Effects of Age, Phase Variation and Pheromones on Male Sperm Storage in the Desert Locust, Schistocerca gregaria
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Sperm Storage
2.3. Phase Variation
2.4. Effect of Pheromone
2.5. Statistics
3. Results
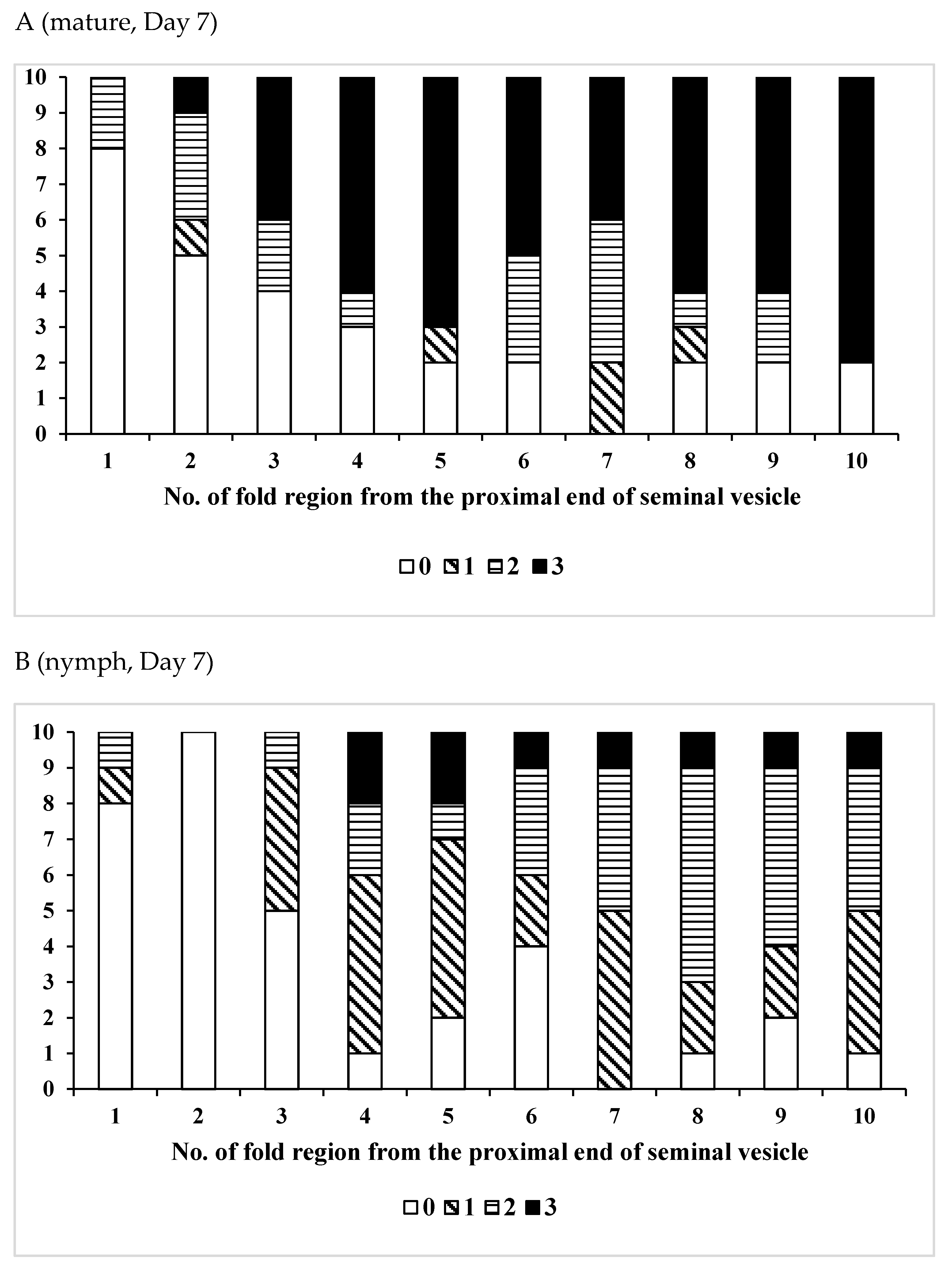
3.1. Change Over Time in Sperm Storage
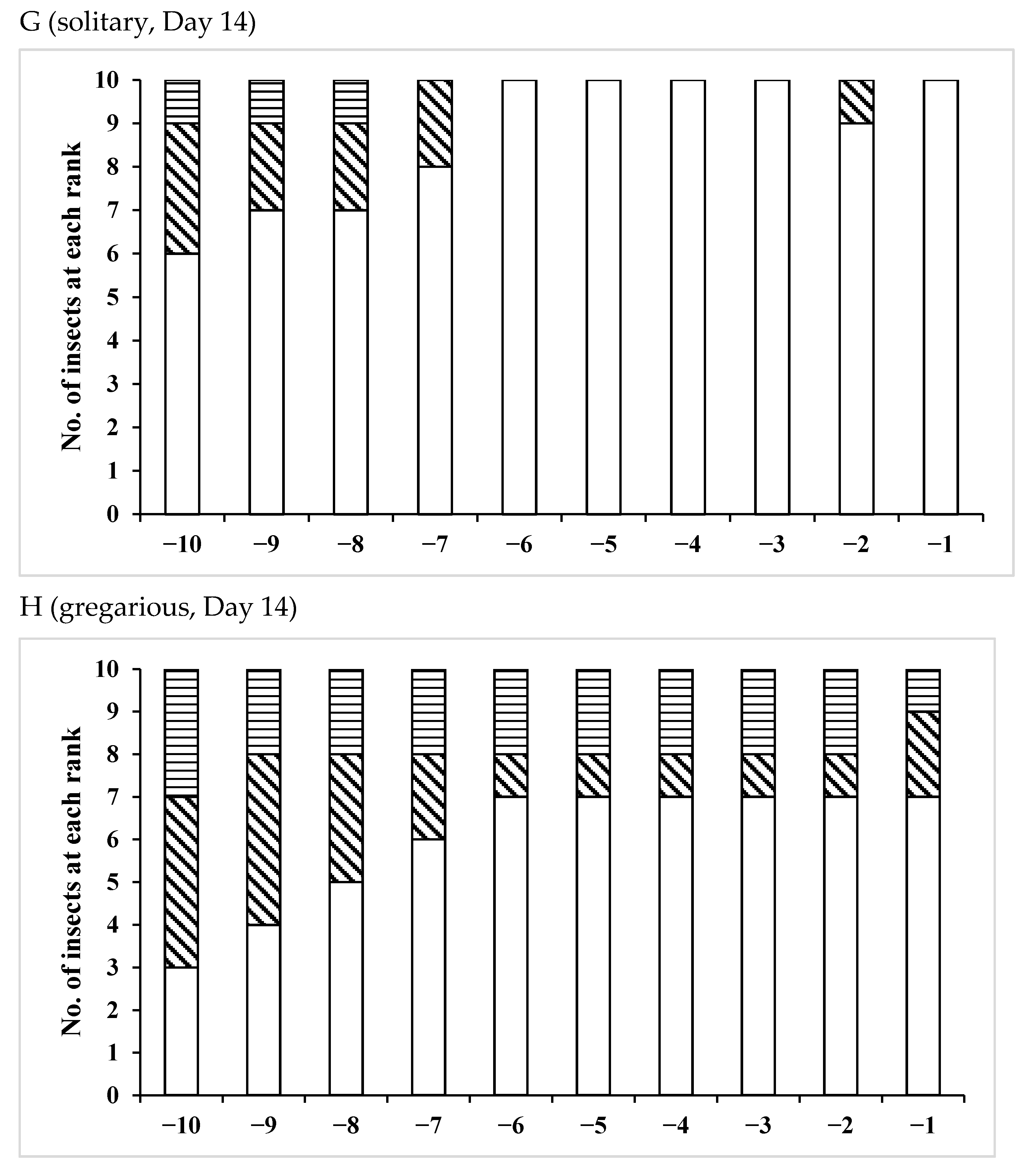
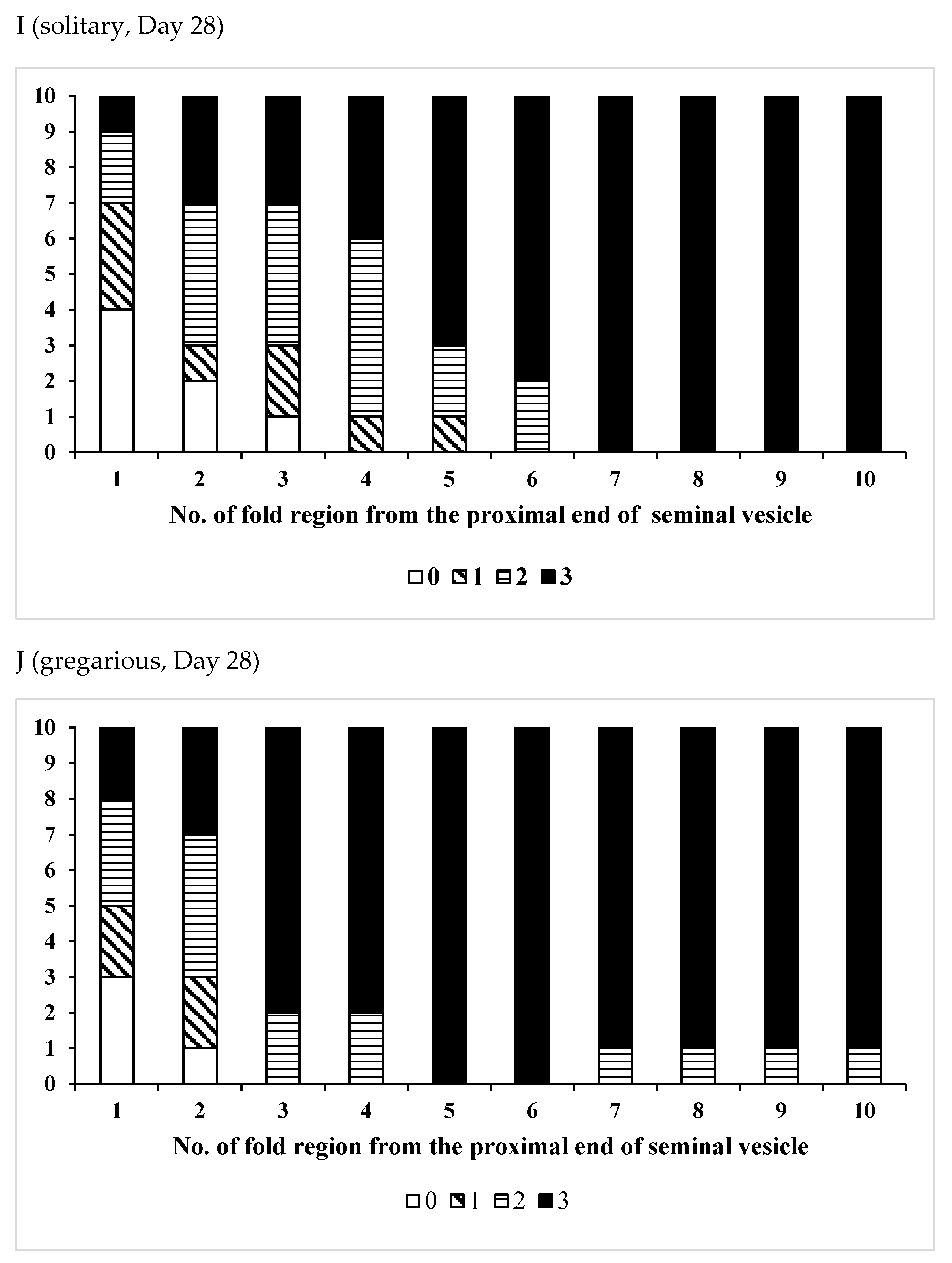
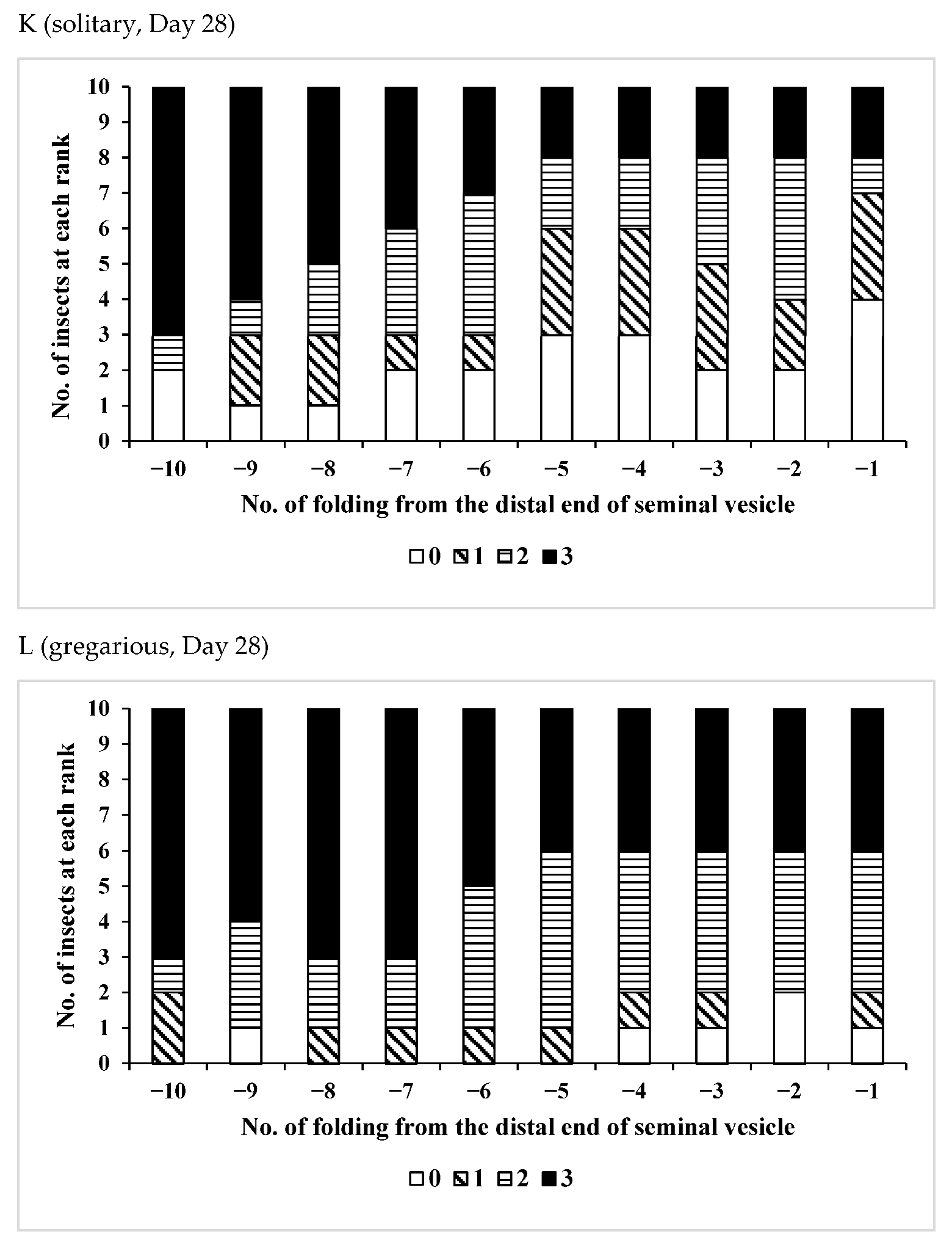
3.2. Effects of Phase Variation on Sperm Storage
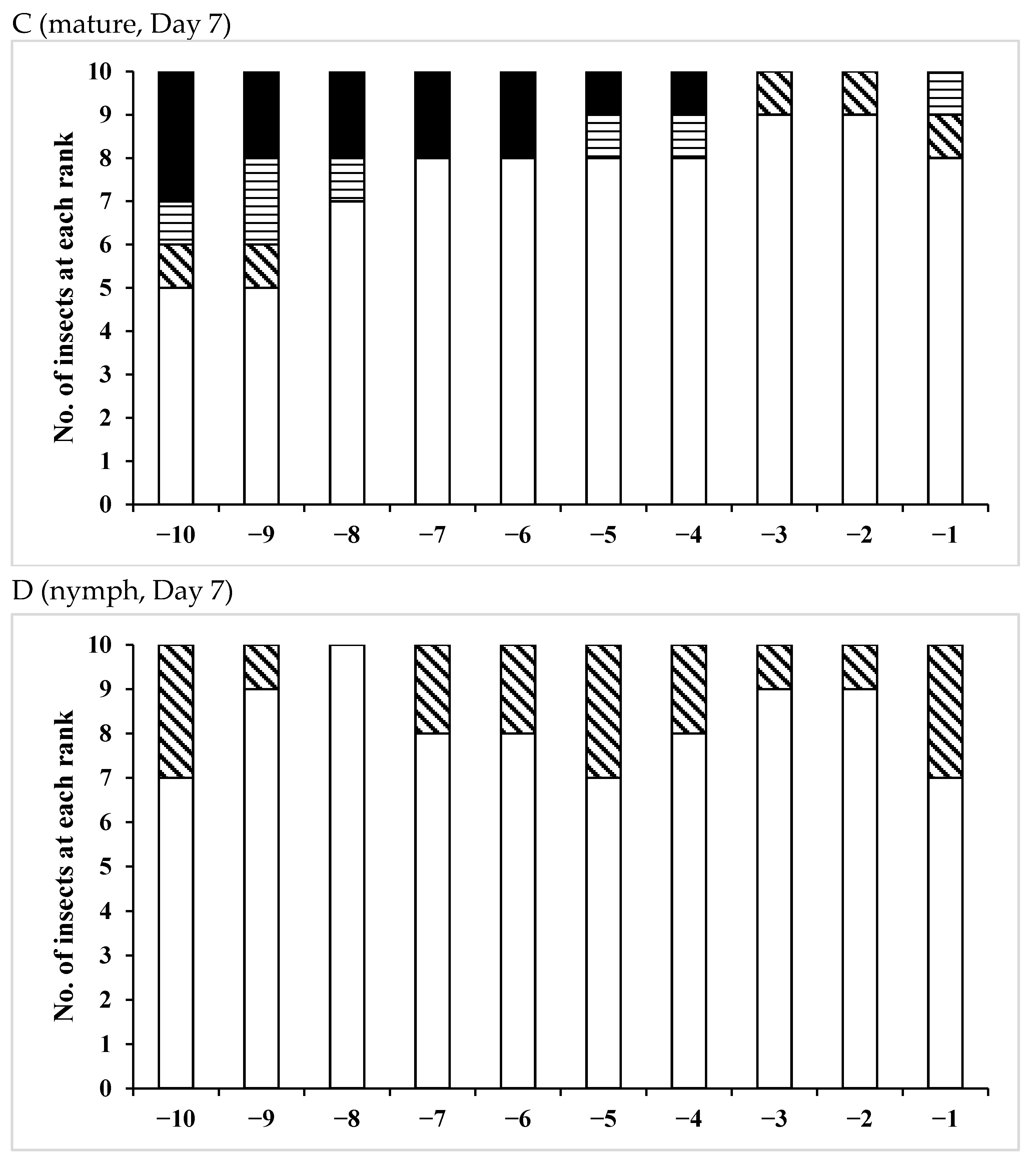
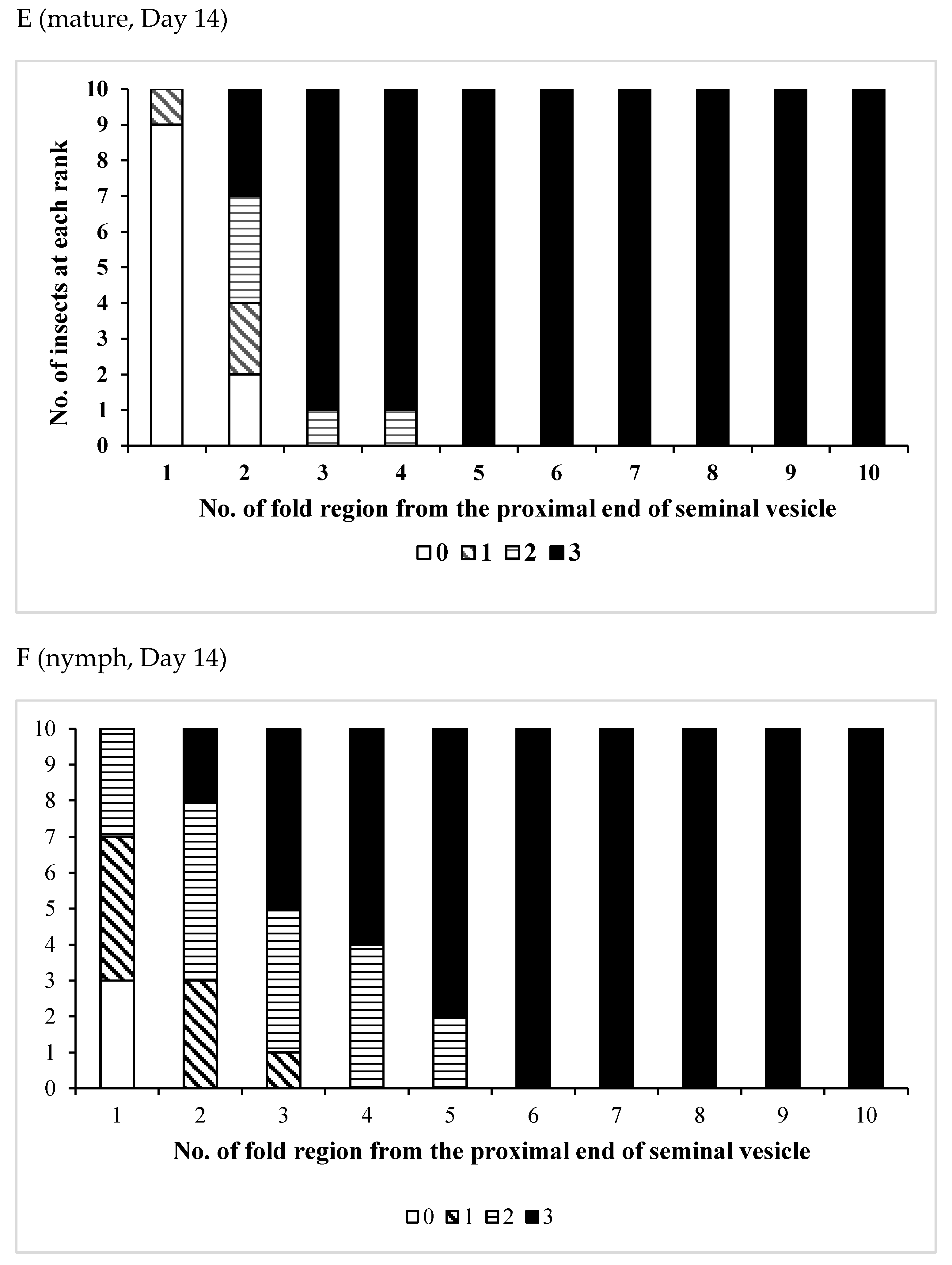
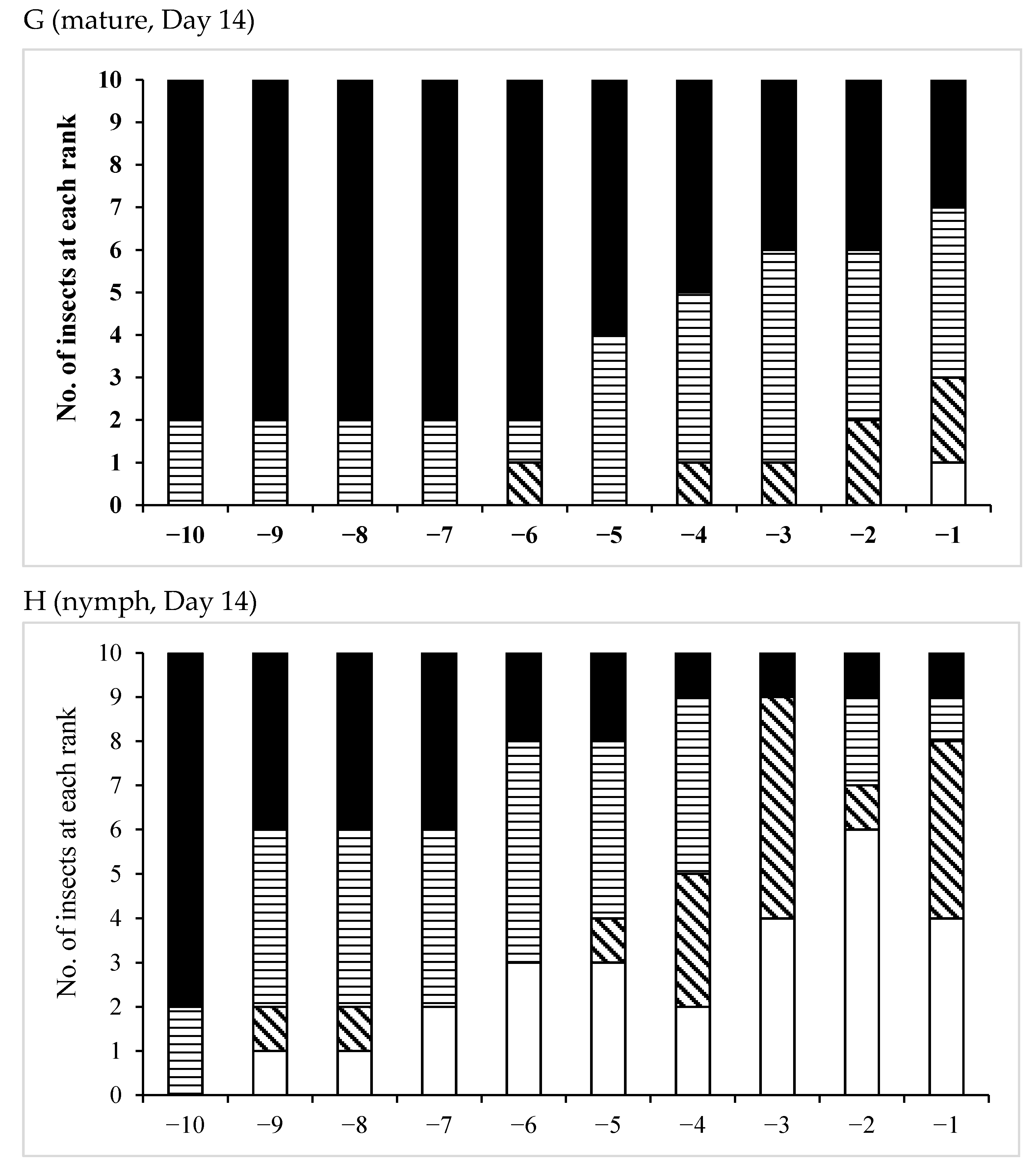
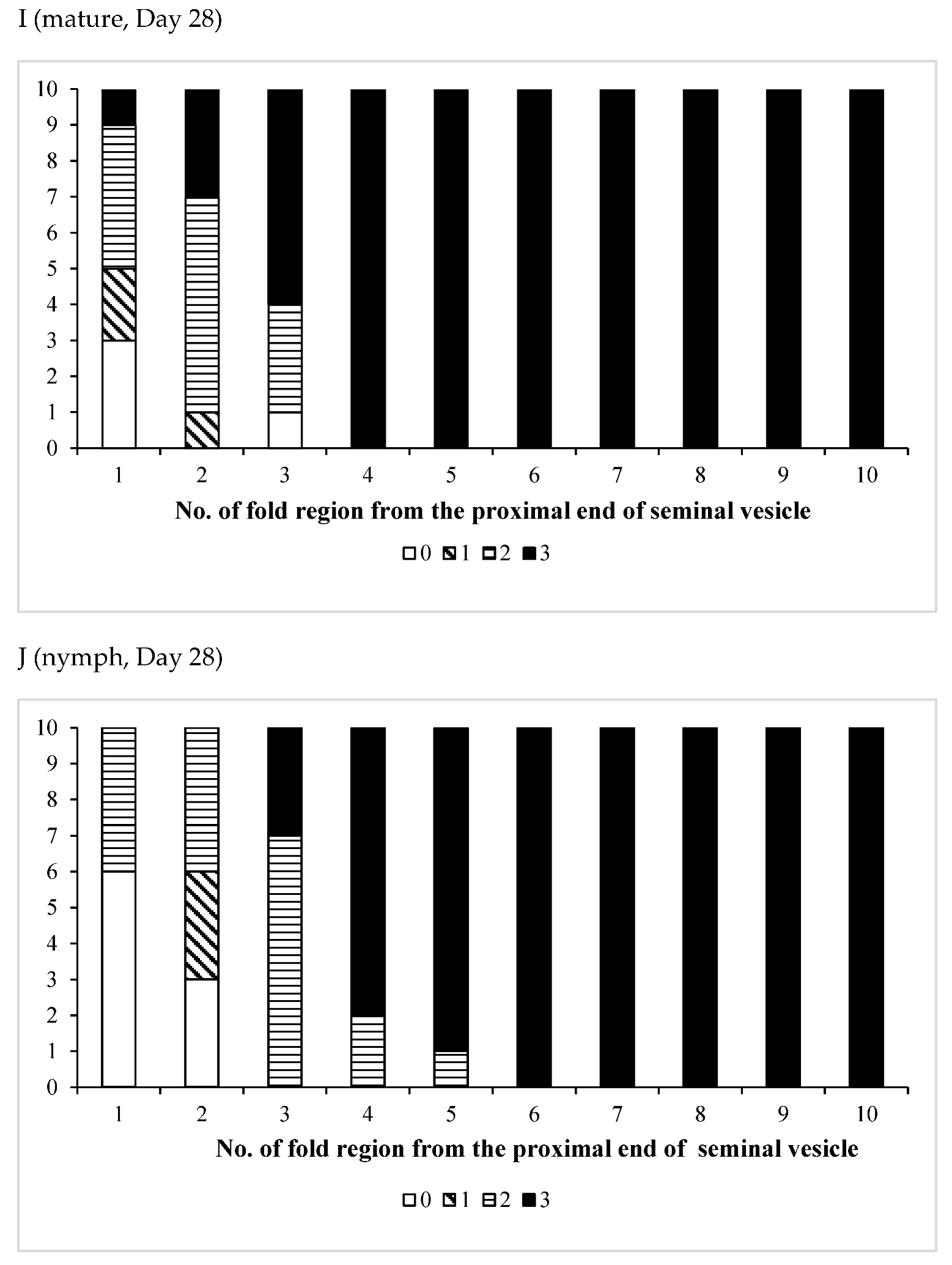
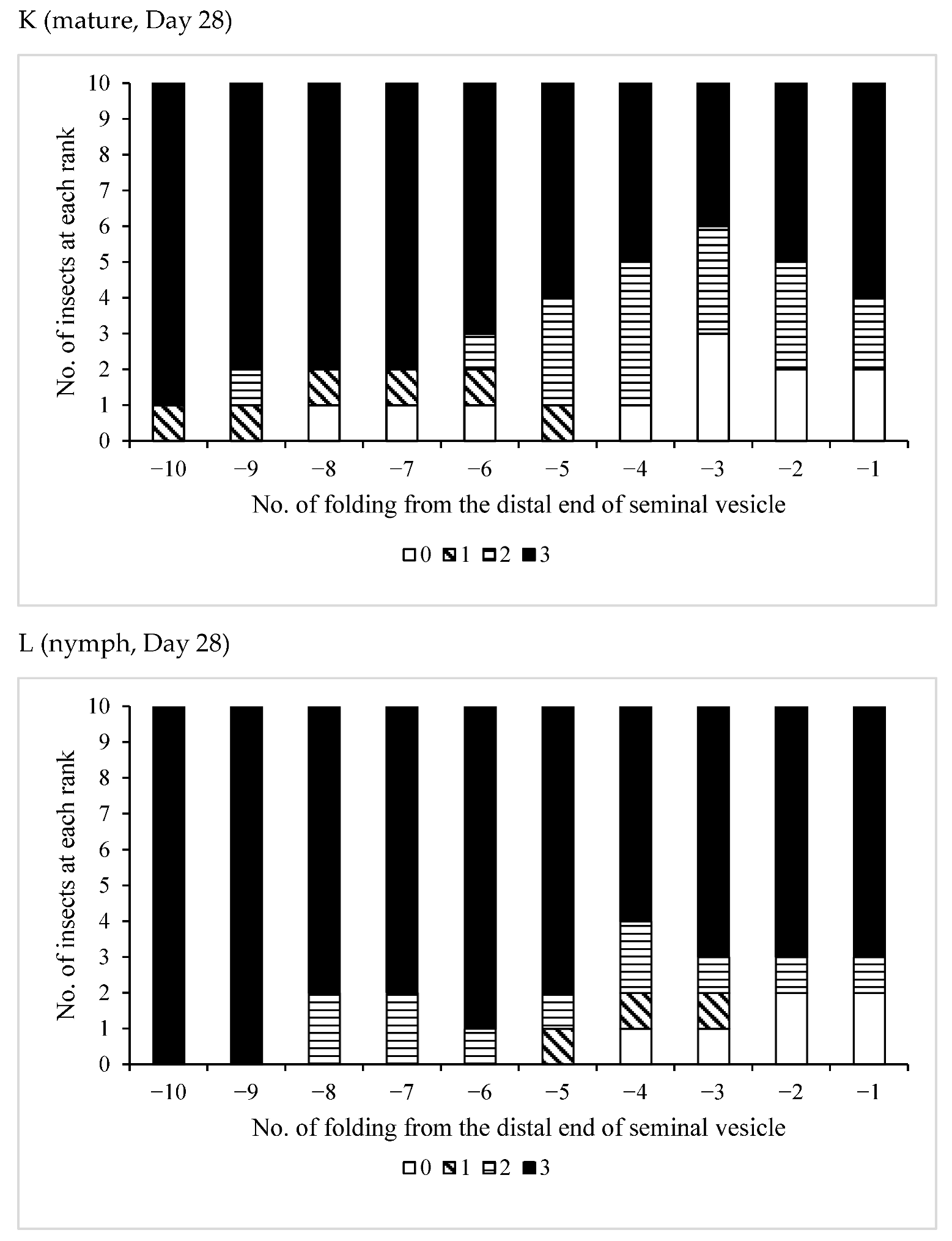
3.3. Effects of Pheromones on Sperm Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pener, M.P. Locust phase polymorphism and its endocrine relations. Adv. Insect Physiol. 1991, 23, 1–79. [Google Scholar]
- Ignell, R.; Couillaud, F.; Anton, S. Juvenile-hormone-mediated plasticity of aggregation behaviour and olfactory processing in adult desert locusts. J. Exp. Biol. 2001, 204, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Breuer, M.; Hoste, B.; de Loof, A. The endocrine control of phase transition: Some new aspects. Physiol. Entomol. 2003, 28, 3–10. [Google Scholar] [CrossRef]
- Tanaka, S. Hormonal control of phase polyphenism in locusts. Formosan. Entomol. 2005, 25, 131–143. [Google Scholar]
- Buhl, J.; Sumpter, D.J.T.; Couzin, I.D.; Hale, J.J.; Despland, E.; Miller, E.R.; Simpson, S.J. From disorder to order in marching locusts. Science 2006, 312, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Pener, M.P.; Simpson, S.J. Locust phase polymorphism: An update. Adv. Insect Physiol. 2009, 36, 1–272. [Google Scholar]
- Ernst, U.R.; van Hiel, M.B.; Depuydt, G.; Boerjan, B.; de Loof, A.; Schoofs, L. Epigenetics and locust life phase transitions. J. Exp. Biol. 2015, 218, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Pener, M.P. Effects of allatectomy and sectioning of the nerves of the corpora allata on oöcyte growth, male sexual behaviour, and colour change in adults of Schistocerca gregaria. J. Insect Physiol. 1967, 13, 665–684. [Google Scholar] [CrossRef]
- Inayatullah, C.; Bashir, S.E.; Hassanali, A. Sexual behavior communication in the desert locust, Shistocerca gregaria (Orthoptera: Acrididae): Sex pheromone in solitaria. Environ. Entomol. 1994, 23, 1544–1551. [Google Scholar] [CrossRef]
- Seidelmann, K.; Ferenz, H.-J. Courtship inhibition pheromone in desert locusts, Schistocerca gregaria. J. Insect Physiol. 2002, 48, 991–996. [Google Scholar] [CrossRef]
- Golov, Y.; Rillich, J.; Harari, A.; Ayali, A. Precopulatory behavior and sexual conflict in the desert locust. Peer J. 2018, 6, e44356. [Google Scholar] [CrossRef]
- Odhiambo, T.R. The architecture of the accessory reproductive glands of the desert locust IV. Fine structure of the glandular epithelium. Trans. R. Soc. Lond. B 1969, 256, 85–114. [Google Scholar]
- Odhiambo, T.R. The architecture of the accessory reproductive glands of the male desert locust III. Components of the Muscular wall. Tissue Cell 1970, 2, 233–248. [Google Scholar] [CrossRef]
- Avruch, L.I.; Tobe, S.S. Juvenile hormone biosynthesis by the corpora allata of the male desert locust, Schistocerca gregaria, during sexual maturation. Can. J. Zool. 1978, 56, 2097–2102. [Google Scholar] [CrossRef]
- Dhadialla, T.S.; Odhiambo, T.R.; Wagner, G.G. Immunochemical ablation of accessory reproductive glands of the male desert locust. Insect Sci. Appl. 1986, 7, 465–470. [Google Scholar] [CrossRef]
- Claeys, I.; Simonet, G.; Breugelmans, B.; van Soest, S.; Franssens, V.; Sas, F.; de Loof, A.; Broeck, J.V. Quantitative real-time RT-PCR analysis in desert locusts reveals p, se dependent differences in neuroparsin transcript levels. Insect Mol. Biol. 2005, 14, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Pickford, R.; Padgham, D.E. Spermatophore formation and sperm transfer in the desert locust, Schistocerca gregaria (Orthoptera: Acrididae). Can. Entomol. 1973, 105, 613–618. [Google Scholar] [CrossRef]
- Boerjan, B.; Tobback, J.; Vandersmissen, H.P.; Huybrechts, R.; Schoofs, L. Fruitless RNAi knockdown in the desert locust, Schistocerca gregaria, influences male fertility. J. Insect Physiol. 2012, 58, 265–269. [Google Scholar] [CrossRef]
- Dushimirimana, S.; Hance, T.; Damiens, D. Comparison of reproductive traits of regular and irradiated male desert locust Schistocerca gregaria (Orthoptera: Acrididae): Evidence of last-male sperm precedence. Biol. Open. 2012, 1, 232–236. [Google Scholar] [CrossRef][Green Version]
- Tobback, J.; Boerjan, B.; Vandersmissen, H.P.; Huybrechts, R. The circadian clock genes affect reproductive capacity in the desert locust Schistocerca gregaria. Insect Biochem. Mol. Biol. 2011, 41, 313–321. [Google Scholar] [CrossRef]
- Van Wielendaele, P.; Wynant, N.; Dillen, S.; Zels, S.; Badisco, L.; Broeck, J.V. Neuropeptide F regulates male reproductive processes in the desert locust, Shistocerca gregaria. Insect Biochem. Mol. Biol. 2013, 43, 252–259. [Google Scholar] [CrossRef]
- Das, N.K.; Siegel, E.P.; Alfert, M. Synthetic activities during spermatogenesis in the locust. J. Cell Biol. 1965, 25, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Cantacuzène, A.M.; Lauverjat, S.; Papillon, M. Influence de la température d’élevage sur les caractères histologiques de l’appareil Génital de Shistocerca gregaria. J. Insect Physiol. 1972, 18, 2077–2093. [Google Scholar] [CrossRef]
- Coggins, P.B. The effect of X-radiation on spermatogenesis and the fertility of Shistocerca gregaria (Forsk.). J. Embryol. Exp. Morph. 1973, 30, 163–177. [Google Scholar] [PubMed]
- Jones, R.T. The blood-germ cell barrier in male Shistocerca gregaria: The time of its establishment and factors affecting its formation. J. Cell Sci. 1978, 31, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Bakr, R.F.A.; Mohammed, M.I.; Elazeem, A.; El-Gammal, M.; Mahdy, N.M. Histopathological change in the testis of the desert locust Shistocerca gregaria (Forskal) induced by the IGR Consult and Lufox. Egypt. Acad. J. Biol. Sci. 2010, 1, 23–28. [Google Scholar]
- Richard, M.J.; El-Mangoury, M.A. Further experiments on the effects of social factors on the rate of sexual maturation in the desert locust. Nature 1968, 219, 865–866. [Google Scholar] [CrossRef]
- Tawfik, A.I.; Osir, E.O.; Hassanali, A.; Ismail, S.H. Effects of juvenile hormone treatment on phase changes and pheromone production in the desert locust, Shistocerca gregaria (Forskal) (Orthoptera: Acrididae). J. Insect Physiol. 1997, 43, 1177–1182. [Google Scholar] [CrossRef]
- Tawfik, A.I.; Treiblmayr, K.; Hassanali, A.; Osir, E.O. Time-course haemolymph juvenile hormone titres in solitarious and gregarious adults of Schistocerca gregaria, and their relation to pheromone emission, CA volumetric changes and oocyte growth. J. Insect Physiol. 2000, 46, 1143–1150. [Google Scholar] [CrossRef]
- Hassanali, A.; Njagi, P.G.N.; Bashir, M.O. Chemical ecology of locusts and related Acridids. Ann. Rev. Entomol. 2005, 50, 223–245. [Google Scholar] [CrossRef]
- Simpson, S.J.; Miller, G.A. Maternal effects on phase characteristics in the desert locust, Shistocerca gregaria: A review of current understanding. J. Insect Physiol. 2007, 53, 869–876. [Google Scholar] [CrossRef]
- Maeno, K.; Tanaka, S. Phase-specific responses to different qualities of food in the desert locust, Schistocerca gregaria: Developmental, morphological and reproductive characteristics. J. Insect Physiol. 2011, 57, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nishide, Y. Do desert locust hoppers develop gregarious characteristics by watching video? J. Insect Physiol. 2012, 58, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, R.; Tanaka, S. Environmental and hormonal control of body color polyphenism in late-instar desert locust nymphs: Role of the yellow protein. Insect Biochem. Mol. Biol. 2018, 93, 27–36. [Google Scholar] [CrossRef]
- Sugawara, R.; Tanaka, S.; Jouraku, A.; Shiotsuki, T. Identification of a transcription factor that functions downstream of corazonin in the control of desert locust gregarious body coloration. Insect Biochem. Mol. Biol. 2018, 93, 27–36. [Google Scholar]
- Norris, M.J. Reproduction in the desert locust (Schistocerca gregaria Forsk.) in relation to density and phase. Anti-Locust Bull. 1952, 13, 1–49. [Google Scholar]
- Norris, M.J. Sexual maturation in the desert locust (Schistocerca gregaria Forsk.) with special reference to the effects of grouping. Anti-Locust Bull. 1954, 18, 1–44. [Google Scholar]
- Norris, M.J. Accelerating and inhibiting effects of crowding on sexual maturation in two species of locusts. Nature 1964, 203, 784–785. [Google Scholar] [CrossRef]
- Loher, W. The chemical acceleration of the maturation process and hormonal control in the male of the desert locust. Proc. R. Soc. Lond. B 1960, 153, 380–397. [Google Scholar]
- Amerasinghe, F.P. Pheromonal effects on sexual maturation, yellowing, and the vibration reaction in immature male desert locusts (Schistocerca gregaria). J. Insect Physiol. 1978, 24, 309–314. [Google Scholar] [CrossRef]
- Amerasinghe, F.P. Effects of J.H.I. and J.H.III. on yellowing, sexual activity and pheromone production in allatectomized male Shistocerca gregaria. J. Insect Physiol. 1978, 24, 603–611. [Google Scholar] [CrossRef]
- Assad, Y.O.H.; Hassanali, A.; Torto, B.; Mahamat, H.; Bashir, N.H.H.; Bashir, S.E. Effects of fifth-instar volatiles on sexual maturation of adult desert locust Schistocerca gregaria. J. Chem. Ecol. 1997, 23, 1377–1388. [Google Scholar] [CrossRef]
- Norris, M.J.; Pener, M.P. An inhibitory effect of allatectomized males and females on the sexual maturation of young male adults of Schistocerca gregaria (Forsk.) (Orthoptera: Acrididae). Nature 1965, 204, 1122. [Google Scholar] [CrossRef]
- Ott, S.R.; Rogers, S.M. Gregarious desert locusts have substantially larger brains with altered proportions compared with the solitarious phase. Proc. R. Soc. Lond. B 2010, 277, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.M.; Cullen, D.A.; Anstey, M.L.; Burrows, M.; Despland, E.; Dodgson, T.; Matheson, T.; Ott, S.R.; Stettin, K.; Sword, G.A.; et al. Rapid behavioural gregarization in the desert locust, Schistocerca gregaria entails synchronous changes in both activity and attraction to conspecifics. J. Insect Physiol. 2014, 65, 9–26. [Google Scholar] [CrossRef]
- Cisse, S.; Ghaout, S.; Mazih, A.; Jourdan-Pineau, H.; Maeno, K.O.; Piou, C. Characterizing phase-related differences in behaviour of Schistocerca gregaria with spatial distribution analysis. Entomol. Exp. Appl. 2015, 156, 128–135. [Google Scholar] [CrossRef]
- Gregory, G.E. The formation and fate of the spermatophore in the African migratory locust, Locusta migratoria migratorioides Reiche and Fairmaire. Trans. R. Entomol. Soc. Lond. 1965, 117, 33–66. [Google Scholar] [CrossRef]
- Pickford, R.; Ewen, A.B.; Gillott, C. Male accessory gland substance: An egg-laying stimulant in Melanoplus sanguinipes (F.) (Orthoptera: Acrididae). Can. J. Zool. 1969, 47, 1199–1203. [Google Scholar] [CrossRef]
- Gillott, C.; Venkatesh, K. Accumulation of secretory proteins in the accessory reproductive glands of the male migratory grasshopper, Melanoplus sanguinipes: A developmental study. J. Insect Physiol. 1985, 31, 195–204. [Google Scholar] [CrossRef]
- Rai, M.M.; Hassanali, A.; Saini, R.K.; Odongo, H.; Kahoro, H. Identification of components of the oviposition aggregation pheromone of the gregarious desert locust, Shistocerca gregaria (Forskal). J. Insect Physiol. 1997, 43, 83–87. [Google Scholar] [CrossRef]
- Szöllösi, A. Relationships between germ and somatic cells in the testes of locusts and moths. In Insect Ultrastructure; King, R.C., Akai, H., Eds.; Plenum Press: New York, NY, USA, 1982; Volume 1, pp. 32–63. [Google Scholar]
- Hamilton, A.G. The relation of humidity and temperature to the development of three species of African locusts-Locusta migratoria migratorioides (R. & F.), Shistocerca gregaria (Forsk.), Nomadacris septemfasciata (Serv.). Trans. R. Entomol. Soc. Lond. 1936, 85, 1–60. [Google Scholar]
- Quesada-Moraga, E.; Santiago-Alvarez, C. Assessment of sexual maturation in the Moroccan locust Dociostaurus maroccanus (Thunberg). J. Ortho. Res. 2001, 10, 1–8. [Google Scholar] [CrossRef]
- Odhiambo, T.R. The architecture of the accessory reproductive glands of the male desert locust. 5: Ultrastructure during maturation. Tissue Cell 1971, 3, 309–324. [Google Scholar] [CrossRef]
- Pickford, R.; Gillott, C. Insemination in the migratory grasshopper, Melanoplus sanguinipes (Fabr.). Can. J. Zool. 1971, 49, 1583–1588. [Google Scholar] [CrossRef]
- Pickford, R.; Gillott, C. Coupling behaviour of the migratory grasshopper, Melanoplus sanguinipes (Orthoptera: Acrididae). Can. Entomol. 1972, 104, 873–879. [Google Scholar] [CrossRef]
- Tsubaki, Y.; Yamagishi, M. “Longevity” of sperm within the female of the melon fly, Dacus cucurbitae (Diptera: Tephritidae), and its relevance to sperm competition. J. Insect Behav. 1991, 4, 243–250. [Google Scholar] [CrossRef]
- Stewart, A.D.; Hannes, A.M.; Rice, W.R. An assessment of sperm survival in Drosophila melanogaster. Evolution 2007, 61, 636–639. [Google Scholar] [CrossRef]
- Bertin, S.; Scolari, F.; Guglielmino, C.R.; Bonizzoni, M.; Bonomi, A.; Marchini, D.; Gomulski, L.M.; Gasperi, G.; Malacrida, A.R.; Matessi, C. Sperm storage and use in polyandrous females of the globally invasive fruitfly, Ceratitis capitata. J. Insect Physiol. 2010, 56, 1452–1551. [Google Scholar] [CrossRef]
- Radhakrishnan, P.; Fedorka, K.M. Influence of female age, sperm senescence and multiple mating on sperm viability in female Drosoph. Melanogaster. J. Insect Physiol. 2011, 57, 778–783. [Google Scholar] [CrossRef]
- Odhiambo, T.R. Growth and the hormonal control of sexual maturation in the desert locust, Schistocerca gregaria (Forkskål). Trans. R. Entomol. Soc. Lond. 1966, 118, 393–412. [Google Scholar] [CrossRef]
- Pener, M.P.; Lazarovici, P. Effect of exogenous juvenile hormones on mating behaviour and yellow colour in atllatectomized adult male desert locusts. Physiol. Entomol. 1979, 4, 251–261. [Google Scholar] [CrossRef]
- Couche, G.A.; Gillott, C. Development of secretory activity in the long hyaline gland of the male migratory grasshopper, Melanoplus sanguinipes (Fabr.) (Orthoptera: Acrididae). Int. J. Insect Morphol. Embryol. 1987, 16, 355–367. [Google Scholar] [CrossRef]
- Couche, G.A.; Gillott, C. Development of secretory activity in the seminal vesicle of the male migratory grasshopper, Melanoplus sanguinipes (Fabr.) (Orthoptera: Acrididae). Int. J. Insect Morphol. Embryol. 1988, 17, 51–61. [Google Scholar] [CrossRef]
- Venkatesh, K.; Gillott, C. Protein production in components of the accessory gland complex of male Melanoplus sanguinipes (Insecta: Orthoptera). Int. J. Invert. Reprod. 1983, 6, 317–325. [Google Scholar] [CrossRef]
- Thorson, B.J.; Riemann, J.G. Effects of 20-hydroxyecdysone on sperm release from the testes of the Mediterranean flour moth, Anagasta kuehniella (Zeller). J. Insect Physiol. 1982, 28, 1013–1019. [Google Scholar] [CrossRef]
- Gillott, C. Male insect accessory glands: Functions and control of secretory activity. Invert. Reprod. Dev. 1996, 30, 199–205. [Google Scholar] [CrossRef]
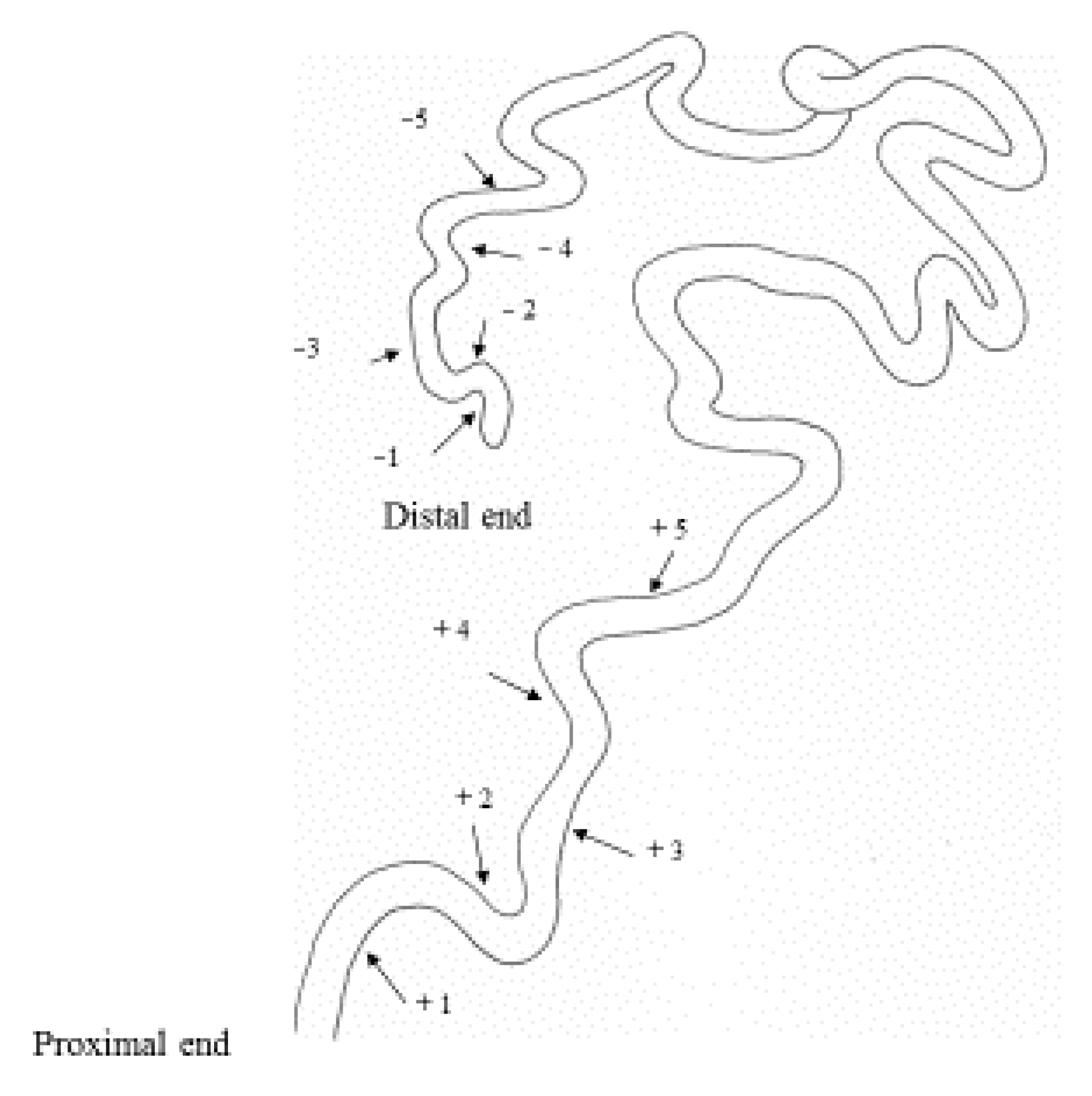
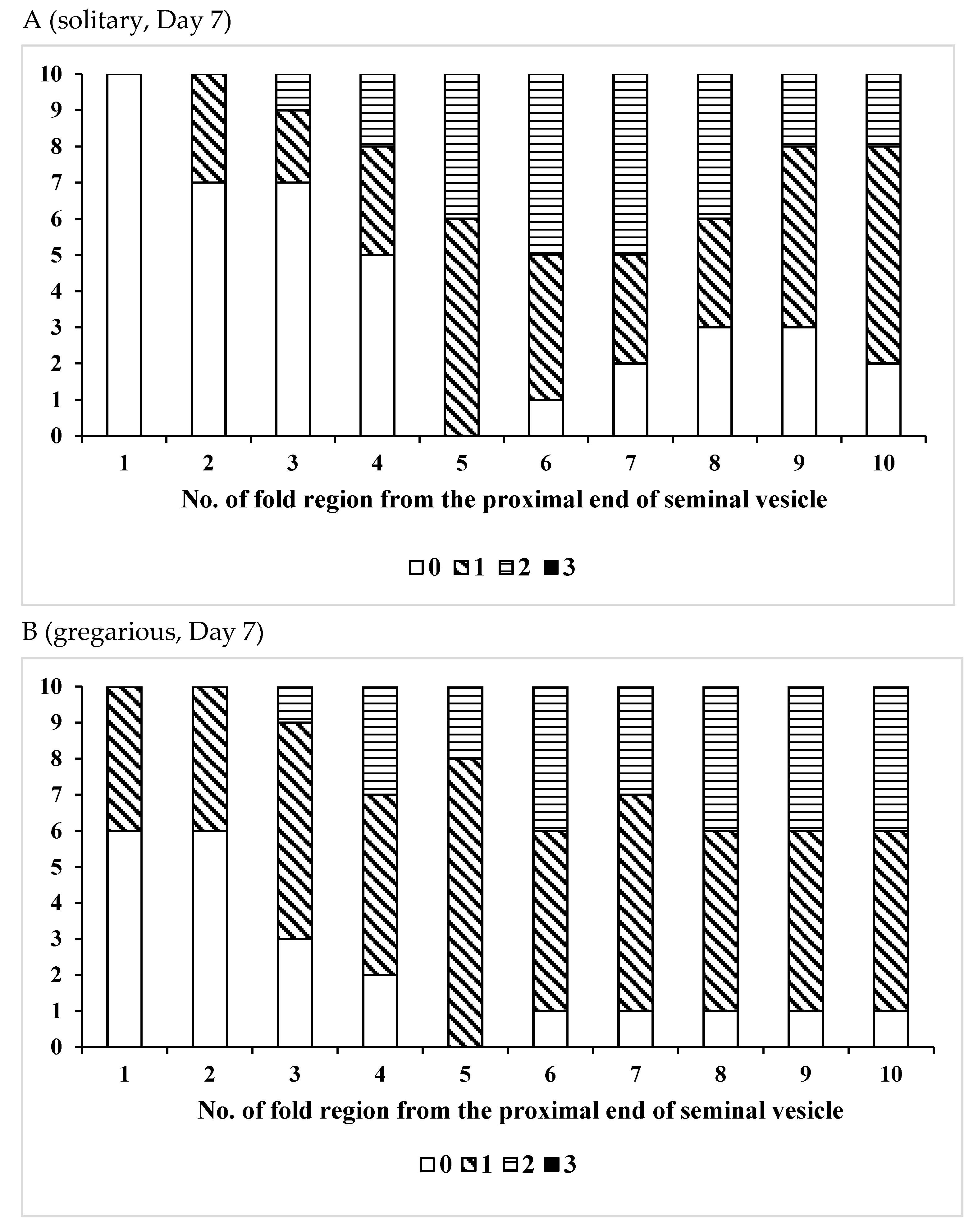
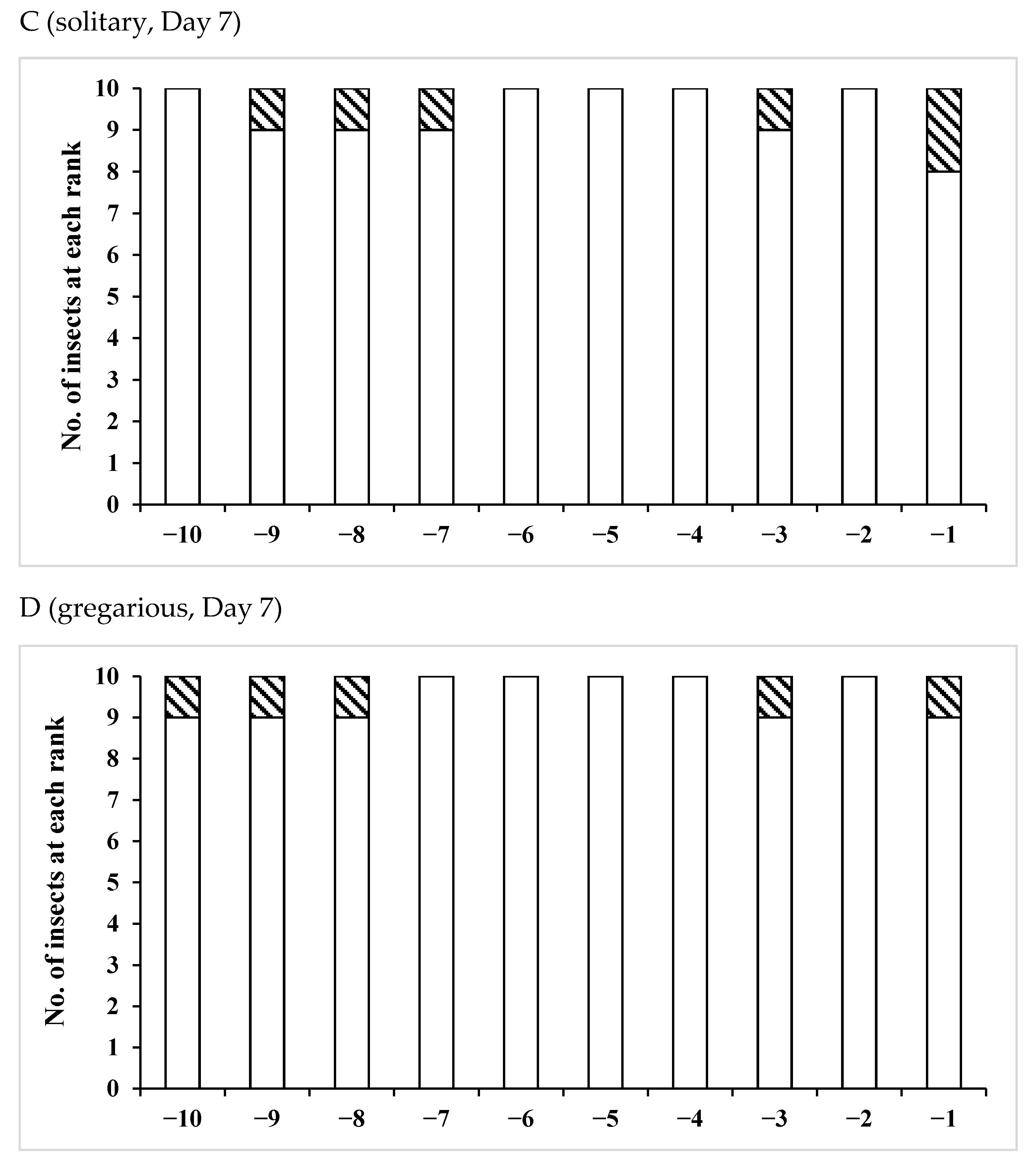
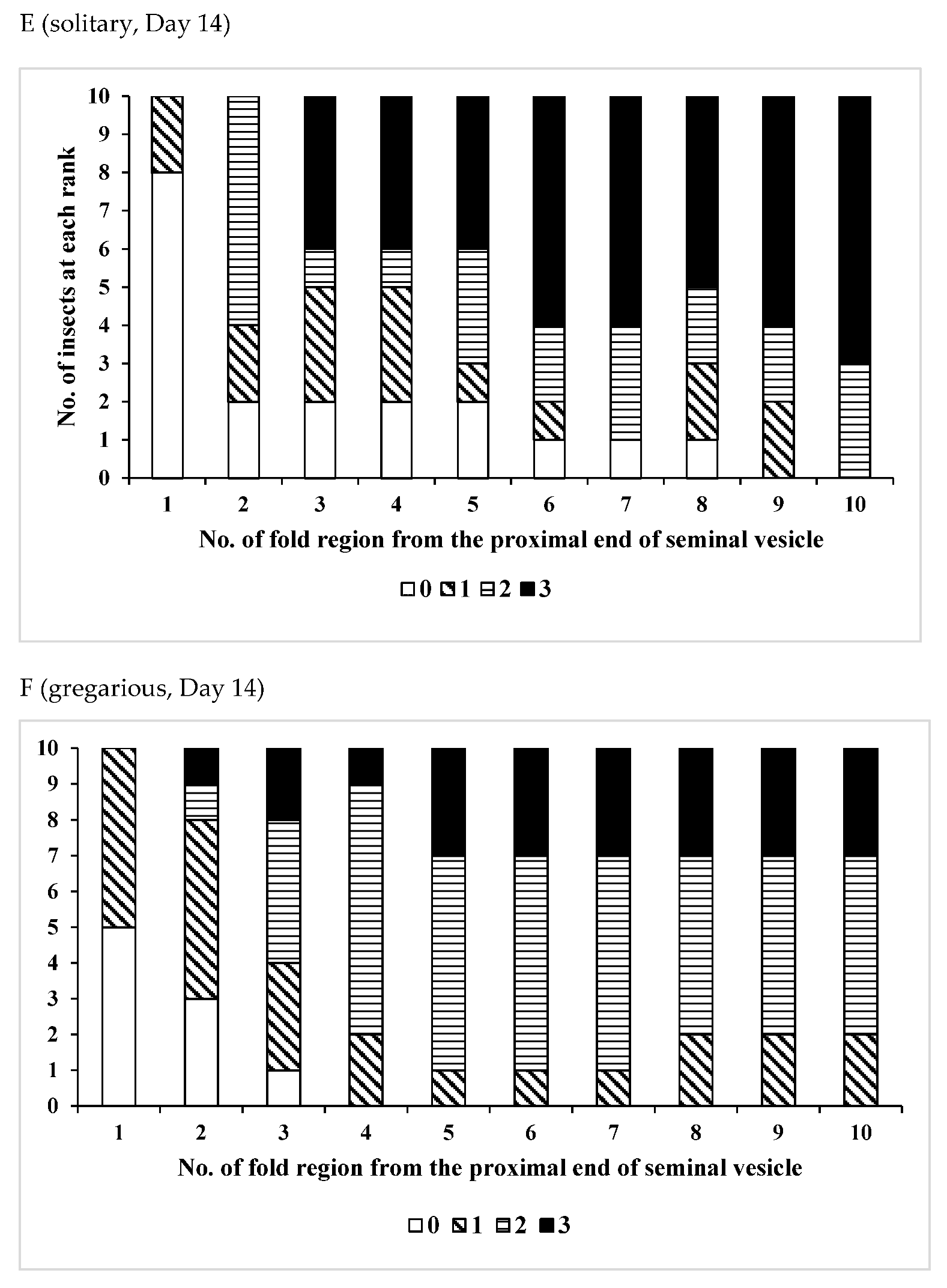
| Age (Days)/Phase | No. of Insects Used | Vas Deferens − | Presence of Sperm + | Seminal Vesicle − | + |
|---|---|---|---|---|---|
| 0 | |||||
| /Solitary | 8 | 8 | 0 | 8 | 0 |
| /Gregarious | 6 | 6 | 0 | 6 | 0 |
| 5 /Gregarious 7 | 5 | 4 | 1 | 4 | 1 |
| /Solitary | 11 | 1 | 10 | 1 | 10 |
| /Gregarious | 14 | 2 | 12 | 1 | 13 |
| 14 | |||||
| /Solitary | 10 | 0 | 10 | 0 | 10 |
| /Gregarious | 8 | 1 | 7 | 0 | 8 |
| 28 | |||||
| /Solitary | 10 | 2 | 8 | 0 | 10 |
| /Gregarious | 9 | 0 | 9 | 0 | 9 |
| Organ/Factor | Parameter | df | χ2 | p-Value |
|---|---|---|---|---|
| Vas deferens/Age | 1 | 1 | 14.8269711 | 0.0001 * |
| /Phase | 1 | 1 | 0.97583528 | 0.3232 |
| /Age × Phase | 1 | 1 | 1.11782763 | 0.2904 |
| Seminal vesicle/Age | 1 | 1 | 27.8032765 | <0.0001 * |
| /Phase | 1 | 1 | 0.00023428 | 0.9878 |
| /Age × Phase | 1 | 1 | 0.06424243 | 0.7999 |
| Age(Days)/Phase | No. of Insects Used | No of Fold Region Range | Average ± SD | p (S vs. G) |
|---|---|---|---|---|
| 0 | ||||
| /Solitary | 10 | 26–41 | 33.7 ± 5.12 | 0.0110 * |
| /Gregarious | 6 | 19–34 | 25.3 ± 5.09 | |
| 7 | ||||
| /Solitary | 11 | 27–37 | 32.5 ± 4.06 | 0.0217 * |
| /Gregarious | 10 | 23–36 | 27.5 ± 4.35 | |
| 14 | ||||
| /Solitary | 10 | 28–38 | 32.3 ± 2.91 | 0.0068 * |
| /Gregarious | 7 | 22–31 | 26.3 ± 3.55 | |
| 28 | ||||
| /Solitary | 15 | 22–38 | 31.7 ± 5.12 | 0.0548 |
| /Gregarious | 6 | 24–32 | 27.3 ± 2.94 |
| Factor | Parameter | df | χ2 | p Value |
|---|---|---|---|---|
| Phase | 1 | 1 | 0.00045826 | 0.9829 |
| Age | 1 | 1 | 356.79833 | <0.0001 * |
| Phase × Age | 1 | 1 | 0.74347209 | 0.3886 |
| Fold region from proximal end (FPE) | 1 | 1 | 200. 358912 | <0.0001 * |
| Phase × FPE | 1 | 1 | 10.3517704 | 0.0013 * |
| Age × FPE | 1 | 1 | 38.0121077 | <0.0001 * |
| Phase × Age × FPE | 1 | 1 | 4.65029832 | 0.0310 * |
| Phase | 1 | 1 | 21.519381 | <0.0001 * |
| Age | 1 | 1 | 424.940556 | <0.0001 * |
| Phase × Age | 1 | 1 | 0.111168 | 0.7388 |
| Fold region from distal end (FDE) | 1 | 1 | 18.5427795 | <0.0001 * |
| Phase × FDE | 1 | 1 | 0.87103289 | 0.3507 |
| Age × FDE | 1 | 1 | 0.76035844 | 0.3832 |
| Phase × Age × FDE | 1 | 1 | 0.16038184 | 0.6888 |
| Age (Days)/Pheromone | No. of Insects Used | Vas Deferens − | Presence of Sperm + | Seminal Vesicle − | + |
|---|---|---|---|---|---|
| 3 | |||||
| /Mature | 10 | 4 | 6 | 5 | 5 |
| /Nymph | 9 | 8 | 1 | 7 | 2 |
| 7 | |||||
| /Mature | 15 | 3 | 12 | 2 | 13 |
| /Nymph | 14 | 2 | 12 | 0 | 14 |
| 14 | |||||
| /Mature | 12 | 0 | 12 | 0 | 12 |
| /Nymph | 13 | 0 | 13 | 0 | 13 |
| 28 | |||||
| /Mature | 11 | 0 | 11 | 0 | 11 |
| /Nymph | 13 | 1 | 12 | 0 | 13 |
| Organ/Factor | Parameter | df | χ2 | p-Value |
|---|---|---|---|---|
| Vas deferens/Age | 1 | 1 | 16.5741729 | 0.0001 * |
| /Pheromone | 1 | 1 | 0.59408121 | 0.4408 |
| /Age × Pheromone | 1 | 1 | 0.00217653 | 0.9628 |
| Seminal vesicle/Age | 1 | 1 | 22.5726214 | 0.0001 * |
| /Pheromone | 1 | 1 | 0.03213105 | 0.8577 |
| /Age × Pheromone | 1 | 1 | 0.41065097 | 0.5216 |
| Age (Days)/Phase | No. of Insects Used | No of Fold Region Range | Average ± SD | p (S vs. G) |
|---|---|---|---|---|
| 3 | ||||
| /Mature | 9 | 17–35 | 25.6 ± 6.15 | 0.7962 |
| /Nymph | 9 | 20–33 | 25.8 ± 4.32 | |
| 7 | ||||
| /Mature | 15 | 18–35 | 25.3 ± 4.88 | 0.5537 |
| /Nymph | 14 | 20–32 | 26.1 ± 3.68 | |
| 14 | ||||
| /Mature | 12 | 21–29 | 26.7 ± 2.10 | 0.1197 |
| /Nymph | 13 | 24–28 | 25.8 ± 1.57 | |
| 28 | ||||
| /Mature | 10 | 24–35 | 30.5 ± 3.44 | 0.1604 |
| /Nymph | 13 | 25–33 | 28.7 ± 2.50 |
| Factor | Parameter | df | χ2 | p Value |
|---|---|---|---|---|
| Pheromone | 1 | 1 | 9.02784363 | 0.0027 * |
| Age | 1 | 1 | 244.925256 | <0.0001 * |
| Pheromone × Age | 1 | 1 | 1.10510193 | 0.2931 |
| Fold region from proximal end (FPE) | 1 | 1 | 350.413422 | <0.0001 * |
| Pheromone × FPE | 1 | 1 | 3.07012175 | 0.0797 * |
| Age × FPE | 1 | 1 | 126.245783 | <0.0001 * |
| Pheromone × Age × FPE | 1 | 1 | 0.2856723 | 0.5930 |
| Pheromone | 1 | 1 | 4.19837727 | 0.0405 * |
| Age | 1 | 1 | 288.467967 | <0.0001 * |
| Pheromone × Age | 1 | 1 | 13.5989836 | 0.0002 * |
| Fold region from distal end (FDE) | 1 | 1 | 53.2676067 | <0.0001 * |
| Pheromone × FDE | 1 | 1 | 0.27928405 | 0.5972 |
| Age × FDE | 1 | 1 | 7.75091207 | 0.0054 * |
| Pheromone × Age × FDE | 1 | 1 | 2.15718014 | 0.1419 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiroyoshi, S.; Mitsunaga, T.; Ganaha-Kikumura, T.; Reddy, G.V.P. Effects of Age, Phase Variation and Pheromones on Male Sperm Storage in the Desert Locust, Schistocerca gregaria. Insects 2021, 12, 642. https://doi.org/10.3390/insects12070642
Hiroyoshi S, Mitsunaga T, Ganaha-Kikumura T, Reddy GVP. Effects of Age, Phase Variation and Pheromones on Male Sperm Storage in the Desert Locust, Schistocerca gregaria. Insects. 2021; 12(7):642. https://doi.org/10.3390/insects12070642
Chicago/Turabian StyleHiroyoshi, Satoshi, Takayuki Mitsunaga, Tomoko Ganaha-Kikumura, and Gadi V. P. Reddy. 2021. "Effects of Age, Phase Variation and Pheromones on Male Sperm Storage in the Desert Locust, Schistocerca gregaria" Insects 12, no. 7: 642. https://doi.org/10.3390/insects12070642
APA StyleHiroyoshi, S., Mitsunaga, T., Ganaha-Kikumura, T., & Reddy, G. V. P. (2021). Effects of Age, Phase Variation and Pheromones on Male Sperm Storage in the Desert Locust, Schistocerca gregaria. Insects, 12(7), 642. https://doi.org/10.3390/insects12070642







