Improvement of the Mass-Rearing Protocols for the South American Fruit Fly for Application of the Sterile Insect Technique
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of the Incubation Time for Eggs in Water
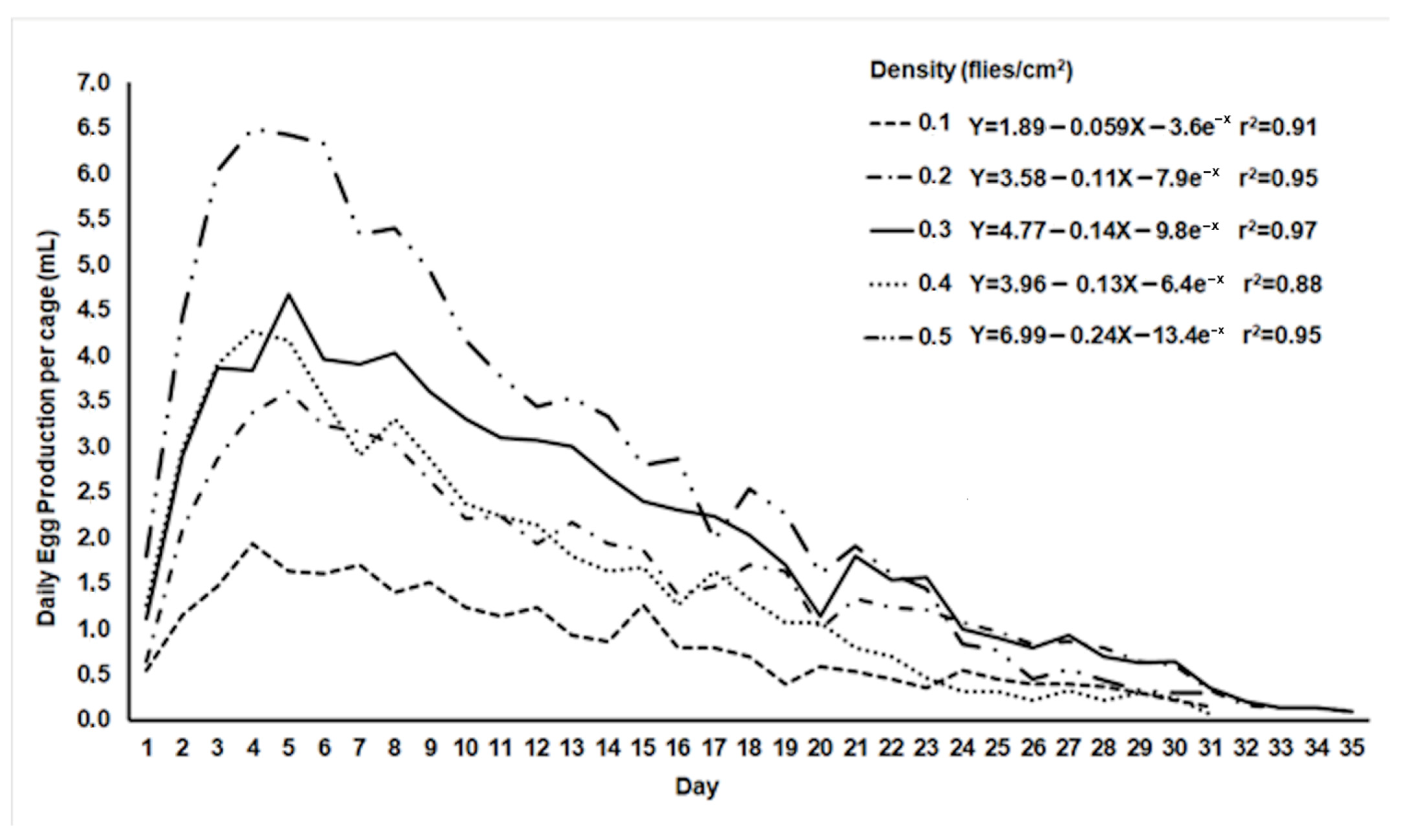
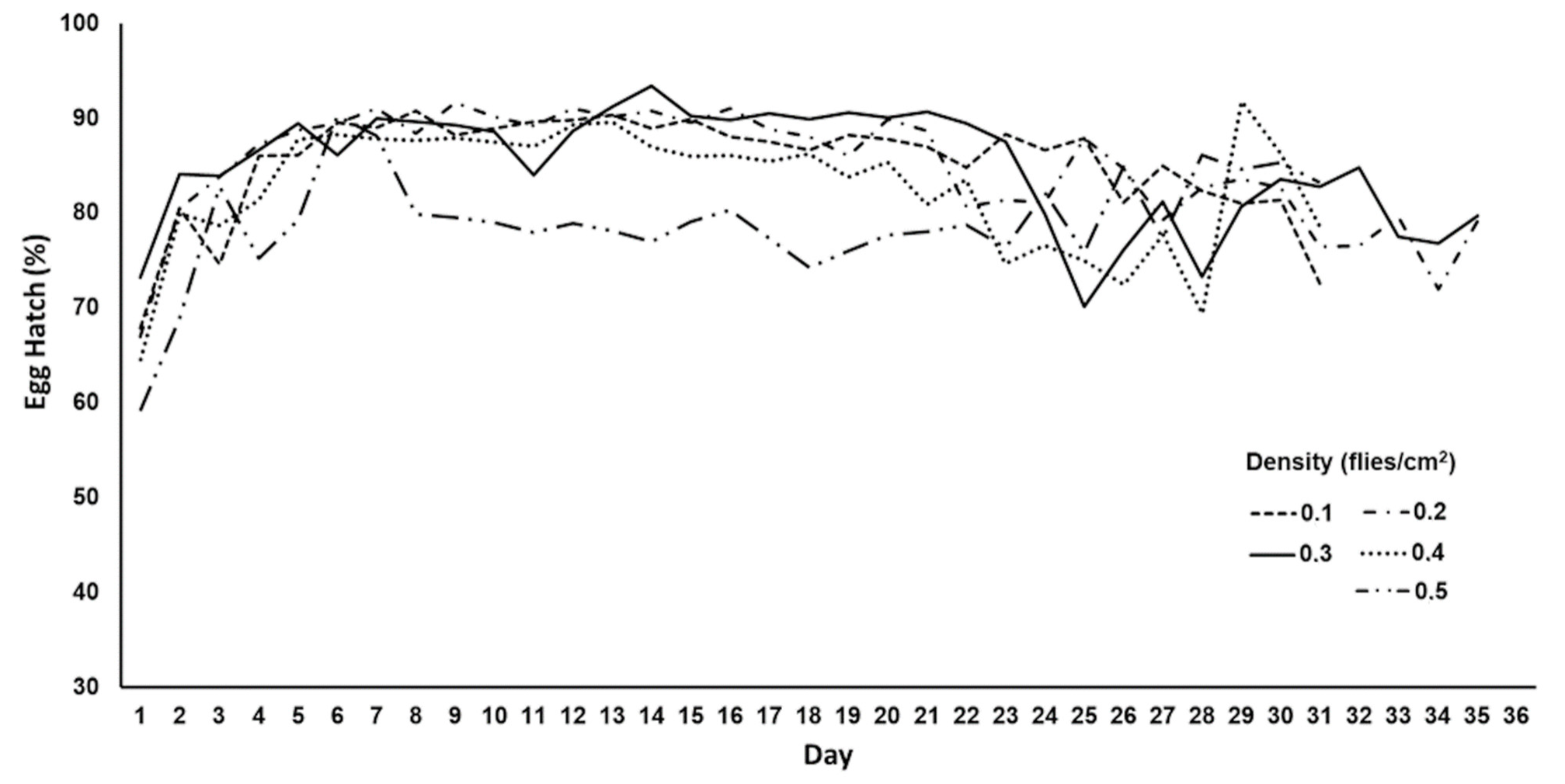
2.2. Assessment of Egg Production in Ovipositing Cages with Different Adult Densities
2.3. Evaluation of Larval Density and Comparison of Diets
2.4. Mating Compatibility Tests
2.5. Data Analysis
3. Results
3.1. Incubation Time of the Eggs in Water
3.2. Egg Production in Oviposition Cages with Different Adult Densities
3.3. Larval Diets
3.4. Mating Compatibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malavasi, A.; Zucchi, R.A. Moscas-das-Frutas de Importância Econômica no Brasil: Conhecimento Básico e Aplicado, 1st ed.; Holos Editora: Ribeirão Preto, Brazil, 2000; p. 327p. [Google Scholar]
- Zucchi, R.A.; Moraes, R.C.B. Fruit Flies (Diptera: Tephritidae) in Brazil: Anastrepha Species Their Host Plants and Parasitoids. Available online: http://www.lea.esalq.usp.br/anastrepha/ (accessed on 17 September 2020).
- Costa-Silva, F.C.; Acioli, A.N.S.; Silva, N.M.; Uramoto, K.; Savaris, M.; Zucchi, R.A. New records of Anastrepha Schiner, 1868 (Diptera, Tephritidae) in an urban forest fragment in Manaus, Amazonas, Brazil. Check List 2020, 16, 853–857. [Google Scholar] [CrossRef]
- Raga, A.; Prestes, D.; Souza Filho, M.; Sato, M.; Siloto, R.; Guimarães, J.; Zucchi, R. Fruit fly (Diptera: Tephritoidea) infestation in citrus in the State of São Paulo, Brazil. Neotrop. Entomol. 2004, 33, 85–89. [Google Scholar] [CrossRef] [Green Version]
- [Fundecitrus] Fundo de Defesa da Citricultura. Estimativa da safra de Laranja 2020/2021 do Cinturão Citrícola de São Paulo e Triângulo/Sudoeste Mineiro: Sumário Executivo 2020/21. Available online: https://www.fundecitrus.com.br/pdf/pes_relatorios/2020_05_11_Sumario-Executivo-da-Estimativa-da-Safra-de-Laranja-2020-2021.pdf (accessed on 20 November 2020).
- Kovaleski, A.; Sugayama, R.L.; Malavasi, A. Controle Químico em Macieiras. In Moscas-das-Frutas de Importância Econômica no Brasil (Conhecimento Básico e Aplicado), 1st ed.; Malavasi, A., Zucchi, R.A., Eds.; FAPESP-Holos: Ribeirão Preto, Brazil, 2000; pp. 135–141. [Google Scholar]
- Rupp, L.C.; Boff, M.I.; Boff, P.; Gonçalves, P.A.D.S.; Botton, M. High dilution of Staphysagria and fruit fly biotherapic preparations to manage South American fruit fly, Anastrepha fraterculus, in organic peach orchards. Biol. Agric. Hortic. 2012, 28, 41–48. [Google Scholar] [CrossRef]
- Machota, R.; Bortoli, L.C.; Cavalcanti, F.R.; Botton, M.; Grützmacher, A.D. Assessment of injuries caused by Anastrepha fraterculus (Wied.) (Diptera: Tephritidae) on the incidence of bunch rot diseases in table grape. Neot. Entomol. 2016, 45, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, P.; Toledo, J.; Hernández, E. Moscas de la Fruta: Fundamentos y Procedimientos para su Manejo; S y G Editores: Coyoacán, México D.F., México, 2010; p. 395. [Google Scholar]
- Cladera, J.; Vilardi, J.; Juri, M.; Paulin, L.; Giardini, M.; Gómez Cendra, P.; Segura, D.; Lanzavecchia, S. Genetics and biology of Anastrepha fraterculus: Research supporting the use of the sterile insect technique (SIT) to control this pest in Argentina. BMC Genet. 2014, 15, S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrichs, J.P.; Vreysen, M.J.B.; Enkerlin, W.R.; Cayol, J.P. Strategic options in using sterile insects for area-wide integrated pest management. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2021; pp. 841–884. [Google Scholar]
- Mastrangelo, T.; Kovaleski, A.; Botteon, V.; Scopel, W.; Costa, M.d.L.Z. Optimization of the sterilizing doses and overflooding ratios for the South American fruit fly. PLoS ONE 2018, 13, e0201026. [Google Scholar] [CrossRef] [PubMed]
- Kovaleski, A.; Mastrangelo, T. Moscasul Programme: First Steps of a Pilot Project to Suppress the South American Fruit Fly in Southern Brazil. In Area-Wide Integrated Pest Management, 2nd ed.; Hendrichs, J., Pereira, R., Vreysen, M.J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 215–230. [Google Scholar]
- International Atomic Energy Agency. The South American fruit fly, Anastrepha fraterculus (Wied.); advances in artificial rearing, taxonomic status and biological studies. In Proceedings of the Workshop Organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Vina del Mar, Chile, 1–2 November 1996; IAEA Tech-Doc 1064: Vienna, Austria, 1999; p. 202. [Google Scholar]
- Jaldo, H.E.; Gramajo, M.C.; Willink, E. Mass-rearing of Anastrepha fraterculus (Diptera: Tephritidae): A preliminary strategy. Fla. Entomol. 2001, 84, 716–718. [Google Scholar] [CrossRef]
- Vera, T.; Abraham, S.; Oviedo, A.; Willink, E. Demographic and quality control parameters of Anastrepha fraterculus (Diptera: Tephritidae) maintained under artificial rearing. Fla. Entomol. 2007, 90, 53–57. [Google Scholar] [CrossRef]
- Walder, J.; Morelli, R.; Costa, K.; Faggioni, K.; Sanches, P.; Paranhos, B.; Bento, J.; Costa, M. Large scale artificial rearing of Anastrepha sp.1 aff. fraterculus (Diptera: Tephritidae) in Brazil. Sci. Agric. 2014, 71, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Vera, M.T.; Cáceres, C.; Wornoayporn, V.; Islam, A.; Robinson, A.S.; De La Vega, M.H.; Hendrichs, J.; Cayol, J.P. Mating incompatibility among populations of the South American fruit fly Anastrepha fraterculus (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2006, 99, 387–397. [Google Scholar] [CrossRef]
- Břízová, R.; Mendonça, A.L.; Vaníčková, L.; Lima-Mendonça, A.; da Silva, C.E.; Tomčala, A.; Paranhos, B.A.J.; Dias, V.S.; Joachim-Bravo, I.S.; Hoskovec, M.; et al. Pheromone analyses of the Anastrepha fraterculus (Diptera: Tephritidae) cryptic species complex. Fla. Entomol. 2013, 96, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Vaníčková, L.; Hernández-Ortiz, V.; Bravo, I.S.J.; Dias, V.; Roriz, A.K.P.; Laumann, R.; Mendonça, A.D.L.; Paranhos, B.A.J.; Nascimento, R.R.D. Current knowledge of the species complex Anastrepha fraterculus (Diptera, Tephritidae) in Brazil. ZooKeys 2015, 540, 211–237. [Google Scholar] [CrossRef]
- Hernández-Ortiz, V.; Barradas-Juanz, N.; Díaz-Castelazo, C. A Review of the Natural Host Plants of the Anastrepha fraterculus Complex in the Americas. In Area-Wide Management of Fruit Fly Pests; Pérez-Staples, D., Diíaz-Fleischer, F., Montoya, P., Vera, M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 90–122. [Google Scholar] [CrossRef]
- Selivon, D.; Perondini, A.L.P.; Morgante, J.S. A genetic-morphological characterization of two cryptic species of the Anastrepha fraterculus complex (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2005, 98, 367–381. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Ortiz, V.; Canal, N.A.; Tigrero Salas, J.O.; Ruiz-Hurtado, F.M.; Dzul-Cauich, J.F. Taxonomy and phenotypic relationships of the Anastrepha fraterculus complex in the Mesoamerican and Pacific Neotropical dominions (Diptera, Tephritidae). ZooKeys 2015, 540, 95–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cáceres, C.; Segura, D.; Vera, M.T.; Wornoayporn, V.; Cladera, J.L.; Teal, P.; Sapountzis, P.; Bourtzis, K.; Zacharopoulou, A.; Robinson, A.S. Incipient speciation revealed in Anastrepha fraterculus (Diptera; Tephritidae) by studies on mating compatibility, sex pheromones, hybridization, and cytology. Biol. J. Linn. Soc. 2009, 97, 152–165. [Google Scholar] [CrossRef]
- Rull, J.; Abraham, S.; Kovaleski, A.; Segura, D.; Mendoza, M.; Liendo, M.C.; Vera, M.T. Evolution of pre-zygotic and post-zygotic barriers to gene flow among three cryptic species within the Anastrepha fraterculus complex. Entomol. Exp. Appl. 2013, 148, 213–222. [Google Scholar] [CrossRef]
- Roriz, A.K.P.; Japyassú, H.F.; Joachim-Bravo, I.S. Incipient speciation in the Anastrepha fraterculus cryptic species complex: Reproductive compatibility between A. sp. 1 aff. fraterculus and A. sp. 3 aff. fraterculus. Entomol. Exp. Appl. 2017, 162, 346–357. [Google Scholar] [CrossRef]
- Dias, V.S.; Silva, J.G.; Lima, K.M.; Petitinga, C.S.; Hernandez-Ortiz, V.; Laumann, R.A.; Paranhos, B.J.; Uramoto, K.; Zucchi, R.A.; Joachim-Bravo, I.S. An integrative multidisciplinary approach to understanding cryptic divergence in Brazilian species of the Anastrepha fraterculus complex (Diptera: Tephritidae). Biol. J. Linn. Soc. 2016, 117, 725–746. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.G.; Vreysen, M.J.B.; Bouyer, J.; Calkins, C.O. Sterile Insect Quality Control/Assurance. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2021; pp. 399–440. [Google Scholar]
- Liedo, P.; Carey, J.R. Mass rearing of Anastrepha (Diptera: Tephritidae) fruit flies: A demography analysis. J. Econ. Entomol. 1994, 87, 176–180. [Google Scholar] [CrossRef]
- Schutze, M.K.; Dammalage, T.; Jessup, A.; Vreysen, M.J.B.; Wornoayporn, V.; Clarke, A.R. Effects of laboratory colonization on Bactrocera dorsalis (Diptera, Tephritidae) mating behaviour: ‘What a difference a year makes’. ZooKeys 2015, 540, 369–383. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Ross, P.A. Rates and patterns of laboratory adaptation in (mostly) insects. J. Econ. Entomol. 2018, 111, 501–509. [Google Scholar] [CrossRef]
- Salles, L.A.B. Rearing technique for Anastrepha fraterculus (Wiedemann, 1830) (Diptera: Tephritidae) in laboratory. An. Soc. Entomológica Bras. 1992, 21, 479–486. [Google Scholar] [CrossRef]
- Taylor, D.B. Comparison of two gelling agents for screwworm (Diptera: Calliphoridae) larval diets. J. Econ. Entomol. 1988, 81, 1414–1419. [Google Scholar] [CrossRef] [Green Version]
- Ongaratto, S.; Pinto, K.J.; Mânica-Berto, R.; Nörnberg, S.D.; Gonçalves, R.S.; Garcia, M.S.; Nava, D.E. Influence of the host diet on the performance of Doryctobracon areolatus (Hymenoptera: Braconidae). Braz. J. Biol. 2020, 80, 727–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations; International Atomic Energy Agency; United States Department of Agriculture. Product Quality Control for Sterile Mass-Reared and Released Tephritid Fruit Flies; Version 7.0.; IAEA: Vienna, Austria, 2019; 148p.
- Abraham, S.; Goane, L.; Rull, J.; Cladera, J.; Willink, E.; Vera, M.T. Multiple mating in Anastrepha fraterculus females and its relationship with fecundity and fertility. Entomol Exp. Appl. 2011, 141, 15–24. [Google Scholar] [CrossRef]
- Kikawa, C.R.; Shatalov, M.; Kloppers, P.H. A Method for Computing Initial Approximations for a 3-Parameter Exponential Function. Phys. Sci. Int. J. 2015, 6, 203–208. [Google Scholar] [CrossRef]
- Bartlett, M.S. Properties of sufficiency and statistical tests. Proc. R. Stat. Soc. A 1937, 160, 268–282. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Renaud, O.; Victoria-Feser, M.P. A robust coefficient of determination for regression. J. Stat. Plan. Inference 2010, 140, 1852–1862. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R i386 3.2.2: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org/ (accessed on 17 September 2020).
- Sivinski, J.M.; Calkins, C.O.; Baranowski, R.; Harris, D.; Brambila, J.; Diaz, J.; Burns, R.E.; Holler, T.; Dodson, G. Suppression of Caribbean fruit fly (Anastrepha suspensa (Loew) Diptera: Tephritidae) population through releases of the parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control. 1996, 6, 177–185. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture. United States and Mexico Lower Rio Grande Valley Mexican Fruit Fly Eradication Program Review: Final Report; USDA/APHIS: Washington, DC, USA, 2010.
- Hernandez, E.; Rivera, J.P.; Artiaga-Lopez, T. Generic larval diet for mass-rearing of three species of Anastrepha (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2014, 34, S13–S18. [Google Scholar] [CrossRef]
- Orozco-Dávila, D.; Quintero, L.; Hernández, E.; Solís, E.; Artiaga, T.; Hernández, R.; Ortega, C.; Montoya, P. Mass rearing and sterile insect releases for the control of Anastrepha spp. pests in Mexico—A review. Entomol. Exp. Appl. 2017, 164, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Aceituno-Medina, M.; Rivera-Ciprian, J.P.; Hernández, E. Evaluation of a pelleted diet for larval mass-rearing of Anastrepha ludens and Anastrepha obliqua. Entomol. Exp. Appl. 2020, 168, 502–512. [Google Scholar] [CrossRef]
- Caceres, C. Mass rearing of temperature sensitive genetic sexing strains in the Mediterranean fruit fly (Ceratitis capitata). Genetica 2002, 116, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Liedo, P.; Salgado, S.; Oropeza, A.; Toledo, J. Improving mating performance of mass-reared sterile Mediterranean fruit flies (Diptera: Tephritidae) through changes in adult holding conditions: Demography and mating competitiveness. Fla. Entomol. 2007, 90, 33–40. [Google Scholar] [CrossRef]
- Orozco-Davila, D.; Hernandez, R.; Solis, E.; Quintero, J.L.; Dominguez, J. Establishment of a colony of Anastrepha ludens(Diptera: Tephritidae) under relaxed mass-rearing conditions in Mexico. In Fruit Flies of Economic Importance: From Basic to Applied Knowledge, Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance, Salvador, Brazil, 10–15 September 2006; SBPC: São Paulo, Brazil, 2008; pp. 335–339. [Google Scholar]
- Orozco-Davila, D.; Artiaga-Lopez, T.; Hernandez, M.R.; Dominguez, J.; Hernandez, E. Anastrepha obliqua (Diptera: Tephritidae) mass-rearing: Effect of relaxed colony management. Int. J. Trop. Insect Sci. 2014, 34, S19–S27. [Google Scholar] [CrossRef]
- Vera, M.T.; Oviedo, A.; Abraham, S.; Ruiz, M.J.; Mendoza, M.; Chang, C.L.; Willink, E. Development of a larval diet for the South American fruit fly Anastrepha fraterculus (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2014, 34, S73–S81. [Google Scholar] [CrossRef]
- González, J.B.; Vargas, C.V.; Jara, B.P. Estudios sobre la aplicación de la técnica de machos estériles en el control de la mosca sudamericana de la fruta, Anastrepha fraterculus (Wied.). Rev. Peru. de Entomol. 1971, 14, 66–86. [Google Scholar]
- Burk, T.; Webb, J.C. Effect of male size on calling propensity, song parameters, and mating success in Caribbean fruit flies, Anastrepha suspensa (Loew) (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1983, 76, 678–682. [Google Scholar] [CrossRef]
- Segura, D.; Petit-Marty, N.; Sciurano, R.; Vera, T.; Calcagno, G.; Allinghi, A.; Gómez Cendra, P.; Cladera, J.; Vilardi, J. Lekking behavior of Anastrepha fraterculus (Diptera: Tephritidae). Fla. Entomol. 2007, 90, 154–162. [Google Scholar] [CrossRef]
- Sciurano, R.; Segura, D.; Rodriguero, M.; Gómez Cendra, P.; Allinghi, A.; Cladera, J.L.; Vilardi, J. Sexual selection on multivariate phenotypes in Anastrepha fraterculus (Diptera: Tephritidae) from Argentina. Fla. Entomol. 2007, 90, 163–170. [Google Scholar] [CrossRef]
- Tejeda, M.T.; Arredondo, J.; Díaz-Fleischer, F.; Pérez-Staples, D. Does Size Matter? Mate Choice in Two Lekking Flies. J. Insect Sci. 2020, 20, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cresoni-Pereira, C.; Zucoloto, F.S. Moscas-das-frutas (Diptera). In Bioecologia e Nutrição de Insetos: Base para o Manejo Integrado de Pragas; Panizzi, A.R., Parra, J.R.P., Eds.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009; pp. 733–766. [Google Scholar]
- Nestel, D.; Nemny-Lavy, E. Nutrient balance in medfly, Ceratitis capitata, larval diets affects the ability of the developing insect to incorporate lipid and protein reserves. Entomol. Exp. Appl. 2007, 126, 53–60. [Google Scholar] [CrossRef]
- Moreno, D.S.; Zaleta, D.A.O.; Mangan, R.L. Development of Artificial Larval Diets for West Indian Fruit Fly (Diptera: Tephritidae). J. Econ. Entomol. 1997, 90, 427–434. [Google Scholar] [CrossRef]
- Chang, C.L. Evaluation of yeasts and yeast products in larval and adult diets for the oriental fruit fly, Bactrocera dorsalis, and adult diets for the medfly, Ceratitis capitata, and the melon fly, Bactrocera cucurbitae. J. Insect Sci. 2009, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aceituno-Medina, M.; Rincón-Betancurt, O.; Martínez-Salgado, R.T.; Hernández, E. A novel, low-cost coconut fiber larval diet for mass rearing Anastrepha (Diptera: Tephritidae). J. Econ. Entomol. 2019, 112, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Pascacio-Villafán, C.; Williams, T.; Birke, A.; Aluja, M. Nutritional and non-nutritional food components modulate phenotypic variation but not physiological trade-offs in an insect. Sci. Rep. 2016, 6, 29413. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Ciprian, J.P.; Aceituno-Medina, M.; Guillen, K.; Hernández, E.; Toledo, J. Midgut Protease Activity During Larval Development of Anastrepha obliqua (Diptera: Tephritidae) Fed with Natural and Artificial Diet. J. Insect Sci. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Deutscher, A.T.; Chapman, T.A.; Shuttleworth, L.A.; Riegler, M.; Reynolds, O.L. Tephritid-microbial interactions to enhance fruit fly performance in sterile insect technique programs. BMC Microbiol. 2019, 19 (Suppl. 1), 287. [Google Scholar] [CrossRef]
- Sørensen, J.; Addison, M.; Terblanche, J. Mass-rearing of insects for pest management: Challenges, synergies and advances from evolutionary physiology. Crop. Prot. 2012, 38, 87–94. [Google Scholar] [CrossRef]
- Parker, A.G.; Mamai, W.; Maiga, H. Mass-Rearing for the Sterile Insect Technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2021; pp. 283–316. [Google Scholar]
- Pascacio-Villafan, C.; Guillen, L.; Aluja, M. Agar and Carrageenan as Cost-Effective Gelling Agents in Yeast-Reduced Artificial Diets for Mass-Rearing Fruit Flies and Their Parasitoids. Insects 2020, 11, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zygouridis, N.E.; Argov, Y.; Nemny-Lavy, E.; Augustinos, A.A.; Nestel, D.; Mathiopoulos, K.D. Genetic changes during laboratory domestication of an olive fly SIT strain. J. Appl. Entomol. 2014, 138, 423–432. [Google Scholar] [CrossRef]


| Quality Control Parameters | No. of Hours under Aeration | ANOVA | ||
|---|---|---|---|---|
| 48 h | 60 h | 72 h | ||
| Egg hatch (%) | 83.4 ± 3.6 a 1 | 49.8 ± 8.0 b | 55.4 ± 4.6 b | F2,24 = 10.1; p = 0.01 |
| First instar larvae before the end of incubation (%) | 0.9 ± 0.8 a | 50.8 ± 4.6 b | 50.7 ± 3.6 b | F2,24 = 71.5; p < 10−3 |
| Number of larvae | 10,880 ± 1251 a 2 | 9560 ± 941 a | 7360 ± 424 a | F2,24 = 3.6; p = 0.09 |
| Number of pupae | 7100 ± 662 a | 6200 ± 391 ab | 4450 ± 181 b | F2,24 = 8.8; p = 0.02 |
| Pupal weight (mg) | 10.4 ± 0.3 a | 9.8 ± 0.2 a | 10.3 ± 0.1 a | F2,24 = 2.3; p = 0.19 |
| Adult emergence (%) | 89.0 ± 5.0 a | 82.7 ± 1.9 a | 90.7 ± 2.4 a | F2,24 = 1.6; p = 0.29 |
| Sex ratio (♀/♂ + ♀) | 0.5 ± 0.02 a | 0.5 ± 0.01 a | 0.6 ± 0.03 a | F2,24 = 4.9; p = 0.053 |
| Adult Density (Flies/cm2) | Daily Egg Production (mL) | Total Egg Production (mL) | Egg Hatch (%) |
|---|---|---|---|
| 0.1 | 0.9 ± 0.042 c 1 | 26.01 ± 7.1 a | 85.8 ± 0.8 a |
| 0.2 | 1.79 ± 0.17 bc | 53.5 ± 13.9 ab | 86.2 ± 0.9 a |
| 0.3 | 2.31 ± 0.2 ab | 69.1 ± 14.2 ab | 85.7 ± 1.1 a |
| 0.4 | 1.71 ± 0.2 bc | 50.5 ± 14.2 ab | 82.8 ± 1.2 ab |
| 0.5 | 2.94 ± 0.4 a | 88.1 ± 6.9 b | 78.9 ± 0.7 b |
| ANOVA | F4,145 = 10.3; p < 10−3 | F4,40 = 3.8; p = 0.038 | F4,145 = 8.6; p < 10−3 |
| Quality Control Parameters | Density (mL of Eggs/kg of Diet) | Regression Analyses | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.5 | 0.7 | 1.0 | 1.3 | 1.5 | 1.7 | 2.0 | ||
| Number of larvae | 8761 ± 373 | 12,013 ± 1326 | 22,312 ± 2033 | 19,271 ± 1658 | 28,147 ± 1303 | 27,668 ± 1358 | 23,442± 1385 | Y = −5029.4 + 29,499.3 X − 6778.8 X2, r2 = 0.91 |
| Larval weight (mg) | 19.4 ± 0.3 | 19.7 ± 0.4 | 17.6 ± 0.1 | 17.6 ± 0.6 | 17.4 ± 0.5 | 16.8 ± 0.9 | 14.2 ± 0.5 | Y = 21.4 − 3.1 X, r2 = 0.86 |
| Number of pupae | 7959 ± 244 | 13,448 ± 1859 | 18,557 ± 1016 | 19,100 ± 1493 | 22,451 ± 1439 | 27,593 ± 1364 | 15,357± 1022 | Y = −12,178 + 47,663 X −16,333 X2, r2 = 0.77 |
| Pupal weight (mg) | 13.4 ±0.2 | 12.6 ±0.1 | 11.4 ±0.3 | 11.9 ± 0.4 | 11.4 ±0.2 | 9.4 ± 0.5 | 10.09 ± 0.2 | Y =14.5 − 2.5 X, r2 = 0.78 |
| Larval recovery (%) | 67.7 ± 2.9 | 79.0 ± 11.1 | 86.1 ± 7.9 | 58.7 ± 5.1 | 72.5 ± 3.4 | 62.8 ± 3.1 | 45.3 ± 2.7 | Y = 45.4 + 68.9 X − 34.8 X2, r2 = 0.84 |
| Larval period (days) | 8.0 ± 0.0 | 8.0 ± 0.0 | 7.7 ± 0.3 | 7.0 ± 0.0 | 7.3 ± 0.3 | 7.0 ± 0.0 | 7.0 ± 0.0 | Y = 8.4 − 0.79 X, r2 = 0.88 |
| Diameter of pupa (mm) | 2.2 ± 0.01 | 2.2 ± 0.03 | 2.1 ± 0.02 | 2.2 ± 0.03 | 2.1 ± 0.04 | 2.0 ± 0.03 | 2.1 ± 0.03 | Linear regression not significant (p = 0.11) |
| Pupal recovery (%) | 91.0 ± 1.6 | 94.0 ± 0.6 | 84.3 ± 4.3 | 99.3 ± 0.73 | 79.6 ± 2.0 | 99.7 ± 0.3 | 65.4 ± 0.6 | Y = 71.2 + 48.6 X − 24.9 X2, r2 = 0.63 |
| Egg–pupa recovery (%) | 61.5 ± 1.9 | 74.2 ± 10.3 | 71.7 ± 3.9 | 58.2 ± 4.6 | 57.8 ± 3.7 | 62.7 ± 3.1 | 29.7 ± 2.0 | Y = 39.7 + 67.5 X − 35.8 X2, r2 = 0.89 |
| Pupal period (days) | 15.0 ± 0.0 | 15.0 ± 0.0 | 14.0 ± 0.0 | 14.3 ± 0.3 | 15.3 ± 0.3 | 14.3 ± 0.3 | 15.0 ± 0.0 | Linear regression not significant (p = 0.98) |
| Adult emergence (%) | 92.3 ± 1.0 | 96.7 ± 2.0 | 91.7 ± 1.4 | 95.1 ± 1.9 | 94.7 ± 0.7 | 90.7 ± 1.9 | 93.0 ± 1.3 | Linear regression not significant (p = 0.64) |
| Sex ratio (♀/♂ + ♀) | 0.50 ± 0.02 | 0.55 ± 0.02 | 0.50 ± 0.03 | 0.57 ± 0.04 | 0.52 ± 0.03 | .53 ± 0.05 | 0.55 ± 0.03 | Linear regression not significant (p = 0.34) |
| Flight ability (%) | 72.1 ± 0.2 | 75.0 ± 1.6 | 70.9 ± 1.1 | 74.3 ± 1.6 | 73.3 ± 0.6 | 71.2 ± 1.2 | 73.0 ± 0.8 | Linear regression not significant (p = 0.85) |
| Quality Control Parameters | Larval Diets | ANOVA | |
|---|---|---|---|
| Embrapa | CENA | ||
| No. of larvae | 6688 ± 297 b 1,2 | 28,147 ± 1303 a | F1,5 = 1280.3; p < 10−4 |
| Larval weight (mg) | 17.8 ± 0.2 a | 17.4 ± 0.5 a | F1,6 = 1.1; p = 0.33 |
| Larval recovery (%) | 11.5 ± 0.5 b | 72.5 ± 3.4 a | F1,5 = 1732.5; p < 10−4 |
| Larval period (days) | 8.0 ± 0.0 a | 7.3 ± 0.3 a | F1,6 = 6.43; p = 0.0522 |
| No. of pupae | 6554 ± 134 b | 22,451 ± 1439 a | F1,6 = 181.7; p < 10−4 |
| Pupal weight (mg) | 15.6 ± 1.1 a | 11.4 ± 0.2 b | F1,5 = 71.03; p = 0.0011 |
| Diameter of pupa (mm) | 2.3 ± 0.04 a | 2.1 ± 0.04 b | F1,6 = 8.03; p = 0.0365 |
| Pupal recovery (%) | 98.0 ± 1.0 a | 79.6 ± 2.0 b | F1,6 = 75.76; p = 0.0003 |
| Egg–pupa recovery (%) | 11.5 ± 0.5 b | 57.8 ± 3.7 a | F1,5 = 4634.22; p < 10−4 |
| Pupal period (days) | 16.0 ± 0.0 a | 15.3 ± 0.3 a | F1,6 = 6.43; p = 0.0522 |
| Emergence (%) | 88.7 ± 1.7 b | 94.7 ± 0.7 a | F1,6 = 13.86; p = 0.0137 |
| Sex ratio (♀/♂ + ♀) | 0.46 ± 0.03 a | 0.52 ± 0.03 a | F1,6 = 1.39; p = 0.2920 |
| Flight ability (%) | 69.1 ± 14.8 a | 74.0 ± 0.8 a | F1,5 = 1.81; p = 0.1697 |
| Larval Diet | USD/kg of Diet 1 | Pupae/kg of Diet 2 | Pupae/USD 1.00 of Diet 3 | kg of Diet for 1 Million Pupae 4 | USD per 1 Million Pupae 5 | USD per 1 Million Flying Adults 6 |
|---|---|---|---|---|---|---|
| Embrapa | 0.46 | 2185 | 4750.0 | 457.7 | 210.54 | 275.22 |
| CENA | 0.44 | 7484 | 17,009.1 | 133.6 | 58.78 | 76.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrangelo, T.; Kovaleski, A.; Maset, B.; Costa, M.d.L.Z.; Barros, C.; Lopes, L.A.; Caceres, C. Improvement of the Mass-Rearing Protocols for the South American Fruit Fly for Application of the Sterile Insect Technique. Insects 2021, 12, 622. https://doi.org/10.3390/insects12070622
Mastrangelo T, Kovaleski A, Maset B, Costa MdLZ, Barros C, Lopes LA, Caceres C. Improvement of the Mass-Rearing Protocols for the South American Fruit Fly for Application of the Sterile Insect Technique. Insects. 2021; 12(7):622. https://doi.org/10.3390/insects12070622
Chicago/Turabian StyleMastrangelo, Thiago, Adalecio Kovaleski, Bruno Maset, Maria de Lourdes Zamboni Costa, Claudio Barros, Luis Anselmo Lopes, and Carlos Caceres. 2021. "Improvement of the Mass-Rearing Protocols for the South American Fruit Fly for Application of the Sterile Insect Technique" Insects 12, no. 7: 622. https://doi.org/10.3390/insects12070622
APA StyleMastrangelo, T., Kovaleski, A., Maset, B., Costa, M. d. L. Z., Barros, C., Lopes, L. A., & Caceres, C. (2021). Improvement of the Mass-Rearing Protocols for the South American Fruit Fly for Application of the Sterile Insect Technique. Insects, 12(7), 622. https://doi.org/10.3390/insects12070622







