Using Chemical Ecology to Enhance Weed Biological Control
Abstract
:Simple Summary
Abstract
1. Introduction
2. Discovery and Development of Semiochemicals for Weed Biocontrol
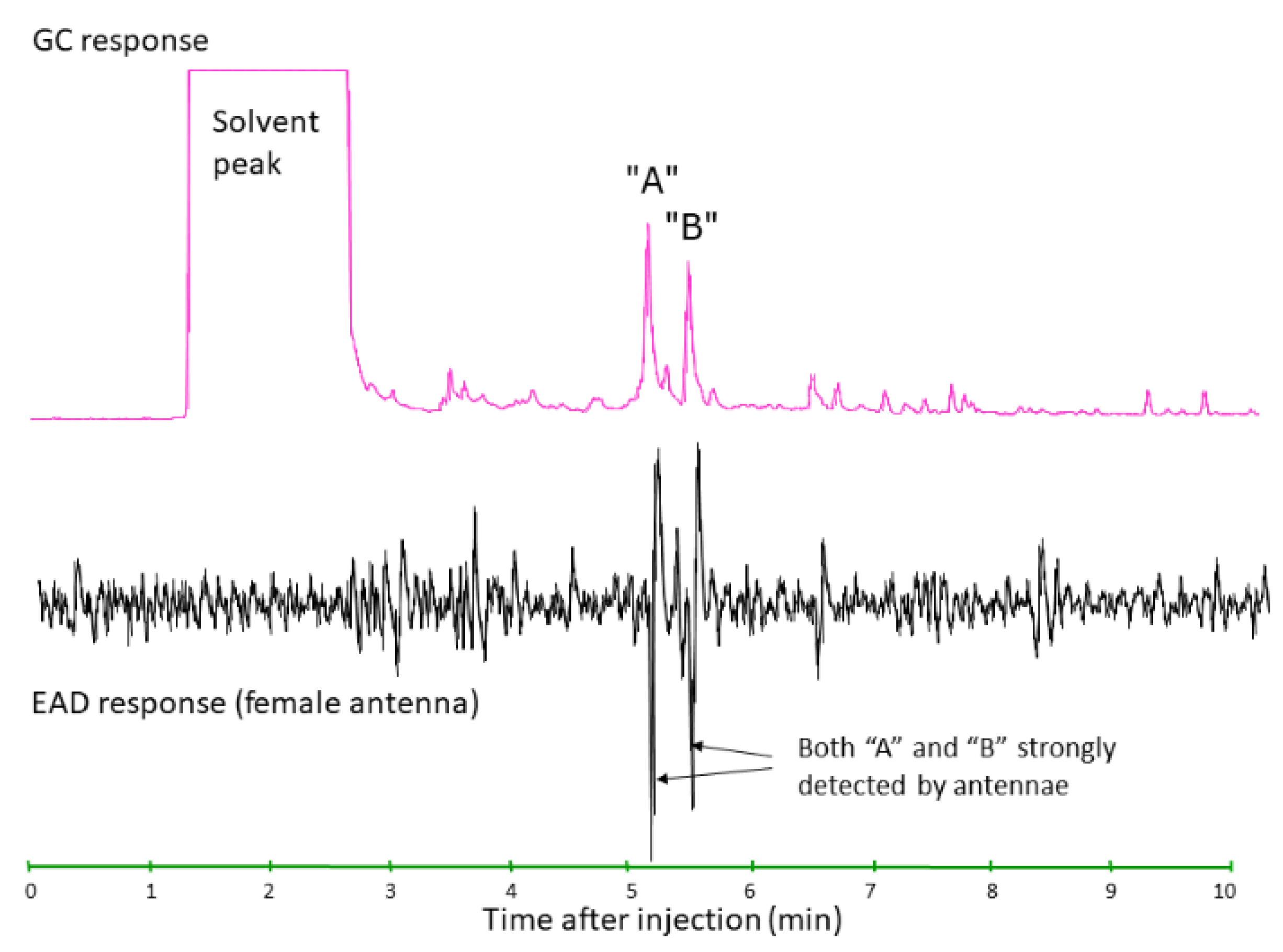
3. Sex Pheromones
4. Aggregation Pheromones
5. Plant-Produced Semiochemicals
6. Using Semiochemicals and the Principles of Chemical Ecology to Enhance Weed Biocontrol
6.1. Enhancing Establishment: Importance of Pheromones during Rearing and Releases
6.2. Sentinel Trapping and Population Monitoring
- The use of the sex attractants allowed researchers to determine that five pairs of the gorse pod moth, Cydia succedana were sufficient to get establishment, thus eliminated costly mass release. The discovery that so few adults were needed for establishment enabled researcher to target many more sites for release [10]. In Hawaii, the pheromone-based monitoring for Acleris (=Croesia) zimmermani Clarke 1978 (Lepidoptera: Tortricidae) and Schreckensteinia festaliella Hübner (Lepidoptera: Schreckensteiniidae) resulted in better evaluation of the Rubus spp. [11] biocontrol programs by providing cost-effective presence/absence data, as well as data on density, phenology, host plant synchrony, and dispersal rates [27].
- Aggregation-causing semiochemicals, especially the aggregation pheromone, were successfully deployed in the field to monitor for the presence of the biocontrol agent D. carinulata. The pheromone was deployed in conjunction with passive, yellow sticky card traps and resulted in detecting the early establishment of D. carinulata at six locations in the southwestern United States, allowing land managers to plan accordingly to incorporate the biological control program into their broader land management strategy [T. Dudley, unpublished data]. The deployment of pheromone-baited traps for detection of D. carinulata also provides an example of how semiochemical baits can be effectively used to detect low-level presence during the initial range expansion of a newly established agent. In the case of Tamarix biocontrol D. carinulata-induced defoliation was readily apparent and detectable using remote sensing (e.g., [53]) but the initial colonization events, presumably driven by dispersal from areas with more dense populations, were extremely difficult to detect without semiochemical baits [16]. This is due to the vast areas covered by Tamarix, and the patchy distribution of Diorhabda spp. during initial colonization, which means that sweep sampling using insect nets can easily miss early colonizing populations.
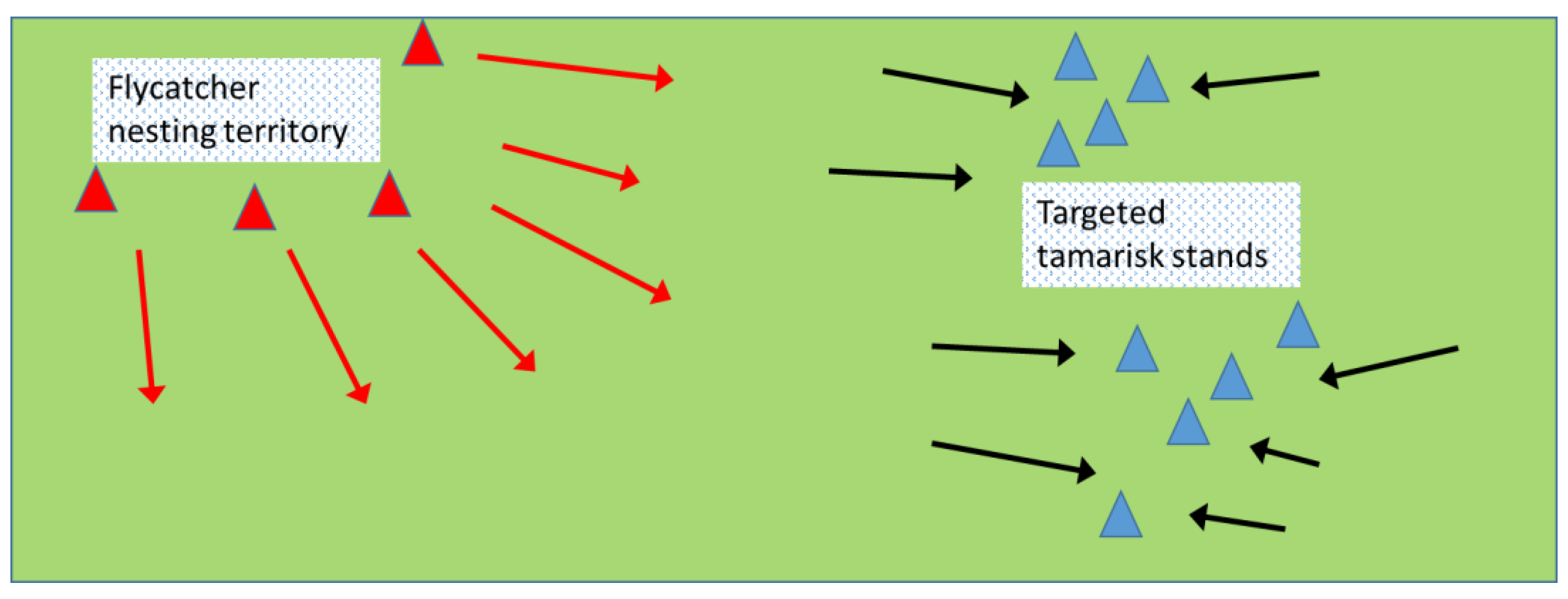
6.3. Directing Activity: Manipulating Population Density with Attractants & Deterrents
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [Green Version]
- Bradley, B.A.; Laginhas, B.B.; Whitlock, R.; Allen, J.M.; Bates, A.E.; Bernatchez, G.; Diez, J.M.; Early, R.; Lenoir, J.; Vilà, M.; et al. Disentangling the abundance–impact relationship for invasive species. Proc. Natl. Acad. Sci. USA 2019, 116, 9919–9924. [Google Scholar] [CrossRef] [Green Version]
- Hinz, H.L.; Winston, R.L.; Schwarzländer, M. A global review of target impact and direct nontarget effects of classical weed biological control. Curr. Opin. Insect Sci. 2020, 38, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zwölfer, H.; Harris, P. Host specificity determination of insects for biological control of weeds. Annu. Rev. Entomol. 1971, 16, 159–178. [Google Scholar] [CrossRef]
- Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Developing sustainable pest control from chemical ecology. Agr. Ecosys. Environ. 1997, 64, 149–156. [Google Scholar] [CrossRef]
- Saha, T.; Chandran, N. Chemical ecology and pest management: A review. Int. J. Chem. Stud. 2017, 5, 618–621. [Google Scholar]
- Heard, T.A. Concepts in insect host-plant selection behavior and their application to host specificity testing. In Host Specificity of Exotic Arthropod Biological Control Agents: The Biological Basis for Improvement in Safety, Proceedings of the Session: X International Symposium on Biological Control of Weeds; van Driesche, R.G., Heard, T., McClay, A., Reardon, R., Eds.; US Forest Service, Forest Health Enterprise Technology Team: Morgantown, WV, USA, 2000; pp. 1–10. [Google Scholar]
- Wheeler, G.S.; Schaffner, U. Improved Understanding of Weed Biological Control Safety and Impact with Chemical Ecology: A Review. Invasive Plant Sci. Manag. 2013, 6, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Suckling, D.M.; Hill, R.L.; Gourlay, A.H.; Witzgall, P. Sex attractant-based monitoring of a biological control agent of gorse. Biocontrol Sci. Technol. 1999, 9, 99–104. [Google Scholar] [CrossRef]
- Suckling, D.M.; Gibb, A.R.; Gourlay, H.; Conant, P.; Hirayama, C.; Leen, R.; Szöcs, G. Sex attractant for the gorse biocontrol agent Agonopterix ulicetella (Oecophoridae). N. Z. Plant Prot. 2000, 53, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Suckling, D.M.; Gibb, A.R.; Johnson, T.; Hall, D.R. Examination of sex attractants for monitoring weed biological control agents in Hawaii. Biocontrol Sci. Technol. 2006, 16, 919–927. [Google Scholar] [CrossRef]
- Cao, W.H.; Charlton, R.E.; Nechols, J.R.; Horak, M.J. Sex pheromone of the noctuid moth, Tyta luctuosa (Lepidoptera: Noctuidae), a candidate biological control agent of field bindweed. Environ. Entomol. 2003, 32, 17–22. [Google Scholar] [CrossRef]
- Tóth, M.; Guerin, P.M.; Buser, H.-R.; Műller, H.; Szöcs, G.; Sziráki, G.; Arn, H. Z-11-Tetradecenyl acetate: Sex attractant of Agapeta zoegana (Lepidoptera: Tortricidae), a potential species for the biological control of knapweed. Can. Entomol. 1985, 117, 1163–1165. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.R.; Teal, P.E.; Epsky, N.; Dueben, B.; Hight, S.D.; Bloem, S.; Carpenter, J.E.; Weissling, T.J.; Kendra, P.E.; Cibrian-Tovar, J.; et al. Pheromone-Based Attractant for Males of Cactoblastis cactorum (Lepidoptera: Pyralidae). Environ. Entomol. 2006, 35, 1469–1476. [Google Scholar] [CrossRef]
- Cossé, A.A.; Bartelt, R.J.; Zilkowski, B.W.; Bean, D.W.; Petroski, R.J. The aggregation pheromone of Diorhabda elongata, a biological control agent of saltcedar (Tamarix spp.): Identification of two behaviorally active components. J. Chem. Ecol. 2005, 31, 657–670. [Google Scholar] [CrossRef]
- Cossé, A.A.; Bartelt, R.J.; Zilkowski, B.W.; Bean, D.W.; Andress, E.R. Behaviorally active green leaf volatiles for monitoring the leaf beetle, Diorhabda elongata, a biocontrol agent of saltcedar, Tamarix spp. J. Chem. Ecol. 2006, 32, 2695–2708. [Google Scholar] [CrossRef] [PubMed]
- Gaffke, A.M.; Sing, S.E.; Millar, J.G.; Dudley, T.L.; Bean, D.W.; Peterson, R.K.; Weaver, D.K. An Herbivore-Induced Plant Volatile from Saltcedar (Tamarix spp.) is Repellent to Diorhabda carinulata (Coleoptera: Chrysomelidae). Environ. Entomol. 2020, 49, 1063–1070. [Google Scholar] [CrossRef]
- Bartelt, R.J.; Cossé, A.A.; Zilkowski, B.W.; Weisleder, D.; Grode, S.H.; Wiedenmann, R.N.; Post, S.L. Dimethylfuran-lactone pheromone from males of Galerucella calmariensis and Galerucella pusilla. J. Chem. Ecol. 2006, 32, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, R.J.; Cossé, A.A.; Zilkowski, B.W.; Wiedenmann, R.N.; Raghu, S. Early-summer pheromone biology of Galerucella calmariensis and relationship to dispersal and colonization. Biol. Control 2008, 46, 409–416. [Google Scholar] [CrossRef]
- Arn, S.; Stadler, E.; Rauscher, S. The electroantennographic detector-a selective and sensitive tool in the gas chromatographic analysis of insect pheromones. Z. Nat. C 1975, 30, 722–725. [Google Scholar] [CrossRef]
- Bartelt, R.J.; Cossé, A.A.; Zilkowski, B.W.; Weisleder, D.; Momany, F.A. Male-specific sesquiterpenes from Phyllotreta and Aphthona flea beetles. J. Chem. Ecol. 2001, 27, 2397–2423. [Google Scholar] [CrossRef]
- Miller, J.R.; Siegert, P.Y.; Amimo, F.A.; Walker, E.D. Designation of chemicals in terms of the locomotor responses they elicit from insects: An update of Dethier et al. (1960). J. Econ. Entomol. 2009, 102, 2056–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffke, A.M.; Sing, S.E.; Dudley, T.L.; Bean, D.W.; Russak, J.A.; Mafra-Neto, A.; Grieco, P.A.; Peterson, R.K.; Weaver, D.K. Semiochemicals to enhance herbivory by Diorhabda carinulata aggregations in saltcedar (Tamarix spp.) infestations. Pest Manag. Sci. 2018, 74, 1494–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. 2012. Available online: www.pherobase.com. (accessed on 10 April 2021).
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Phillips, T.W. Host plant influences on sex pheromone behavior of phytophagous insects. Annu. Rev. Entomol. 1997, 42, 371–391. [Google Scholar] [CrossRef]
- Suckling, D.M. Pheromones, sex attractants and kairomones in weed and insect biological control: An emerging frontier of tools to manage risk and reward. In Proceedings of the 3rd International Symposium on Biological Control of Arthropods, Christchurch, New Zealand, 8–13 February 2009; Mason, P.G., Gillespie, D.R., Vincent, C., Eds.; USDA, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2009; pp. 30–38. [Google Scholar]
- Wertheim, B.; van Baalen, E.-J.A.; Dicke, M.; Vet, L.E.M. Pheromone-mediated aggregation in nonsocial arthropods: An evolutionary ecological perspective. Annu. Rev. Entomol. 2005, 50, 321–346. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Hinz, H.L.; Winston, R.; Day, M. Biological control of weeds: An analysis of introductions, rates of establishment and estimates of success, worldwide. Biocontrol 2018, 63, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Blossey, B.; Hunt, T.R. Mass rearing methods for Galerucella calmariensis and G. pusilla (Coleoptera: Chrysomelidae), biological control agents of Lythrum salicaria (Lythraceae). J. Econ. Entomol. 1999, 92, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Grevstad, F.; Herzig, A. Quantifying the effects of distance and conspecifics on colonization: Experiments and models using the loosestrife leaf beetle, Galerucella calmariensis. Oecologia 1997, 110, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Grevstad, F.S. Experimental Invasions Using Biological Control Introductions: The Influence of Release Size on the Chance of Population Establishment. Biol. Invasions 1999, 1, 313–323. [Google Scholar] [CrossRef]
- Tansey, J.A.; McClay, A.S.; Cole, D.E.; Keddie, B.A. Evidence for the influence of conspecific chemical cues on Aphthona nigriscutis (Coleoptera: Chrysomelidae) behaviour and distribution. Biocontrol 2005, 50, 343–358. [Google Scholar] [CrossRef]
- Butler, J.L.; Parker, M.S.; Murphy, J.T. Efficacy of flea beetle control of leafy spurge in Montana and South Dakota. Rangel. Ecol. Manag. 2006, 59, 453–461. [Google Scholar] [CrossRef]
- Dickens, J.C.; Oliver, J.E.; Hollister, B.; Davis, J.C.; Klun, J.A. Breaking a paradigm: Male-produced aggregation pheromone for the Colorado potato beetle. J. Exp. Biol. 2002, 205, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Felton, G.W.; Tumlinson, J.H. Plant-insect dialogs: Complex interactions at the plant–insect interface. Curr. Opin. Plant Biol. 2008, 11, 457–463. [Google Scholar] [CrossRef]
- de Moraes, C.M.; Lewis, W.J.; Paré, P.W.; Alborn, H.T.; Tumlinson, J.H. Herbivore-infested plants selectively attract parasitoids. Nature 1998, 393, 570–573. [Google Scholar] [CrossRef]
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.P.; Guerrero, A. Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef]
- Cossé, A.A.; Bartelt, R.J.; Zilkowski, B.W.; Bean, D.W.; Louden, N.; Locke, T. The importance of semiochemicals for Diorhabda spp. (Coleoptera: Chrysomelidae): Biological control agents of invasive tamarisk shrubs. IOBC/Wprs Bull. 2011, 72, 135–138. [Google Scholar]
- Pattison, R.R.; D’Antonio, C.M.; Dudley, T.L.; Allander, K.K.; Rice, B. Early impacts of biological control on canopy cover and water use of the invasive saltcedar tree (Tamarix spp.) in western Nevada, USA. Oecologia 2011, 165, 605–616. [Google Scholar] [CrossRef]
- Hudgeons, J.L.; Knutson, A.E.; Heinz, K.M.; DeLoach, C.J.; Dudley, T.L.; Pattison, R.R.; Kiniry, J.R. Defoliation by introduced Diorhabda elongata leaf beetles (Coleoptera: Chrysomelidae) reduces carbohydrate reserves and regrowth of Tamarix (Tamaricaceae). Biol. Control 2007, 43, 213–221. [Google Scholar] [CrossRef]
- Dudley, T.L.; Bean, D.W. Tamarisk biocontrol, endangered species risk and resolution of conflict through riparian restoration. Biocontrol 2012, 57, 331–347. [Google Scholar] [CrossRef]
- Bean, D.W.; Wang, T.; Bartelt, R.J.; Zilkowski, B.W. Diapause in the leaf beetle Diorhabda elongata (Coleoptera: Chrysomelidae), a biological control agent for tamarisk (Tamarix spp.). Environ. Entomol. 2007, 36, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Fors, L.; Liblikas, I.; Andersson, P.; Borg-Karlson, A.-K.; Cabezas, N.; Mozuraitis, R.; Hambäck, P.A. Chemical communication and host search in Galerucella leaf beetles. Chemoecology 2015, 25, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Seybold, S.J.; Bentz, B.J.; Fettig, C.J.; Lundquist, J.E.; Progar, R.A.; Gillette, N.E. Management of Western North American Bark Beetles with Semiochemicals. Annu. Rev. Entomol. 2018, 63, 407–432. [Google Scholar] [CrossRef]
- Gascoigne, J.; Berec, L.; Gregory, S.; Courchamp, F. Dangerously few liaisons: A review of mate-finding Allee effects. Popul. Ecol. 2009, 51, 355–372. [Google Scholar] [CrossRef]
- Tobin, P.C.; Berec, L.; Liebhold, A.M. Exploiting Allee effects for managing biological invasions. Ecol. Lett. 2011, 14, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Winston, R.; Schwarzländer, M.; Hinz, H.; Day, M.; Cock, M.J.; Julien, M. Biological Control of Weeds: A World 735 Catalogue of Agents and Their Target Weeds; Forest Health Technology Enterprise Team, USDA Forest Service: Morgantown, WV, USA, 2014; p. 838.
- Gaffke, A.M.; Sing, S.E.; Dudley, T.L.; Bean, D.W.; Russak, J.A.; Mafra-Neto, A.; Peterson, R.K.; Weaver, D.K. Establishing Diorhabda carinulata: Impact of release disturbances on pheromone emission and influence of pheromone lures on establishment. J. Chem. Ecol. 2020, 46, 378–386. [Google Scholar] [CrossRef]
- Balciunas, J.; Coombs, E.M. International code of best practices for classical biological control of weed. In Biological Control of Invasive Plants in the US; Coombs, E.M., Clark, J.K., Piper, G.L., Cofrancesco, A.F., Eds.; Oregon State University Press: Corvallis, OR, USA, 2004; pp. 130–136. [Google Scholar]
- Morin, L.; Reid, A.M.; Sims-Chilton, N.M.; Buckley, Y.M.; Dhileepan, K.; Hastwell, G.T.; Nordblom, T.L.; Raghu, S. Review of approaches to evaluate the effectiveness of weed biological control agents. Biol. Control 2009, 51, 1–15. [Google Scholar] [CrossRef]
- Ji, W.; Wang, L.; Knutson, A.E. Detection of the spatiotemporal patterns of beetle-induced tamarisk (Tamarix spp.) defoliation along the Lower Rio Grande using Landsat TM images. Remote. Sens. Environ. 2017, 193, 76–85. [Google Scholar] [CrossRef]
- Gaffke, A.M.; Sing, S.E.; Dudley, T.L.; Bean, D.W.; Russak, J.A.; Mafra-Neto, A.; Peterson, R.K.; Weaver, D.K. Field demonstration of a semiochemical treatment that enhances Diorhabda carinulata biological control of Tamarix spp. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [Green Version]
- Bean, D.W.; Dudley, T.L. A synoptic review of Tamarix biocontrol in North America: Tracking success in the midst of controversy. Biocontrol 2018, 63, 361–376. [Google Scholar] [CrossRef]
- Cusumano, A.; Harvey, J.A.; Bourne, M.E.; Poelman, E.H.; de Boer, J.G. Exploiting chemical ecology to manage hyperparasitoids in biological control of arthropod pests. Pest Manag. Sci. 2020, 76, 423–443. [Google Scholar] [CrossRef] [Green Version]
- Richardson, L.A.; Juricek, C.J.; Lym, R.G.; Kirby, D.R.; Tober, D.A. Integrated Leafy Spurge (Euphorbia esula) Control Using Imazapic, Aphthona spp. Biological Control Agents, and Seeded Native Grasses. Invas. Plant Sci. Mana. 2008, 1, 255–264. [Google Scholar] [CrossRef]
- Muto, S.E.; Bando, M.; Mori, K. Synthesis and stereochemistry of the four himachalene-type sesquiterpenes isolated from the flea beetle (Aphthona flava) as pheromone candidates. Eur. J. Org. Chem. 2004, 9, 1946–1952. [Google Scholar] [CrossRef]
- Miller, J.R.; Gut, L.J. Mating disruption for the 21st century: Matching technology with mechanism. Environ. Entomol. 2015, 44, 427–453. [Google Scholar] [CrossRef] [Green Version]
- Park, I.; Eigenbrode, S.D.; Cook, S.P.; Harmon, B.L.; Hinz, H.L.; Schaffner, U.; Schwarzländer, M. Examining olfactory and visual cues governing host-specificity of a weed biological control candidate species to refine pre-release risk assessment. Biocontrol 2018, 63, 377–389. [Google Scholar] [CrossRef]
- Park, I.; Schwarzländer, M.; Hinz, H.L.; Schaffner, U.; Eigenbrode, S.D. A simple approach to evaluate behavioral responses of insect herbivore to olfactory and visual cues simultaneously: The double stacked y-tube device and portable volatile collection system. Arthropod Plant Int. 2019, 13, 139–149. [Google Scholar] [CrossRef]
- Fung, J.M.; Nepal, K.; Kafle, B.D.; Eigenbrode, S.D.; Schwarzländer, M. Locomotory responses to olfactory cues during host-finding can inform environmental safety assessments of biological weed control agents. Entomol. Exp. Appl. 2021. [Google Scholar] [CrossRef]
- Wheeler, G.S.; David, A.S.; Lake, E.C. Volatile chemistry, not phylogeny, predicts host range of a biological control agent of Old-World climbing fern. Biol. Control 2021, 159, 104636. [Google Scholar] [CrossRef]
- Park, I.; Thompson, D.C. Host recognition by Rhinocyllus conicus of floral scents from invasive and threatened thistles. Biol. Invasions 2021, 23, 1663–1668. [Google Scholar] [CrossRef]
- Suckling, D.M.; Walker, J.T.S.; Clare, G.K.; Boyd Wilson, K.S.H.; Hall, C.; El-Sayed, A.M.; Stevens, P.S. Development and commercialisation of pheromone products in New Zealand. N. Z. Plant Protect. 2012, 65, 267–273. [Google Scholar] [CrossRef] [Green Version]



| Scientific Name | Order: Family | Agent Common Name and Host Plant | Semiochemical Type and Uses | Reference |
|---|---|---|---|---|
| Agonopterix ulicitella | Lepidoptera: Oecophoridae | gorse shoot moth/Ulex europaeus | sex attractant/STM | Suckling et al. [9] |
| Cydia succedana | Lepidoptera: Tortricidae | gorse pod moth/Ulex europaeus | sex attractant/STM | Suckling et al. [10] |
| Acleris (=Croesia) zimmermani | Lepidoptera: Tortricidae | none/Rubus spp. | sex attractant/STM | Suckling et al. [11] |
| Schreckensteinia festaliella | Lepidoptera: Schreckensteiniidae | blackberry skeletonizer/Rubus spp. | sex attractant/STM | Suckling et al. [11] |
| Tyta luctuosa | Lepidoptera: Noctuidae | field bindweed moth/Convolvulus arvensis | sex pheromone/STM | Cao et al. [12] |
| Agapeta zoegana | Lepidoptera: Tortricidae | sulphur knapweed moth/Centaurea spp. | sex pheromone/STM | Tóth et al. [13] |
| Cactoblastis cactorum | Lepidoptera: Pyralidae | cactus moth/Opuntia spp. | sex pheromone/STM | Heath et al. [14] |
| Diorhabda carinulata | Coleoptera: Chrysomelidae | northern tamarisk beetle/Tamarix spp. | aggregation pheromone blend/EE, STM, DA | Cossé et al. [15] |
| D. carinulata | Coleoptera: Chrysomelidae | northern tamarisk beetle/Tamarix spp. | Tamarix HIPVs attractants/ STM | Cossé et al. [16] |
| D. carinulata | Coleoptera: Chrysomelidae | northern tamarisk beetle/Tamarix spp. | Tamarix HIPV deterrent/ DA | Gaffke et al., [17] |
| Galerucella calmariensis and G. pusilla | Coleoptera: Chrysomelidae | black-margined and golden loosestrife beetles/Lythrum salicaria | aggregation pheromone/STM | Bartelt et al. [18,19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaffke, A.M.; Alborn, H.T.; Dudley, T.L.; Bean, D.W. Using Chemical Ecology to Enhance Weed Biological Control. Insects 2021, 12, 695. https://doi.org/10.3390/insects12080695
Gaffke AM, Alborn HT, Dudley TL, Bean DW. Using Chemical Ecology to Enhance Weed Biological Control. Insects. 2021; 12(8):695. https://doi.org/10.3390/insects12080695
Chicago/Turabian StyleGaffke, Alexander M., Hans T. Alborn, Tom L. Dudley, and Dan W. Bean. 2021. "Using Chemical Ecology to Enhance Weed Biological Control" Insects 12, no. 8: 695. https://doi.org/10.3390/insects12080695
APA StyleGaffke, A. M., Alborn, H. T., Dudley, T. L., & Bean, D. W. (2021). Using Chemical Ecology to Enhance Weed Biological Control. Insects, 12(8), 695. https://doi.org/10.3390/insects12080695







