Evol. 2020, 10, 11657–11670. [Google Scholar] [CrossRef]
- Kimura, M. The Neutral Theory of Molecular Evolution; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1983. [Google Scholar]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Nakamine, H.; Takeda, M. Molecular phylogeny and phylogeography of flightless beetles Parechthistatus gibber and Hayashiechthistatus inexpectus (Coleoptera: Cerambycidae) inferred from mitochondrial COI gene sequences. Entomol. Sci. 2008, 11, 239–246. [Google Scholar] [CrossRef]
- Petit-Marty, N.; Vázquez-Luis, M.; Hendriks, I.E. Use of the nucleotide diversity in COI mitochondrial gene as an early diagnostic of conservation status of animal species. Conserv. Lett. 2020, 14, e12756. [Google Scholar] [CrossRef]
- Pruett, C.L.; Winker, K. The effects of sample size on population genetic diversity estimates in song sparrows Melospiza melodia. J. Avian Biol. 2008, 39, 252–256. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Shreeve, T.G. Climatic change and the British butterfly fauna: Opportunities and constraints. Biol. Conserv. 1991, 55, 1–16. [Google Scholar] [CrossRef]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hillk, J.K.; Thomas, C.D.; Descimon, H.; Huntleyk, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Volney, W.J.A.; Fleming, R.A. Climate change and impacts of boreal forest insects. Agric. Ecosyst. Environ. 2000, 82, 283–294. [Google Scholar] [CrossRef]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding pole wards. Glob. Change Biol. 2005, 12, 450–455. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Pelini, S.L.; Prior, K.M.; Dzurisin, J.D.K. The response of two butterfly species to climatic variation at the edge of their range and the implications for poleward range shifts. Oecologica 2008, 157, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, M.; Hufnagel, L. Impacts of climate change on Lepidoptera species and communities. Appl. Ecol. Environ. Res. 2011, 9, 43–72. [Google Scholar] [CrossRef]
- Wermelinger, B.; Seifert, M. Analysis of temperature dependent development of the spruce bark beetle Ips typographus (L.) (Col. Scol.). J. Appl. Entomol. 1998, 122, 185–191. [Google Scholar] [CrossRef]
- Yi, D.A.; Kuprin, A.V.; Lee, Y.H.; Bae, Y.J. Newly developed fungal diet for artificial rearing of the endangered long-horned beetle Callipogon relictus (Coleoptera: Cerambycidae). Entomol. Res. 2017, 47, 373–379. [Google Scholar] [CrossRef]
- Honda, K.; Akutsu, K.; Arai, S. Photoperiodic response of Acalolepta luxuriosa Bates (Coleoptera: Cerambycidae): Effect of photoperiodic change for induction of larval diapose. Jpn. J. Appl. Entomol. Zool. 1981, 25, 108–112. [Google Scholar] [CrossRef]
- Shintani, Y.; Ishikawa, Y.; Tatsuki, S. Larval daiapause in the Yellow-spotted longicorn beetle, Pascothea hilais (Pascoe) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1996, 31, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Faccoli, M. Effect of weather on Ips typographus (Coleoptera Curculionidae) Phenology, Voltinism, and Associated Spruce Mortality in the Southeastern Alps. Environ. Entomol. 2009, 38, 307–316. [Google Scholar] [CrossRef]
- Štefková, K.; Okrouhlik, J.; Doležal, P. Development and survival of the spruce bark beetle, Ips typographus (Coleoptera: Curculionidae: Scolytinae) at low temperatures in the laboratory and the field. Eur. J. Entomol. 2017, 114, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yi, D.A.; Kuprin, A.V.; Bae, Y.J. Effects of temperature on instar number and larval development in the endangered longhorn beetle Callipogon relictus (Coleoptera: Cerambycidae) raised on an artificial diet. Can. Entomol. 2019, 151, 537–544. [Google Scholar] [CrossRef]

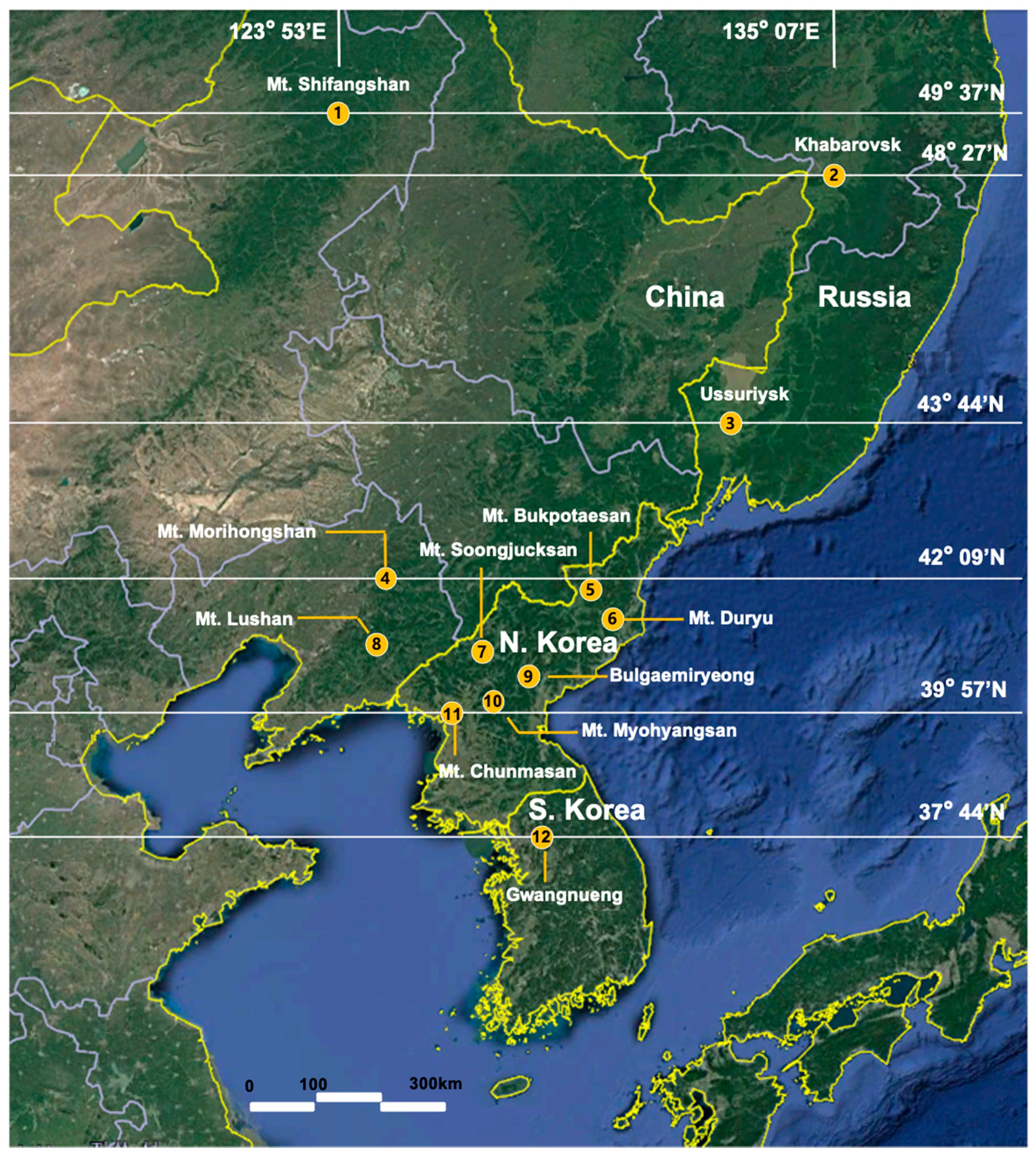

| Site | Locality (Abbreviation) | GPS | Region | Specimen | n | NH | h(SD) | π (SD) | Tajima’s D | Fu’s Fs |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mt. Shifangshan, Inner Mongolia, China (CHSF) | 49°37′ N/123°53′ E | Northwest (NW) | CPD-026 CPD-027 | 2 | 2 | 1.000 (0.500) | 0.013 (0.014) | 0 | 2.303 |
| 2 | Khabarovsk Region, Russia (RUKB) | 48°27′ N/135°05′ E | Northeast (NE) | CPD-196 | 1 | 1 | 1.000 (0.000) | 0.000 (0.000) | 0 | 0 |
| 3 | Ussuriysk, Primoskij Kra, Russia (RUPR) | 43°44′ N/131°56′ E | Central North (CN) | CPD-003 CPD-004 CPD-189 CPD-190 CPD-191 CPD-197 CPD-198 | 7 | 7 | 1.000 (0.000) | 0.020 (0.076) | 1.586 | −0.9891 |
| 4 | Mt. Morihongshan, Fushun, Liaoning, China (CHMR) | 41°47′ N/123°58′ E | Central (C) | CPD-180 CPD-181 CPD-034 CPD-035 | 4 | 4 | 1.000 (0.177) | 0.025 (0.017) | −0.522 | 1.096 |
| 5 | Mt. Bukpotaesan, Ryanggang-do, North Korea (NKBT) | 41°41′ N/128°18′ E | Central (C) | CPD-161 CPD-162 CPD-163 CPD-164 | 4 | 2 | 0.667 (0.204) | 0.002 (0.002) | 1.633 | 1.530 |
| 6 | Mt. Duryusan, Hamgyeongnam-do, North Korea (NKDR) | 41°08′ N/128°07′ E | Central (C) | CPD-007 CPD-010 | 2 | 1 | 0.000 (0.000) | 0.000 (0.000) | 0 | 0 |
| 7 | Mt. Soongjucksan, Jagang-do, North Korea (NKSJ) | 40°27′ N/126°23′ E | Central (C) | CPD-013 CPD-015 | 2 | 2 | 1.000 (0.500) | 0.007 (0.007) | 0 | 1.609 |
| 8 | Mt. Lushan, Beining West, Liaonin, China (CHLS) | 41°36′ N/121°42′ E | Central (C) | CPD-028 | 1 | 1 | 1.000 (0.000) | 0.000 (0.000) | 0 | 0 |
| 9 | Bulgaemiryeong, Hamgyeongnam-do, North Korea (NKBG) | 40°08′ N/127°53′ E | Central (C) | CPD-016 CPD-018 CPD-020 CPD-022 | 4 | 2 | 0.667 (0.204) | 0.002 (0.002) | 1.893 | 1.530 |
| 10 | Mt. Myohyangsan, Pyeonganbuck-do, North Korea (NKMH) | 39°57′ N/126°16′ E | Central (C) | CPD-011 | 1 | 1 | 1.000 (0.000) | 0.000 (0.000) | 0 | 0 |
| 11 | Mt. Chunmasan, Pyeonganbuk-do, North Korea (NKCM) | 40°00′ N/124°56′ E | Central (C) | CPD-144 CPD-145 CPD-146 CPD-147 | 4 | 4 | 1.000 (0.000) | 0.024 (0.016) | −0.683 | 1.029 |
| 12 | Gwangneung Forest South Korea | 37°44′ N/127°09′ E | South (S) | JN093124 (COI) MF521835 (COII) | 1 | 1 | 1.000 (0.000) | 0.000 (0.000) | 0 | 0 |
| Mexico (C. barbartum) | CPD102 | |||||||||
| Mexico (C. senex) (Outgroup) | CPD103 | |||||||||
| Mexico (C. lemoinei) (Outgroup) | CPD104 |
| Grouping | Source of Variation | d.f. | Sum of Squares | Variance Components | Percentage of Variation | ϕ-Statistics |
|---|---|---|---|---|---|---|
| 2 groups (I) (Upper vs. lower from the latitude 40°00′ N) | Among groups | 1 | 16.410 | −0.66370 | −7.53 | ϕCT = −0.075 |
| Among populations Within groups | 10 | 155.610 | 4.24547 | 48.19 | ϕSC = 0.448 | |
| Within populations | 212 | 109.786 | 5.22789 | 59.34 | ϕST = 0.407 | |
| 2 groups (II) (Upper vs. lower from the latitude 43°00′ N) | Among groups | 1 | 2.679 | −1.95599 | −25.85 | ϕCT = −0.259 |
| Among populations Within groups | 10 | 169.081 | 4.29419 | 56.76 | ϕSC = 0.451 | |
| Within populations | 21 | 109.786 | 5.22789 | 69.10 | ϕST = 0.309 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.H.; Yi, D.-A.; Kuprin, A.V.; Han, C.; Bae, Y.J. Phylogeographic Investigation of an Endangered Longhorn Beetle, Callipogon relictus (Coleoptera: Cerambycidae), in Northeast Asia: Implications for Future Restoration in Korea. Insects 2021, 12, 555. https://doi.org/10.3390/insects12060555
Kang JH, Yi D-A, Kuprin AV, Han C, Bae YJ. Phylogeographic Investigation of an Endangered Longhorn Beetle, Callipogon relictus (Coleoptera: Cerambycidae), in Northeast Asia: Implications for Future Restoration in Korea. Insects. 2021; 12(6):555. https://doi.org/10.3390/insects12060555
Chicago/Turabian StyleKang, Ji Hyoun, Dae-Am Yi, Alexander V. Kuprin, Changdo Han, and Yeon Jae Bae. 2021. "Phylogeographic Investigation of an Endangered Longhorn Beetle, Callipogon relictus (Coleoptera: Cerambycidae), in Northeast Asia: Implications for Future Restoration in Korea" Insects 12, no. 6: 555. https://doi.org/10.3390/insects12060555





