Comparative Antennal Morphometry and Sensilla Organization in the Reproductive and Non-Reproductive Castes of the Formosan Subterranean Termite
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Morphometric Analyses
2.3. Antennal Sensilla Analyses
2.4. Statistical Analyses
3. Results
3.1. Morphometric Analyses
3.2. Types of Antennal Sensilla
3.3. Spatial Organization of Antennal Sensilla
3.4. Composition of Antennal Sensilla in Different Castes
4. Discussion
4.1. Antennal Morphology
4.2. Antennal Sensillar Types and Spatial Organization
4.3. Comparison of Antennal Sensilla among Castes
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zacharuk, R.Y. Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Elgar, M.A.; Zhang, D.; Wang, Q.; Wittwer, B.; Pham, H.T.; Johnson, T.L.; Freelance, C.B.; Coquilleau, M. Focus: Ecology and evolution: Insect antennal morphology: The evolution of diverse solutions to odorant perception. Yale J. Biol. Med. 2018, 91, 457. [Google Scholar]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef] [Green Version]
- Korb, J.; Hartfelder, K. Life history and development-a framework for understanding developmental plasticity in lower termites. Biol. Rev. 2008, 83, 295–313. [Google Scholar] [CrossRef]
- McKenzie, S.K.; Fetter-Pruneda, I.; Ruta, V.; Kronauer, D.J.C. Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. Proc. Natl. Acad. Sci. USA 2016, 113, 14091–14096. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, A.; Nishino, H.; Watanabe, H.; Yokohari, F.; Nishikawa, M. Sex-specific antennal sensory system in the ant Camponotus japonicus: Structure and distribution of sensilla on the flagellum. Cell Tissue Res. 2009, 338, 79–97. [Google Scholar] [CrossRef]
- Ravaiano, S.V.; de Paiva Ferreira, R.; de Oliveira Campos, L.A.; Martins, G.F. The antennal sensilla of Melipona quadrifasciata (Hymenoptera: Apidae: Meliponini): A study of different sexes and castes. Naturwissenschaften 2014, 101, 603–611. [Google Scholar] [CrossRef]
- Babu, M.J.; Ankolekar, S.M.; Rajashekhar, K.P. Castes of the weaver ant Oecophylla smaragdina (Fabricius) differ in the organization of sensilla on their antennae and mouthparts. Curr. Sci. 2011, 101, 755–764. [Google Scholar]
- Month-Juris, E.; Ravaiano, S.V.; Lopes, D.M.; Fernandes Salomão, T.M.; Martins, G.F. Morphological assessment of the sensilla of the antennal flagellum in different castes of the stingless bee Tetragonisca fiebrigi. J. Zool. 2020, 310, 110–125. [Google Scholar] [CrossRef]
- Stort, A.C.; Moraes-Alves, M.M.B. Differences in the number of antennal sensory structures of males of three honey bee types. Rev. Bras. Biol. 1999, 59, 161–166. [Google Scholar] [CrossRef]
- Grüter, C.; Segers, F.H.I.D.; Santos, L.L.G.; Hammel, B.; Zimmermann, U.; Nascimento, F.S. Enemy recognition is linked to soldier size in a polymorphic stingless bee. Biol. Lett. 2017, 13, 20170511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Renthal, R.; Velasquez, D.; Olmos, D.; Hampton, J.; Wergin, W.P. Structure and distribution of antennal sensilla of the red imported fire ant. Micron 2003, 34, 405–413. [Google Scholar] [CrossRef]
- Kidokoro-Kobayashi, M.; Iwakura, M.; Fujiwara-Tsujii, N.; Fujiwara, S.; Sakura, M.; Sakamoto, H.; Higashi, S.; Hefetz, A.; Ozaki, M. Chemical discrimination and aggressiveness via cuticular hydrocarbons in a supercolony-forming ant, Formica yessensis. PLoS ONE 2012, 7, e46840. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.R.; Enzmann, B.L.; Schmidt, Y.; Moore, D.; Jones, G.R.; Parker, J.; Berger, S.L.; Reinberg, D.; Zwiebel, L.J.; Breit, B. Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Rep. 2015, 12, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Nutting, W.L. Flight and colony foundation. In Biology of Termites; Krishna, K., Weesner, F.M., Eds.; Academic Press: Cambridge, MA, USA, 1969; Volume 1, pp. 233–282. [Google Scholar]
- Hartke, T.R.; Baer, B. The mating biology of termites: A comparative review. Anim. Behav. 2011, 82, 927–936. [Google Scholar] [CrossRef]
- Bordereau, C.; Pasteels, J.M. Pheromones and Chemical Ecology of Dispersal and Foraging in Termites. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 279–320. [Google Scholar]
- Tian, L.; Zhou, X. The soldiers in societies: Defense, regulation, and evolution. Int. J. Biol. Sci. 2014, 10, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Preisser, E.L.; Haynes, K.F.; Zhou, X. Social buffering in a eusocial invertebrate: Termite soldiers reduce the lethal impact of competitor cues on workers. Ecology 2017, 98, 952–960. [Google Scholar] [CrossRef] [Green Version]
- Krishna, K.; Weesner, F.M. Biology of Termites; Academic Press: New York, NY, USA, 1969; Volume 1. [Google Scholar]
- Hager, F.A.; Krausa, K.; Kirchner, W.H. Vibrational Behavior in Termites (Isoptera). In Biotremology: Studying Vibrational Behavior; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 309–327. [Google Scholar]
- Tokoro, M.; Takahashi, M.; Tsunoda, K.; Yamaoka, R. Isolation and Primary Structure of Trail Pheromone of the Termite, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). Wood Res. Bull. Wood Res. Inst. Kyoto Univ. 1989, 76, 29–38. [Google Scholar]
- Matsuura, K.; Himuro, C.; Yokoi, T.; Yamamoto, Y.; Vargo, E.L.; Keller, L. Identification of a pheromone regulating caste differentiation in termites. Proc. Natl. Acad. Sci. USA 2010, 107, 12963–12968. [Google Scholar] [CrossRef] [Green Version]
- Funaro, C.F.; Böröczky, K.; Vargo, E.L.; Schal, C. Identification of a queen and king recognition pheromone in the subterranean termite Reticulitermes flavipes. Proc. Natl. Acad. Sci. USA 2018, 115, 3888–3893. [Google Scholar] [CrossRef] [Green Version]
- Hanus, R.; Luxová, A.; Šobotník, J.; KalinovÁ, B.; Jiroš, P.; Křeček, J.; Bourguignon, T.; Bordereau, C. Sexual communication in the termite Prorhinotermes simplex (Isoptera, Rhinotermitidae) mediated by a pheromone from female tergal glands. Insectes Soc. 2009, 56, 111–118. [Google Scholar] [CrossRef]
- Chouvenc, T.; Sillam-Dussès, D.; Robert, A. Courtship behavior confusion in two subterranean termite species that evolved in allopatry (Blattodea, Rhinotermitidae, Coptotermes). J. Chem. Ecol. 2020, 46, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Haynes, K.F.; Zhou, X. Dynamic changes in death cues modulate risks and rewards of corpse management in a social insect. Funct. Ecol. 2017, 31, 697–706. [Google Scholar] [CrossRef]
- Sun, Q.; Hampton, J.D.; Merchant, A.; Haynes, K.F.; Zhou, X. Cooperative policing behaviour regulates reproductive division of labour in a termite. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200780. [Google Scholar] [CrossRef]
- Howse, P.E. An investigation into the mode of action of the subgenual organ in the termite, Zootermopsis angusticollis Emerson, and in the cockroach, Periplaneta americana L. J. Insect Physiol. 1964, 10, 409–424. [Google Scholar] [CrossRef]
- Huang, Q. Aggressive behavior and the role of antennal sensillae in the termite Reticulitermes chinensis (Isoptera: Rhinotermitidae). Sociobiology 2014, 59, 1239–1251. [Google Scholar]
- Raina, A.K.; Bland, J.M.; Dickens, J.C.; Park, Y.I.; Hollister, B. Premating behavior of dealates of the Formosan subterranean termite and evidence for the presence of a contact sex pheromone. J. Insect Behav. 2003, 16, 233–245. [Google Scholar] [CrossRef]
- Yanagawa, A.; Yokohari, F.; Shimizu, S. The role of antennae in removing entomopathogenic fungi from cuticle of the termite, Coptotermes formosanus. J. Insect Sci. 2009, 9. [Google Scholar] [CrossRef] [Green Version]
- Chouvenc, T.; Su, N.Y. Colony age-dependent pathway in caste development of Coptotermes formosanus Shiraki. Insectes Soc. 2014, 61, 171–182. [Google Scholar] [CrossRef]
- Prestage, J.J.; Slifer, E.H.; Stephens, L.B. Thin-walled sensory pegs on the antenna of the termite worker, Reticulitermes flavipes. Ann. Entomol. Soc. Am. 1963, 56, 874–878. [Google Scholar] [CrossRef]
- Costa-Leonardo, A.M.; Soares, H.X. Morphological aspects of neotropical termite antenna under scanning microscopy. Rev. Bras. Entomol. 1997, 41, 47–52. [Google Scholar]
- Ishikawa, Y.; Koshikawa, S.; Miura, T. Differences in mechanosensory hairs among castes of the damp-wood termite Hodotermopsis sjostedti (Isoptera: Termopsidae). Sociobiology 2007, 50, 895–907. [Google Scholar]
- Tarumingkeng, R.C.; Coppel, H.C.; Matsumura, F. Morphology and ultrastructure of the antennal chemoreceptors and mechanoreceptors of worker Coptotermes formosanus Shiraki. Cell Tissue Res. 1976, 173, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.F.; Mo, J.C.; He, H.Y.; Pan, C.Y.; Cheng, J.A. Differences of morphology of antennae between soldiers and workers in Coptotermes formosanus (Isoptera: Rhinotermitidae). Sociobiology 2006, 48, 689–700. [Google Scholar]
- Yanagawa, A.; Shimizu, S.; Noma, K.; Nishikawa, M.; Kazumasa, O.; Yokohari, F. Classification and distribution of antennal sensilla of the termite Coptotermes formosanus (Isoptera: Rhinotermitidae). Sociobiology 2009, 54, 327. [Google Scholar]
- Fu, B.X.; Rong, N.H.; Hong, J.; Zhu, Z.R.; Mo, J.C.; Zhang, D. Comparative study with scanning electron microscopy on the antennal sensilla of two main castes of Coptotermes formosanus Shiraki (Blattaria: Rhinotermitidae). Micron 2020, 129, 102777. [Google Scholar] [CrossRef]
- Evans, T.A.; Forschler, B.T.; Grace, J.K. Biology of invasive termites: A worldwide review. Annu. Rev. Entomol. 2013, 58, 455–474. [Google Scholar] [CrossRef] [Green Version]
- Ross, I.; Gentleman, R. R: A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Sall, J.; Lehman, A.; Stephens, M.; Loring, S. JMP Start Statistics: A Guide to Statistics and Data Analysis Using JMP; SAS Institute: Cary, NC, USA, 2017. [Google Scholar]
- Scholtz, O.I.; Macleod, N.; Eggleton, P. Termite soldier defence strategies: A reassessment of Prestwich’s classification and an examination of the evolution of defence morphology using extended eigenshape analyses of head morphology. Zool. J. Linn. Soc. 2008, 153, 631–650. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Henderson, G.; Laine, R.A. Lignoceric acid and hexacosanoic acid: Major components of soldier frontal gland secretions of the Formosan subterranean termite (Coptotermes formosanus). J. Chem. Ecol. 1999, 25, 817–824. [Google Scholar] [CrossRef]
- Parsons, P.A. Fluctuating asymmetry: An epigenetic measure of stress. Biol. Rev. Camb. Philos. Soc. 1990, 65, 131–145. [Google Scholar] [CrossRef] [PubMed]
- De Coster, G.; Van Dongen, S.; Malaki, P.; Muchane, M.; Alcántara-Exposito, A.; Matheve, H.; Lens, L. Fluctuating asymmetry and environmental stress: Understanding the role of trait history. PLoS ONE 2013, 8, e57966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaki, M.; Wada-Katsumata, A.; Fujikawa, K.; Iwasaki, M.; Yokohari, F.; Satoji, Y.; Nisimura, T.; Yamaoka, R. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 2005, 309, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.; Drijfhout, F. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 2009, 35, 1151–1161. [Google Scholar] [CrossRef]
- Van Oystaeyen, A.; Oliveira, R.C.; Holman, L.; van Zweden, J.S.; Romero, C.; Oi, C.A.; d’Ettorre, P.; Khalesi, M.; Billen, J.; Wäckers, F. Conserved class of queen pheromones stops social insect workers from reproducing. Science 2014, 343, 287–290. [Google Scholar] [CrossRef]
- Sun, Q.; Haynes, K.F.; Zhou, X. Temporal changes in cuticular hydrocarbons during worker-reproductive transition in the eastern subterranean termite (Blattodea: Rhinotermitidae). Ann. Entomol. Soc. Am. 2020. [Google Scholar] [CrossRef]
- Gautam, B.K.; Henderson, G. Effects of sand moisture level on food consumption and distribution of Formosan subterranean termites (Isoptera: Rhinotermitidae) with different soldier proportions. Entomol. Sci. 2011, 46, 1–13. [Google Scholar] [CrossRef]
- Gautam, B.K.; Henderson, G. Relative humidity preference and survival of starved Formosan subterranean termites (Isoptera: Rhinotermitidae) at various temperature and relative humidity conditions. Environ. Entomol. 2011, 40, 1232–1238. [Google Scholar] [CrossRef] [Green Version]
- Yanagawa, A.; Yoshimura, T.; Yanagawa, T.; Yokohari, F. Detection of a humidity difference by antennae in the termite Coptotermes formosanus (Isoptera: Rhinotermitidae). Sociobiology 2010, 56, 255–270. [Google Scholar]
- Nalepa, C.A.; Evans, T.A.; Lenz, M. Antennal cropping during colony foundation in termites. ZooKeys 2011, 148, 185–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaguni, Y.; Sugio, K.; Tsuji, K. Antennal cropping in the Asian dry-wood termite, Neotermes koshunensis. Insectes Soc. 2013, 60, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-I.; Neoh, K.-B.; Li, H.-F. Colony-founding success of pleometrosis in a fungus-growing termite Odontotermes formosanus. Behav. Ecol. Sociobiol. 2018, 72, 13. [Google Scholar] [CrossRef]
- Chouvenc, T.; Scheffrahn, R.H.; Mullins, A.J.; Su, N.Y. Flight phenology of two Coptotermes species (Isoptera: Rhinotermitidae) in southeastern Florida. J. Econ. Entomol. 2017, 110, 1693–1704. [Google Scholar] [CrossRef]
- Chouvenc, T.; Su, N.Y. Irreversible transfer of brood care duties and insights into the burden of caregiving in incipient subterranean termite colonies. Ecol. Entomol. 2017, 42, 777–784. [Google Scholar] [CrossRef]
- Gordon, J.M.; Šobotník, J.; Chouvenc, T. Colony-age-dependent variation in cuticular hydrocarbon profiles in subterranean termite colonies. Ecol. Evol. 2020, 10, 10095–10104. [Google Scholar] [CrossRef]
- Bardunias, P.M.; Su, N.Y. Queue size determines the width of tunnels in the Formosan subterranean termite (Isoptera: Rhinotermitidae). J. Insect Behav. 2010, 23, 189–204. [Google Scholar] [CrossRef]
- Crosland, M.W.J.; Traniello, J.F.A. Behavioral plasticity in division of labor in the lower termite Reticulitermes fukienensis. Naturwissenschaften 1997, 84, 208–211. [Google Scholar] [CrossRef]
- Ferguson, S.T.; Bakis, I.; Zwiebel, L.J. Advances in the study of olfaction in eusocial ants. Insects 2021, 12, 252. [Google Scholar] [CrossRef]
- Yan, H.; Liebig, J. Genetic basis of chemical communication in eusocial insects. Genes Dev. 2021, 35, 470–482. [Google Scholar] [CrossRef]
- Yanagawa, A.; Yokohari, F.; Shimizu, S. Influence of fungal odor on grooming behavior of the termite, Coptotermes formosanus. J. Insect Sci. 2010, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, Y.; Aonuma, H.; Sasaki, K.; Miura, T. Tyraminergic and octopaminergic modulation of defensive behavior in termite soldier. PLoS ONE 2016, 11, e0154230. [Google Scholar] [CrossRef] [PubMed]
- Mitaka, Y.; Kobayashi, K.; Mikheyev, A.; Tin, M.M.Y.; Watanabe, Y.; Matsuura, K. Caste-specific and sex-specific expression of chemoreceptor genes in a termite. PLoS ONE 2016, 11, e0146125. [Google Scholar] [CrossRef]
- Harrison, M.C.; Jongepier, E.; Robertson, H.M.; Arning, N.; Bitard-Feildel, T.; Chao, H.; Childers, C.P.; Dinh, H.; Doddapaneni, H.; Dugan, S. Hemimetabolous genomes reveal molecular basis of termite eusociality. Nat. Ecol. Evol. 2018, 2, 557–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Oi, F.M.; Scharf, M.E. Social exploitation of hexamerin: RNAi reveals a major caste-regulatory factor in termites. Proc. Natl. Acad. Sci. USA 2006, 103, 4499–4504. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Yu, S.; Merchant, A.; Lei, C.; Zhou, X.; Huang, Q. Downregulation of Orco and 5-HTT alters nestmate discrimination in the subterranean termite Odontotermes formosanus (Shiraki). Front. Physiol. 2019, 10, 714. [Google Scholar] [CrossRef]
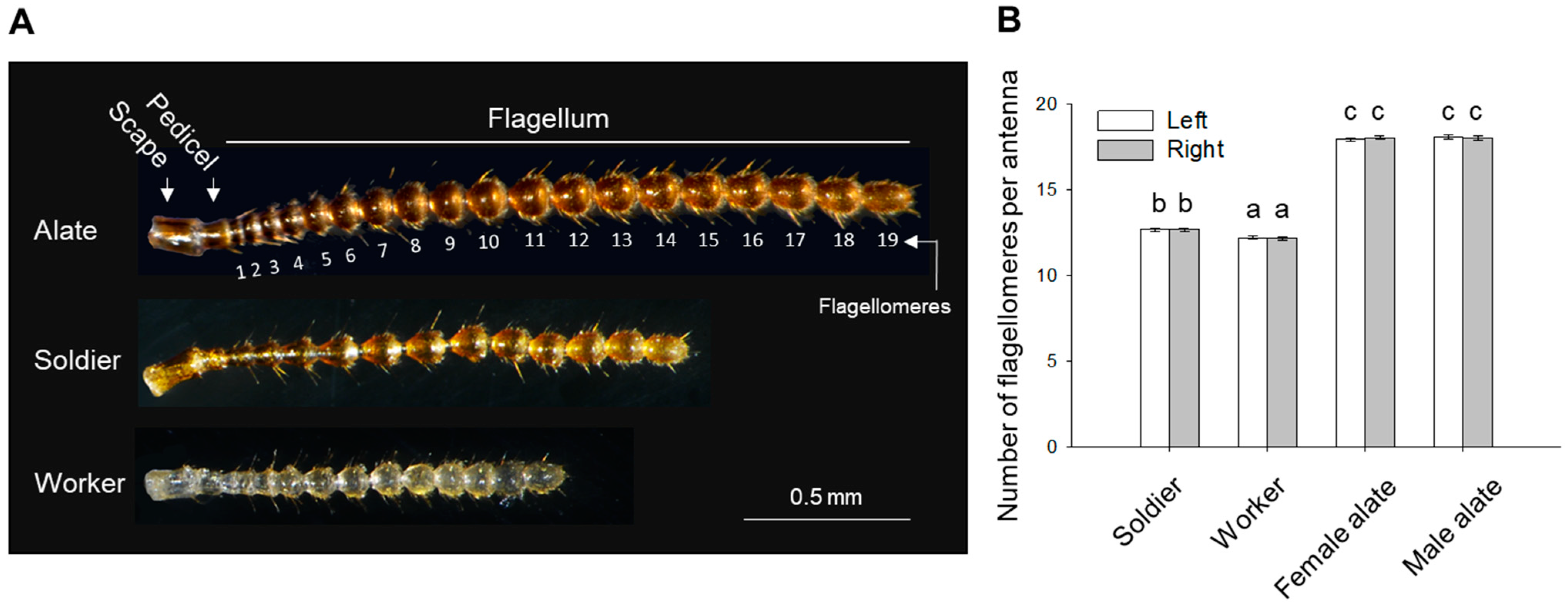





| Side | Soldier | Worker | Female Alate | Male Alate | |
|---|---|---|---|---|---|
| Total antennal length (mm) | Left | 1.643 ± 0.019 (b) | 1.277 ± 0.017 (a) | 2.309 ± 0.014 (c) | 2.284 ± 0.020 (c) |
| Right | 1.648 ± 0.019 (b) | 1.265 ± 0.020 (a) | 2.335 ± 0.016 (c) | 2.284 ± 0.026 (c) | |
| Antennal length normalized to body length (%) | Left | 45.78 ± 0.540 (b) | 36.42 ± 0.555 (a) | 34.45 ± 0.215 (a) | 35.61 ± 0.310 (a) |
| Right | 45.93 ± 0.540 (b) | 36.11 ± 0.660 (a) | 34.84 ± 0.251 (a) | 35.60 ± 0.409 (a) | |
| Length of scape (µm) | Left | 163.50 ± 3.053 (ab) | 159.13 ± 2.750 (a) | 173.16 ± 4.160 (c) | 179.66 ± 2.360 (bc) |
| Right | 165.43 ± 2.906 (b) | 148.27 ± 3.635 (a) | 179.03 ± 2.202 (b) | 173.08 ± 2.974 (b) | |
| Length of pedicel (µm) | Left | 90.23 ± 1.506 (b) | 84.50 ± 0.816 (a) | 91.97 ± 0.131 (b) | 92.52 ± 1.372 (b) |
| Right | 89.40 ± 1.252 (b) | 82.47 ± 1.462 (a) | 95.38 ± 1.285 (b) | 94.72 ± 1.285 (b) | |
| Width of scape (µm) | Left | 95.60 ± 0.601 (a) | 94.90 ± 0.929 (a) | 120.38 ± 1.097 (b) | 124.00 ± 1.350 (c) |
| Right | 96.20 ± 0.904 (a) | 93.03 ± 1.287 (a) | 124.55 ± 1.344 (b) | 121.40 ± 1.316 (b) | |
| Width of pedicel (µm) | Left | 72.90 ± 0.597 (a) | 78.10 ± 0.422 (b) | 94.36 ± 0.984 (c) | 93.55 ± 0.930 (c) |
| Right | 72.90 ± 0.524 (a) | 77.23 ± 0.644 (b) | 95.45 ± 0.628 (c) | 93.76 ± 0.722 (c) | |
| Width of proximal flagellomere (µm) | Left | 64.03 ± 0.885 (a) | 71.20 ± 0.643 (b) | 86.80 ± 1.235 (c) | 87.41 ± 1.604 (c) |
| Right | 65.83 ± 1.092 (a) | 72.13 ± 0.847 (b) | 88.97 ± 1.283 (c) | 86.40 ± 1.786 (c) | |
| Width of central flagellomere (µm) | Left | 90.83 ± 0.924 (a) | 93.90 ± 1.004 (a) | 115.45 ± 1.294 (c) | 109.40 ± 1.094 (b) |
| Right | 91.63 ± 0.931 (a) | 96.27 ± 0.953 (b) | 115.55 ± 1.110 (c) | 112.76 ± 1.482 (c) | |
| Width of distal flagellomere (µm) | Left | 80.17 ± 0.803 (a) | 83.07 ± 0.694 (a) | 83.34 ± 1.073 (a) | 83.68 ± 1.174 (a) |
| Right | 80.60 ± 0.592 (a) | 83.50 ± 0.805 (b) | 82.41 ± 0.873 (ab) | 83.48 ± 0.818 (ab) |
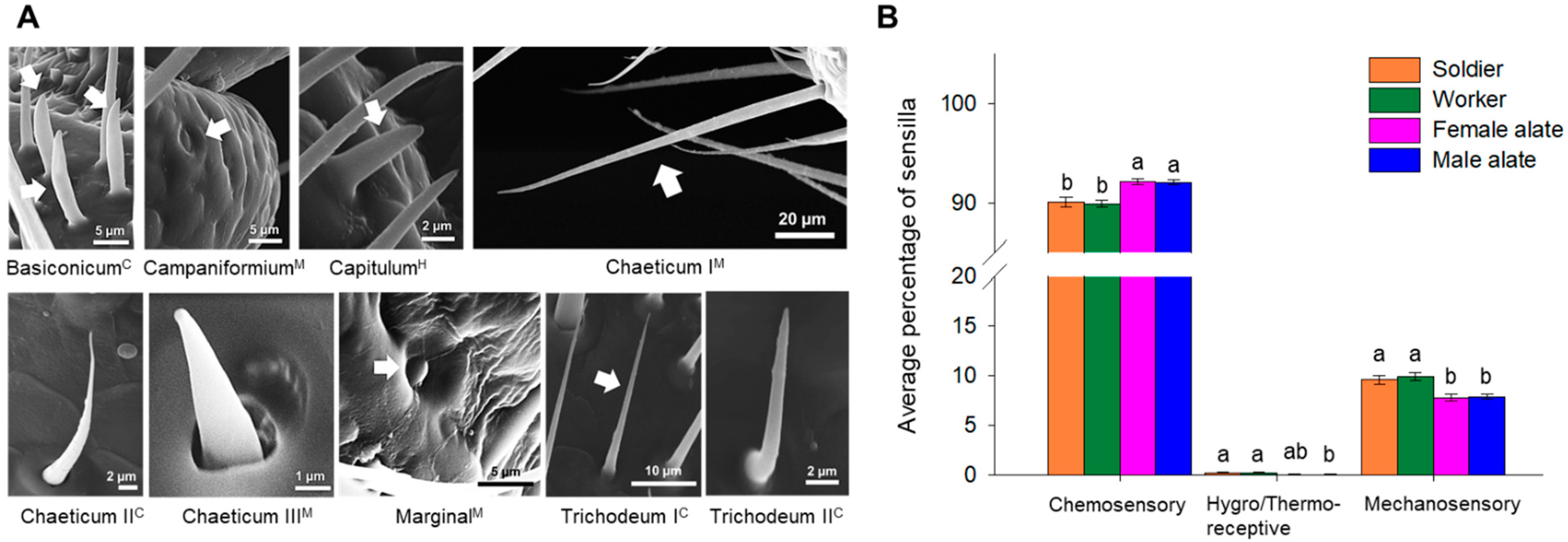
| Sensillar Type | Morphological Characteristics | Putative Function | Total Length (µm) | Basal Diameter (µm) | Tip Diameter (µm) | Socket Diameter (µm) | Central Diameter (µm) |
|---|---|---|---|---|---|---|---|
| Basiconicum | Short, blunt-tipped, cylindrical-shaped with a narrow tip | Chemosensory | 12.916 ± 0.685 | 2.129 ± 0.067 | 0.504 ± 0.068 | 3.559 ± 0.070 | - |
| Campaniformium | Smooth-surfaced oval-shaped pore | Mechanosensory | 4.472 ± 0.127 | - | - | - | 1.702 ± 0.088 |
| Capitulum | Cone-shaped, wider at the base and narrowed towards the tip | Hygro/thermoreceptive | 6.579 ± 0.162 | 2.483 ± 0.087 | 0.608 ± 0.032 | 3.970 ± 0.226 | - |
| Chaeticum I | Longest sensilla; the wide base gives support to its cylindrical shape that ends in a thin tip | Mechanosensory | 114.649 ± 7.595 | 4.209 ± 0.310 | 0.618 ± 0.007 | 5.523 ± 0.226 | - |
| Chaeticum II | Wider at the base and narrower towards the tip; slightly curved towards the surface of the antennae | Chemosensory | 18.790 ± 2.405 | 1.986 ± 0.099 | 0.315 ± 0.006 | 2.593 ± 0.106 | - |
| Chaeticum III | Short, cone-shaped sensilla located in a socket-like structure | Mechanosensory | 5.860 ± 0.500 | 1.636 ± 0.029 | 0.397 ± 0.045 | 2.776 ± 0.150 | - |
| Marginal | Smooth-surfaced dome in a socket | Mechanosensory | - | - | - | 3.360 ± 0.697 | 1.899 ± 0.464 |
| Trichodeum I | Similar in shape to Chaeticum II but straight, without curving towards the surface of the antennae | Chemosensory | 49.093 ± 1.069 | 2.473 ± 0.061 | 0.455 ± 0.015 | 3.546 ± 0.302 | - |
| Trichodeum II | Wider than trichodeum I, slightly curved towards the surface of the antennae | Chemosensory | 11.520 ± 0.207 | 1.840 ± 0.162 | 0.291 ± 0.061 | 2.487 ± 0.235 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, P.; Le, N.; Sun, Q. Comparative Antennal Morphometry and Sensilla Organization in the Reproductive and Non-Reproductive Castes of the Formosan Subterranean Termite. Insects 2021, 12, 576. https://doi.org/10.3390/insects12070576
Castillo P, Le N, Sun Q. Comparative Antennal Morphometry and Sensilla Organization in the Reproductive and Non-Reproductive Castes of the Formosan Subterranean Termite. Insects. 2021; 12(7):576. https://doi.org/10.3390/insects12070576
Chicago/Turabian StyleCastillo, Paula, Nathan Le, and Qian Sun. 2021. "Comparative Antennal Morphometry and Sensilla Organization in the Reproductive and Non-Reproductive Castes of the Formosan Subterranean Termite" Insects 12, no. 7: 576. https://doi.org/10.3390/insects12070576
APA StyleCastillo, P., Le, N., & Sun, Q. (2021). Comparative Antennal Morphometry and Sensilla Organization in the Reproductive and Non-Reproductive Castes of the Formosan Subterranean Termite. Insects, 12(7), 576. https://doi.org/10.3390/insects12070576






