Horizontal Honey-Bee Larvae Rearing Plates Can Increase the Deformation Rate of Newly Emerged Adult Honey Bees
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing Honey Bee Larvae In Vitro
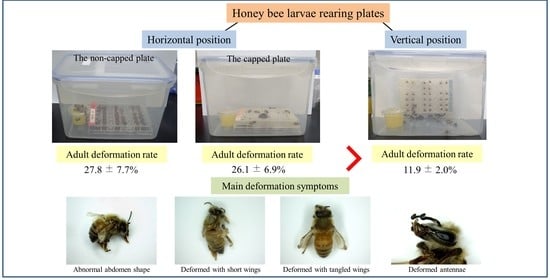
2.2. Experimental Design
2.3. Mortality and Abnormal Symptoms
2.4. Statistical Analysis
3. Results
3.1. In Vitro Mortality, Adult Emergence Rates, and Survival
3.2. Emergence Rates by Time (Days)
3.3. Deformation Rate of Newly Emerged Adult Bees
3.4. Body Weight and Length of Newly Emerged Adult Bees
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissiere, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Lebuhn, G.; Droege, S.; Connor, E.F.; Gemmill-Herren, B.; Potts, S.G.; Minckley, R.L.; Griswold, T.; Jean, R.; Kula, E.; Roubik, D.W.; et al. Detecting insect pollinator declines on regional and globalscales. Conserv. Biol. 2013, 27, 113–120. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Van der Zee, R.; Pisa, L.; Andonov, S.; Brodschneider, R.; Charriere, J.D.; Chlebo, R.; Coffey, M.F.; Crailsheim, K.; Dahle, B.; Gajda, A.; et al. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008-9 and 2009-10. J. Apic. Res. 2012, 51, 100–114. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Baude, M.; Biesmeijer, J.C.; Britton, N.F.; Brown, M.J.F.; Brown, M.; Carvell, C. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Fairbrother, A.; Purdy, J.; Anderson, T.; Fell, R. Risks of neonicotinoid insecticides to honey bees. Environ. Toxicol. Chem. 2014, 33, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Staveley, J.P.; Law, S.A.; Fairbrother, A.; Menzie, C.A. A causal analysis of observed declines in managed honey bees (Apis mellifera). Hum. Ecol. Risk Assess. 2014, 20, 566–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanEngelsdorp, D.; Speybroeck, N.; Evans, J.D.; Nguyen, B.K.; Mullin, C.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; Tarpy, D.R.; et al. Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. J. Econ. Entomol. 2010, 103, 1517–1523. [Google Scholar] [CrossRef] [Green Version]
- Ratnieks, F.L.W.; Carreck, N.L. Clarity on Honey Bee Collapse? Science 2010, 327, 152–153. [Google Scholar] [CrossRef]
- Grillone, G.; Laurino, D.; Manino, A.; Porporato, M. Toxicity of thiametoxam on in vitro reared honey bee brood. Apidologie 2017, 48, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Schmehl, D.R.; Tome, H.V.V.; Mortensen, A.N.; Martins, G.F.; Ellis, J.D. Protocol for the in vitro rearing of honey bee (Apis mellifera L.) workers. J. Apic. Res. 2016, 55, 113–129. [Google Scholar] [CrossRef] [Green Version]
- Blacquière, T.; Smagghe, G.; van Gestel, C.A.M.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef] [Green Version]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple Routes of Pesticide Exposure for Honey Bees Living Near Agricultural Fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef] [PubMed]
- Medrzycki, P.; Giffard, H.; Aupinel, P.; Belzunces, L.P.; Chauzat, M.-P.; Claßen, C.; Colin, M.E.; Dupont, T.; Girolami, V.; Johnson, R.; et al. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 2013, 52, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Alix, A.; Chauzat, M.P.; Duchard, S.; Lewis, G.; Maus, C.; Miles, M.J.; Pilling, E.D.; Thompson, H.M.; Wallner, K. Environmental risk assessment scheme for plant protection products. Chapter 10: Honeybees-proposed scheme. Julius-Kühn-Archiv 2010, 423, 27–33. [Google Scholar]
- Aupinel, P.; Fortini, D.; Michaud, B.; Medrzyck, P.; Padovani, E.; Przygoda, D.; Maus, C.; Charriere, J.D.; Kilchenmann, V.; Riessberger-Galle, U.; et al. Honey bee brood ring-test: Method for testing pesticide toxicity on honeybee brood in laboratory conditions. Julius-Kühn-Archiv 2009, 423, 96–101. [Google Scholar]
- Hendriksma, H.P.; Hartel, S.; Steffan-Dewenter, I. Honey bee risk assessment: New approaches for in vitro larvae rearing and data analyses. Methods Ecol. Evol. 2011, 2, 509–517. [Google Scholar] [CrossRef]
- Mortensen, A.N.; Ellis, J.D. A honey bee (Apis mellifera) colony’s brood survival rate predicts its in vitro-reared brood survival rate. Apidologie 2018, 49, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Brødsgaard, C.J.; Ritter, W.; Hansen, H. Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae larvae spores. Apidologie 1998, 29, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.S.C.; Mussen, E.; Fong, A.; Montague, M.A.; Tyler, T. Effects of chlortetracycline on honey bee worker larvae reared in vitro. J. Invertebr. Pathol. 1992, 60, 127–133. [Google Scholar] [CrossRef]
- Kaftanoglu, O.; Linksvayer, T.A.; Page, R.E. Rearing Honey Bees, Apis mellifera, in vitro 1: Effects of Sugar Concentrations on Survival and Development. J. Insect Sci. 2011, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rembold, H.; Lackner, B. Rearing of honey bee larvae in vitro: Effect of yeast extract on queen differentiation. J. Apic. Res. 1981, 20, 165–171. [Google Scholar] [CrossRef]
- Tavares, D.A.; Roat, T.C.; Carvalho, S.M.; Silva-Zacarin, E.C.M.; Malaspina, O. In vitro effects of thiamethoxam on larvae of Africanized honey bee Apis mellifera (Hymenoptera: Apidae). Chemosphere 2015, 135, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Dai, P.; Jack, C.J.; Mortensen, A.N.; Bustamante, T.A.; Bloomquist, J.R.; Ellis, J.D. Chronic toxicity of clothianidin, imidacloprid, chlorpyrifos, and dimethoate to Apis mellifera L. larvae reared in vitro. Pest Manag. Sci. 2019, 75, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Rhein, W.V. Über die Entstehung des weiblichen Dimorphismus im Bienenstaate. Wilhelm Roux Arch. Entwickl. Mech. Org. 1933, 129, 601–665. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, J.D.; Shimanuki, H. Technique for rearing worker honey bees in the laboratory. J. Apic. Res. 1987, 26, 90–97. [Google Scholar] [CrossRef]
- Wittmann, D.; Engels, W. Development of test procedures for insecticide-induced brood damage in honey bees. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 1981, 3, 187–190. [Google Scholar]
- Davis, A.R.; Solomon, K.R.; Shuel, R.W. Laboratory studies of honey bee larval growth and development as affected by systemic insecticides at adult-sublethal levels. J. Apic. Res. 1988, 27, 146–161. [Google Scholar] [CrossRef]
- OECD. Test No. 237: Honey Bee (Apis mellifera) Larval Toxicity Test, Single Exposure. OECD Guidance for the Testing of Chemicals, Section 2, No. 237; OECD Publishing: Paris, France, 2013. [Google Scholar]
- OECD. Test No. 239: Honey Bee (Apis mellifera) Larval Toxicity Test, Repeated Exposure. OECD Environment, Health and Safety Publications, Series on Testing and Assessment, No.239; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Crailsheim, K.; Brodschneider, R.; Aupinel, P.; Behrens, D.; Genersch, E.; Vollmann, J.; Riessberger-Gallé, U. Standard methods for artificial rearing of Apis mellifera larvae. J. Apic. Res. 2013, 52, 1–16. [Google Scholar] [CrossRef]
- Fukuda, H.; Sakagami, S.F. Worker brood survival in honey bees. Res. Popul. Ecol. 1968, 10, 31–39. [Google Scholar] [CrossRef]
- Medrzycki, P.; Sgolastra, F.; Bortolotti, L.; Bogo, G.; Tosi, S.; Padovani, E.; Porrini, C.; Sabatini, A.G. Influence of brood rearing temperature on honey bee development and susceptibility to poisoning by pesticides. J. Apic. Res. 2010, 49, 52–59. [Google Scholar] [CrossRef]
- Williams, G.R.; Alaux, C.; Costa, C.; Csáki, T.; Doublet, V.; Eisenhardt, D.; Kuhn, R.; McMahon, D.P.; Medrzycki, P.; Murray, T.E. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res. 2013, 52, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Staron, M.; Sabo, R.; Staroňová, D.; Sabová, L.; Abou-Shaara, H.F. The age of honey bee larvae at grafting can affect survival during larval tests. Environ. Exp. Biol. 2019, 17, 1–4. [Google Scholar]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 2014, 9, e77547. [Google Scholar]
- Aupinel, P.; Fortini, D. Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. Bull. Insectol. 2005, 58, 107–111. [Google Scholar]
- Vazquez, D.E.; Farina, W.M. Differences in pre-imaginal development of the honey bee Apis mellifera between in vitro and in-hive contexts. Apidologie 2020, 51, 861–875. [Google Scholar] [CrossRef]
- Riessberger-Gallé, U.; Vollmann, J.; Brodschneider1, R.; Aupinel, P.; Crailsheim, K. Improvement in the pupal development of artificially reared honeybee larvae. Apidologie 2008, 39, 595. [Google Scholar]
- Brodschneider, R.; Riessberger-Gallé, U.; Crailsheim, K. Flight performance of artificially reared honeybees (Apis mellifera). Apidologie 2009, 40, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Fine, J.D.; Cox-Foster, D.L.; Mullin, C.A. An inert pesticide adjuvant synergizes viral pathogenicity and mortality in honey bee larvae. Sci. Rep. 2017, 7, 40499. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, W.F.; Tome, H.V.V.; Bernardes, R.C.; Siqueira, M.A.L.; Smagghe, G.; Guedes, R.N.C. Biopesticide-induced be-havioral and morphological alterations in the stingless bee Melipona quadrifasciata. Environ. Toxicol. Chem. 2015, 34, 2149–2158. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, S.; Liu, F.; Wang, Q.; Dai, P. Acute and chronic toxicity of acetamiprid, carbaryl, cypermethrin and deltamethrin to Apis mellifera larvae reared in vitro. Pest Manag. Sci. 2019, 76, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D.; Morse, R.A. The nest of the honey bee (Apis mellifera L.). Lnsectes Sociaux 1976, 23, 495–512. [Google Scholar] [CrossRef]
- Krainer, S.; Brodschneider, R.; Vollmann, J.; Crailsheim, K.; Riessberger-Gallé, U. Effect of hydroxymethylfurfural (HMF) on mortality of artificially reared honey bee larvae (Apis mellifera carnica). Ecotoxicology 2016, 25, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Tehel, A.; Vu, Q.; Bigot, D.; Gogol-Döring, A.; Koch, P.; Jenkins, C.; Doublet, V.; Theodorou, P.; Paxton, R. The two prevalent genotypes of an emerging infectious disease, deformed wing virus, cause equally low pupal mortality and equally high wing deformities in host honey bees. Viruses 2019, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Oertel, E. Metamorphosis in the honeybee. J. Morphol. 1930, 50, 295–339. [Google Scholar] [CrossRef]





| Day | 1 | 3 | 4, 5, 6 |
|---|---|---|---|
| Volume of diet/larva (μL) | 20 | 20 | 30, 40, 50 |
| Diet component | Diet A | Diet B | Diet C |
| Royal jelly (%) | 50.0 | 50.0 | 50.0 |
| Distilled water (%) | 37.0 | 33.5 | 30.0 |
| Glucose (%) | 6.0 | 7.5 | 9.0 |
| Fructose (%) | 6.0 | 7.5 | 9.0 |
| Yeast extract (%) | 1.0 | 1.5 | 2.0 |
| Symptom | Description |
|---|---|
| Early death (ED) | Sunk in diet, failed to maintain the C-shape, flattening |
| Melanizing death (MD) | Death with darkening internally or externally, having black spots |
| Failed molt (FM) | Failure to evert imaginal discs, but the pupal molt is incomplete |
| Failed adult molt (FA) | Failure to emerge from final molt |
| Surviving normal (SN) | Survived and successfully eclosed as bees |
| Deformed wings (DW) | Eclosed as bees with deformed wings |
| -with short wings (DSW) | Eclosed as bees with short wings |
| -with tangled wings (DTW) | Eclosed as bees with tangled wings |
| Deformed antennae (DA) | Eclosed as bees with deformed antennae |
| Abnormal abdomen shape (AAS) | Eclosed as bees with abnormal abdomen shape (humpback) |
| NHG 1 | CHG 2 | CVG 3 | p Values | |
|---|---|---|---|---|
| Larval mortality (%) | 4.9 (±0.7) | 7.7 (±4.1) | 4.2 (±2.7) | 0.396 |
| Pupal mortality (%) | 16.1 (±2.8) | 13.5 (±3.4) | 15.3 (±2.2) | 0.843 |
| Adult emergence rate (%) | 79.9 (±3.3) | 79.9 (±6.7) | 81.3 (±0.9) | 0.943 |
| Observed Symptoms 1 | NHG 2 | CHG 3 | CVG 4 |
|---|---|---|---|
| SN (%) | 73.0 | 73.0 | 88.0 |
| AAS (%) | 13.9 | 4.3 | 3.4 |
| DSW (%) | 0.9 | 4.3 | 1.7 |
| DTW (%) | 0.9 | 3.5 | 1.7 |
| DTWA (%) | 3.5 | 6.1 | 3.4 |
| DA (%) | 0.0 | 0.9 | 0.9 |
| AAS + DW (%) | 4.3 | 4.3 | 0.0 |
| AAS + DA (%) | 0.0 | 1.7 | 0.0 |
| DW + DA (%) | 0.9 | 0.9 | 0.9 |
| AAS + DW + DA (%) | 2.6 | 0.9 | 0.0 |
| Weight (mg) | Length (mm) | |
|---|---|---|
| NHG 1 | 67.40 (±12.24) a | 10.88 (±0.96) a |
| CHG 2 | 69.77 (±12.63) ab | 10.82 (±0.92) a |
| CVG 3 | 71.95 (±12.12) b | 11.55 (±1.00) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Chon, K.; Kim, B.-S.; Oh, J.-A.; Yoon, C.-Y.; Park, H.-H.; Choi, Y.-S. Horizontal Honey-Bee Larvae Rearing Plates Can Increase the Deformation Rate of Newly Emerged Adult Honey Bees. Insects 2021, 12, 603. https://doi.org/10.3390/insects12070603
Kim J, Chon K, Kim B-S, Oh J-A, Yoon C-Y, Park H-H, Choi Y-S. Horizontal Honey-Bee Larvae Rearing Plates Can Increase the Deformation Rate of Newly Emerged Adult Honey Bees. Insects. 2021; 12(7):603. https://doi.org/10.3390/insects12070603
Chicago/Turabian StyleKim, Juyeong, Kyongmi Chon, Bo-Seon Kim, Jin-A Oh, Chang-Young Yoon, Hong-Hyun Park, and Yong-Soo Choi. 2021. "Horizontal Honey-Bee Larvae Rearing Plates Can Increase the Deformation Rate of Newly Emerged Adult Honey Bees" Insects 12, no. 7: 603. https://doi.org/10.3390/insects12070603
APA StyleKim, J., Chon, K., Kim, B.-S., Oh, J.-A., Yoon, C.-Y., Park, H.-H., & Choi, Y.-S. (2021). Horizontal Honey-Bee Larvae Rearing Plates Can Increase the Deformation Rate of Newly Emerged Adult Honey Bees. Insects, 12(7), 603. https://doi.org/10.3390/insects12070603