Field Effectiveness of Drones to Identify Potential Aedes aegypti Breeding Sites in Household Environments from Tapachula, a Dengue-Endemic City in Southern Mexico
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
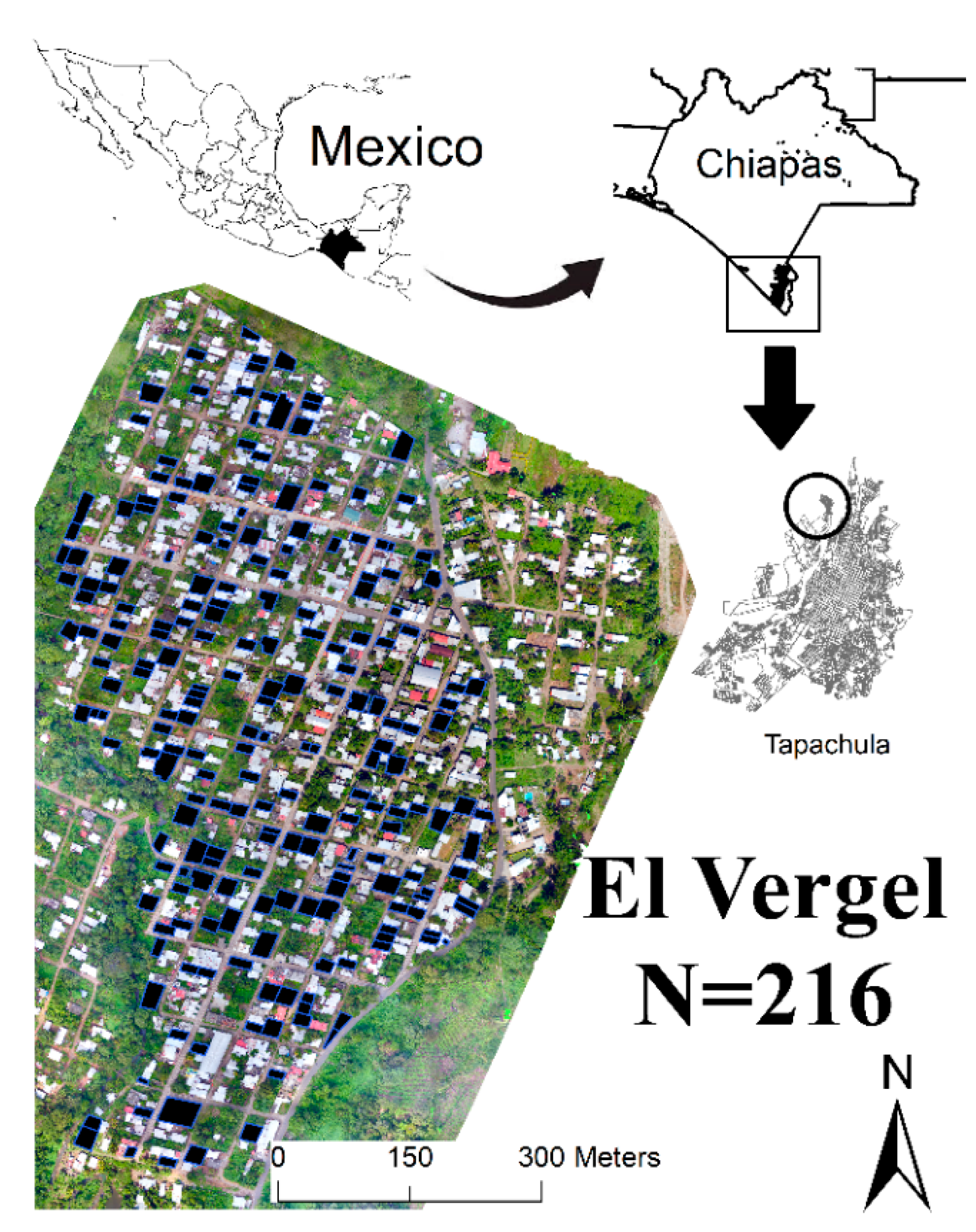
2.1. Study Area
2.2. Breeding Habitats Sampling by Traditional Ground Surveillance (GS)
2.3. Breeding Habitats Inspections by Drone Surveillance (DS)
2.4. Data Analysis
3. Results
3.1. Ground Surveillance (GS) Method
3.2. Drone Surveillance (DS) Method
3.3. Comparison between Drone and Ground Surveillance
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huy, N.T.; Van Giang, T.; Thuy, D.H.D.; Kikuchi, M.; Hien, T.T.; Zamora, J.; Hirayama, K. Factors associated with dengue shock syndrome: A system review and meta-analysis. PLoS Negl. Trop. Dis. 2013, 7, e2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arredondo-García, J.L.; Aguilar-López, E.C.G.; Lugo-Gerez, A.J.J.; Osnaya-Romero, N.; Pérez-Guillé, G.; Medina-Cortina, H. Panorama epidemiológico de dengue en Mexico 2000–2019. Rev. Latinoam. Infect. Pediatrica 2020, 33, 78–83. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy for Dengue Prevention and Control 2012–2020; WHO: Geneva, Switzerland, 2012; p. 14. ISBN 978-92-4-150403-4. [Google Scholar]
- World Health Organization. Report of the Consultation on Key Issues in Dengue Vector Control Toward the Operationalization of a Global Strategy; WHO: Geneva, Switzerland, 1995; p. 48, CTD/FIL(DEN)/IC/95.WP.3.4.1. [Google Scholar]
- World Health Organization. Strengthening Implementation of the Global Strategy for Dengue Fever/Dengue Haemorrhagic Fever Prevention and Control; Report of the Informal Consultation 18–20 October 1999; Document WHO/CDS/(DEN)/ IC/2000.1; WHO: Geneva, Switzerland, 2000; p. 10. [Google Scholar]
- Focks, D.A.; Chadee, D. Pupal survey: An epidemiologically significant surveillance method for Aedes aegypti: An example using from Trinidad. Am. J. Trop. Med. Hyg. 1997, 56, 159–167. [Google Scholar] [CrossRef]
- Tun-Lin, W.; Kay, B.H.; Barnes, A. The Premise Condition Index: A tool for streamlining surveys of Aedes aegypti. Am. J. Trop. Med. Hyg. 1995, 53, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Tun-Lin, W.; Kay, B.H.; Barnes, A.; Forsyth, S. Critical examination of Aedes aegypti indices: Correlations with abundance. Am. J. Trop. Med. Hyg. 1996, 54, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Focks, D.A. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. In A Review of Entomological Sampling Methods and Indicators for Dengue Vectors; TDR/IDE/Den/03.1; WHO: Geneva, Switzerland, 2004; p. 36. Available online: http://whqlibdoc.who.int/hq/2003/TDR_IDE_DEN_03.1.pdf (accessed on 10 October 2020)TDR/IDE/Den/03.1.
- Bowman, L.R.; Runge-Ranzinger, S.; McCall, P.J. Assessing the Relationship between Vector Indices and Dengue Transmission: A Systematic Review of the Evidence. PLoS Negl. Trop. Dis. 2014, 8, e2848. [Google Scholar] [CrossRef] [PubMed]
- Garjito, T.A.; Hidajat, M.C.; Kinansi, R.R.; Setyaningsih, R.; Anggraeni, Y.M.; Trapsilowati, W.; Satoto, T.B.T.; Gavotte, L.; Manguin, S.; Frutos, R. Stegomyia Indices and Risk of Dengue Transmission: A Lack of Correlation. Front. Public Health 2020, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Saide, P.; Che-Mendoza, A.; Rizzo, N.; Arana, B.; Pilger, D.; Lenhart, A.; Kroeger, A. Operational Guide for Assessing the Productivity of Aedes Aegypti Breeding Sites; UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases: Geneva, Switzerland, 2011; p. 30. ISBN 978-92-4-150268-9. [Google Scholar]
- Diario Oficial de la Federación. NORMA Oficial Mexicana NOM-032-SSA2-2014, Para la Vigilancia Epidemiológica, Promoción, Prevención y Control de las Enfermedades Transmitidas por Vectores. DOF: 16/04/2015. Available online: http://www.cenaprece.salud.gob.mx/programas/interior/vectores/descargas/pdf/NOM_032_SSA2_2014.pdf (accessed on 2 December 2020).
- Organización Panamericana de la Salud. Evaluación de las Estrategias Innovadoras para el Control de Aedes aegypti: Desafíos para su Introducción y Evaluación del Impacto; Organización Panamericana de la Salud: Washington, DC, USA, 2019; p. 62. ISBN 978-92-75-32096-9. eISBN 978-92-75-32097-6. [Google Scholar] [CrossRef]
- Dantés, H.G.; Manrique-Saide, P.; Vazquez-Prokopec, G.; Correa Morales, F.; Siqueira Junior, J.B.; Pimenta, F.; Coehlo, G.; Bezerra, H. Prevention and control of Aedes transmitted infections in the post-pandemic scenario of COVID-19: Challenges and opportunities for the region of the Americas. Mem. Inst. Oswaldo Cruz 2020, 115, e200284. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L. Ethical issues with use of Drone aircraft. In Proceedings of the 2014 IEEE International Symposium on Ethics in Science, Technology and Engineering, ETHICS 2014, Chicago, IL, USA, 23–24 May 2014; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Luppicini, R.; So, A. A technoethical review of commercial drone use in the context of governance, ethics, and privacy. Technol. Soc. 2016, 46, 109–119. [Google Scholar] [CrossRef]
- Nex, F.; Remondino, F. UAV for 3D mapping application: A review. Appl. Geomat. 2014, 6, 1–15. [Google Scholar] [CrossRef]
- Landau, K.I.; Van Leeuwen, W.J. Fine scale spatial urban land cover factors associated with adult mosquito abundance and risk in Tucson, Arizona. J. Vector Ecol. 2012, 37, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadistica; Geografía e Informática. Censo de Poblacion y Vivienda 2010. MEX-INEGI 40.201.01-CPV-2010. Available online: https://inegi.org.mx/programas/ccpv/2010/ (accessed on 10 November 2020).
- Arredondo-Jiménez, J.I.; Valdez-Delgado, K.M. Aedes aegypti pupal/demographic surveys in southern Mexico: Consistency and practicality. Ann. Trop. Med. Parasitol. 2006, 100, S17–S32. [Google Scholar] [CrossRef] [PubMed]
- Dirección General de Aeronáutica Civil. Circular Obligatoria que Establece los Requerimientos Para Operar un Sistema de Aeronave Pilotada a Distancia (RPAS) en el Espacio Aéreo Mexicano. 2017, p. 55. Available online: http://www.sct.gob.mx/fileadmin/DireccionesGrales/DGAC-archivo/modulo3/co-av-23-10-r4.pdf (accessed on 16 October 2020).
- Phantom 4 DJI® User Manual. Available online: https://dl.djicdn.com/downloads/phantom_4/en/Phantom_4_User_Manual_en_v1.2_20160805.pdf (accessed on 16 October 2020).
- DJI®GO 4 App. Available online: https://www.dji.com/mx/downloads/products/phantom-4 (accessed on 16 October 2020).
- Case, E.; Shragai, T.; Harrington, L.; Ren, Y.; Morreale, S.; Erickson, D. Evaluation of Unmanned Aerial Vehicles and Neural Networks for Integrated Mosquito Management of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.M.; Skelly, C.; Weinstein, P.; Maguire, M.; Ritchie, S. Domestic Aedes aegypti breeding site surveillance: Limitations of remote sensing as a predictive surveillance tool. Am. J. Trop. Med. Hyg. 1998, 59, 261–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suduwella, C.; Amarasinghe, A.; Niroshan, L.; Elvitigala, C.; De Zoysa, K.; Keppetiyagama, C. Identifying Mosquito Breeding Sites via Drone Images. In Proceedings of the 3rd Workshop on Micro Aerial Vehicle Networks, Systems, and Applications-DroNet’17, Association for Computing Machinery. New York, NY, USA, 23 June 2017; pp. 27–30. [Google Scholar] [CrossRef]
- Team GIMP. GIMP: GNU Image Manipulation Program. GIMP Team. 2019. Available online: https://www.gimp.org.es/ (accessed on 1 October 2020).
- Requena-Méndez, A.; Aldasoro, E.; Muñoz, J.; Moore, D.A.J. Robust and Reproducible Quantification of the Extent of Chest Radiographic Abnormalities (And It’s Free!). PLoS ONE 2015, 10, e0128044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, N.; Schiefenhovel, W.; d’Errico, F.; Banks, W.E.; Vanhaeren, M. Quantitative methods demonstrate that environment alone is an insufficient predictor of present-day language distributions in New Guinea. PLoS ONE 2020, 15, e0239359. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Acevedo, V.; Amador, M. Role of Abandoned and Vacant Houses on Aedes aegypti Productivity. Am. J. Trop. Med. Hyg. 2021, 104, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Control of Aedes Aegypti in the Scenario of Simultaneous Transmission of COVID-19; Technical and Scientific Products; PAHO: Washington, DC, USA, 2020; p. 6. Available online: https://www.paho.org/en/documents/control-aedes-aegypti-scenario-simultaneous-transmission-covid-19 (accessed on 23 January 2021)Technical and Scientific Products.
- Ruiz-Cabrejos, J.; Moreno, M.; Bickersmith, S.; Saavedra, M.; Prussing, C.; Gamboa, D.; Manrique, E.; Conn, J.E.; Alava, F.; Vinetz, J.M.; et al. High-accuracy detection of malaria vector larval habitats using drone-based multispectral imagery. PLoS Negl. Trop. Dis. 2019, 13, e0007105. [Google Scholar] [CrossRef] [Green Version]
- Sarira, T.V.; Clarke, K.; Weinstein, P.; Koh, L.P.; Lewis, M. Rapid identification of shallow inundation for mosquito disease mitigation using drone-derived multispectral imagery. Geospat. Health 2020, 15, 101–108. [Google Scholar] [CrossRef]
- Passos, W.L.; da Silva, E.A.B.; Netto, S.L.; Araujo, G.M.; de Lima, A.A. Spatio-temporal Consistency to Detect Potential Aedes aegypti Breeding Grounds in Aerial Video Sequences. arXiv 2020, arXiv:2007.14863. [Google Scholar]


| Container Type | House Location | Total | ||||
|---|---|---|---|---|---|---|
| Indoor (%) | +/−Larvae Presence (%) | Outdoor (%) | +/−Larvae Presence (%) | Frequency (%) | +/−Larvae Presence (%) | |
| Plastic bucket & tubs | 60 (14.4) | 3 (5.0) | 191 (8.2) | 12 (6.3) | 251 (9.1) | 15 (6.0) |
| Tin bucket | 2 (0.5) | 1 (50.0) | 10 (0.4) | 1 (10.0) | 12 (0.4) | 2 (16.7) |
| 55-Ga drum | 31 (7.4) | 5 (16.1) | 55 (2.4) | 12 (21.8) | 86 (3.1) | 17 (19.8) |
| Non-disposable plastic container | 51 (12.2) | 2 (3.9) | 232 (9.9) | 10 (4.3) | 283 (10.3) | 12 (4.2) |
| Non-disposable glass container | 17 (4.1) | 0 (0.0) | 51 (2.2) | 1 (2.0) | 68 (2.5) | 1 (1.5) |
| Non-disposable pewter container | 1 (0.2) | 0 (0.0) | 3 (0.1) | 1 (33.3) | 4 (0.1) | 1 (25.0) |
| Clay pot | 1 (0.2) | 0 (0.0) | 2 (0.1) | 1 (50.0) | 3 (0.1) | 1 (33.3) |
| Cement washbasin (large) | 97 (23.2) | 42 (43.3) | 108 (4.6) | 37 (34.3) | 205 (7.4) | 79 (38.5) |
| Cement washbasin (small) | 2 (0.5) | 0 (0.0) | 6 (0.3) | 1 (16.7) | 8 (0.3) | 1 (12.5) |
| Elevated storage tank | 2 (0.5) | 0 (0.0) | 36 (1.5) | 3 (8.3) | 38 (1.4) | 3 (7.9) |
| Cistern | 0 (0.0) | 0 (0.0) | 2 (0.1) | 0 (0.0) | 2 (0.1) | 0 (0.0) |
| Flowerpot | 23 (5.5) | 0 (0.0) | 846 (36.2) | 6 (0.7) | 869 (31.6) | 6 (0.7) |
| Animal drinking container (fixed) | 5 (1.2) | 0 (0.0) | 24 (1.0) | 1 (4.2) | 29 (1.1) | 1 (3.4) |
| Animal eating container (fixed) | 0 (0.0) | 0 (0.0) | 4 (0.2) | 0 (0.0) | 4 (0.1) | 0 (0.0) |
| Glass bottle | 1 (0.2) | 0 (0.0) | 39 (1.7) | 1 (2.6) | 40 (1.5) | 1 (2.5) |
| Can | 0 (0.0) | 0 (0.0) | 151 (6.5) | 3 (2.0) | 151 (5.5) | 3 (2.0) |
| Animal drinking (mobile) | 1 (0.2) | 0 (0.0) | 141 (6.0) | 5 (3.5) | 142 (5.2) | 5 (3.5) |
| Animal eating container (mobile) | 0 (0.0) | 0 (0.0) | 2 (0.1) | 0 (0.0) | 2 (0.1) | 0 (0.0) |
| Disposable plastic container | 7 (1.7) | 0 (0.0) | 218 (9.3) | 4 (1.8) | 225 (8.2) | 4 (1.8) |
| Disposable glass container | 2 (0.5) | 0 (0.0) | 11 (0.5) | 0 (0.0) | 13 (0.5) | 0 (0.0) |
| Disposable pewter container | 0 (0.0) | 0 (0.0) | 3 (0.1) | 1 (33.3) | 3 (0.1) | 1 (33.3) |
| Vase | 14 (3.3) | 3 (21.4) | 4 (0.2) | 1 (25.0) | 18 (0.7) | 4 (22.2) |
| Tire | 11 (2.6) | 0 (0.0) | 84 (3.6) | 7 (8.3) | 95 (3.5) | 7 (7.4) |
| Toilet | 82 (19.6) | 1 (1.2) | 66 (2.8) | 2 (3.0) | 148 (5.4) | 3 (2.0) |
| Discarded stove | 0 (0.0) | 0 (0.0) | 2 (0.1) | 0 (0.0) | 2 (0.1) | 0 (0.0) |
| Others | 8 (1.9) | 2 (25.0) | 43 (1.8) | 8 (18.6) | 51 (1.9) | 10 (19.6) |
| Total (n) | 418 (100.0) | 59 (14.1) | 2334 (100.0) | 118 (5.1) | 2752 (100.0) | 177 (6.4) |
| Container Type | Drone Surveillance (DS) | Ground Surveillance (GS) | Proportion of Containers DS in Roofs:GS | Proportion of Containers DS in Backyards:GS | Proportion of Containers DS Total: GS | ||
|---|---|---|---|---|---|---|---|
| Roofs (%) | Backyards (%) | Total (%) | |||||
| Disposable plastic containers | 55 (37.4) | 218 (26.1) | 273 (27.8) | 218 | 1:4.0 | 1:1 | 1.25:1 |
| Plastic bucket & tubs | 20 (13.6) | 378 (45.2) | 398 (40.5) | 191 | 1:10.0 | 1.98:1 | 2.08:1 |
| Elevated storage tanks | 12 (8.2) | 8 (1.0) | 20 (2.0) | 36 | 1:3 | 1:4.5 | 1:1.8 |
| Cement washbasins (large) | 0 (0.0) | 8 (1.0) | 8 (0.8) | 108 | 0:1 | 1:13.5 | 1:13.5 |
| Tires | 10 (6.8) | 15 (1.8) | 25 (2.5) | 84 | 1:8.4 | 1:5.6 | 1:3.4 |
| Toilets | 0 (0.0) | 9 (1.1) | 9 (0.9) | 66 | 0:1 | 1:7.3 | 1:7.3 |
| Flowerpots | 3 (2.0) | 152 (18.2) | 155 (15.8) | 846 | 1:282 | 1:5.6 | 1:5.5 |
| Tin buckets | 2 (1.4) | 20 (2.4) | 22 (2.2) | 10 | 1:5 | 2:1 | 2.20:1 |
| Glass bottles | 0 (0.0) | 9 (1.1) | 9 (0.9) | 39 | 0:1 | 1:4.3 | 1:4.3 |
| Others | 45 (30.6) | 19 (2.3) | 64 (6.5) | 43 | 1.1:1 | 1:2.3 | 1.49:1 |
| Total (n) | 147 (15.0) | 836 (85.0) | 983 (100) | 2752 * | 1:18.7 * | 1:3.3 * | 1:2.8 * |
| Container type | Presence by DS (%) | Absence by DS (%) | Presence by GS (%) | Absence by GS (%) | Presence DS + GS (%) | Absence DS + GS (%) | Concordance DS–GS (%) * |
|---|---|---|---|---|---|---|---|
| Disposable plastic containers | 62 (28.7) | 154 (71.3) | 18 (8.3) | 198 (91.7) | 77 (35.6) | 139 (64.3) | 3 (3.9) |
| Plastic bucket & tubs | 101 (46.8) | 115 (53.2) | 84 (38.9) | 132 (61.1) | 142 (65.7) | 74 (34.3) | 43 (30.3) |
| Elevated storage tanks | 8 (3.7) | 208 (96.3) | 34 (15.7) | 182 (84.3) | 40 (18.5) | 176 (81.5) | 2 (5.0) |
| Cement washbasins (large) | 7 (3.2) | 209 (96.8) | 106 (49.1) | 110 (50.9) | 108 (50.0) | 108 (50.0) | 5 (4.6) |
| Tires | 9 (4.2) | 207 (95.8) | 16 (7.4) | 200 (92.6) | 23 (10.6) | 193 (89.4) | 2 (8.7) |
| Toilets | 8 (3.7) | 208 (96.3) | 64 (29.6) | 152 (70.4) | 69 (31.9) | 147 (68.1) | 3 (4.35) |
| Flowerpots | 48 (22.2) | 168 (77.8) | 101 (46.8) | 115 (53.2) | 127 (58.8) | 89 (41.2) | 22 (17.32) |
| Tin buckets | 16 (7.4) | 200 (92.6) | 10 (4.6) | 206 (95.4) | 24 (11.1) | 192 (88.9) | 2 (8.33) |
| Glass bottles | 3 (1.4) | 213 (98.6) | 12 (5.6) | 204 (94.4) | 15 (6.9) | 201 (93.1) | 0 (0.0) |
| Others | 19 (8.8) | 197 (91.2) | 39 (18.1) | 177 (81.9) | 55 (25.5) | 161 (84.5) | 3 (5.5) |
| Total (n) | 146 (67.6) | 70 (32.4) | 197 (91.2) | 19 (8.8) | 208 (96.3) | 8 (3.7) | 135 (64.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez-Delgado, K.M.; Moo-Llanes, D.A.; Danis-Lozano, R.; Cisneros-Vázquez, L.A.; Flores-Suarez, A.E.; Ponce-García, G.; Medina-De la Garza, C.E.; Díaz-González, E.E.; Fernández-Salas, I. Field Effectiveness of Drones to Identify Potential Aedes aegypti Breeding Sites in Household Environments from Tapachula, a Dengue-Endemic City in Southern Mexico. Insects 2021, 12, 663. https://doi.org/10.3390/insects12080663
Valdez-Delgado KM, Moo-Llanes DA, Danis-Lozano R, Cisneros-Vázquez LA, Flores-Suarez AE, Ponce-García G, Medina-De la Garza CE, Díaz-González EE, Fernández-Salas I. Field Effectiveness of Drones to Identify Potential Aedes aegypti Breeding Sites in Household Environments from Tapachula, a Dengue-Endemic City in Southern Mexico. Insects. 2021; 12(8):663. https://doi.org/10.3390/insects12080663
Chicago/Turabian StyleValdez-Delgado, Kenia Mayela, David A. Moo-Llanes, Rogelio Danis-Lozano, Luis Alberto Cisneros-Vázquez, Adriana E. Flores-Suarez, Gustavo Ponce-García, Carlos E. Medina-De la Garza, Esteban E. Díaz-González, and Ildefonso Fernández-Salas. 2021. "Field Effectiveness of Drones to Identify Potential Aedes aegypti Breeding Sites in Household Environments from Tapachula, a Dengue-Endemic City in Southern Mexico" Insects 12, no. 8: 663. https://doi.org/10.3390/insects12080663








