Colonization and Authentication of the Pyrethroid-Resistant Anopheles gambiae s.s. Muleba-Kis Strain; an Important Test System for Laboratory Screening of New Insecticides
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
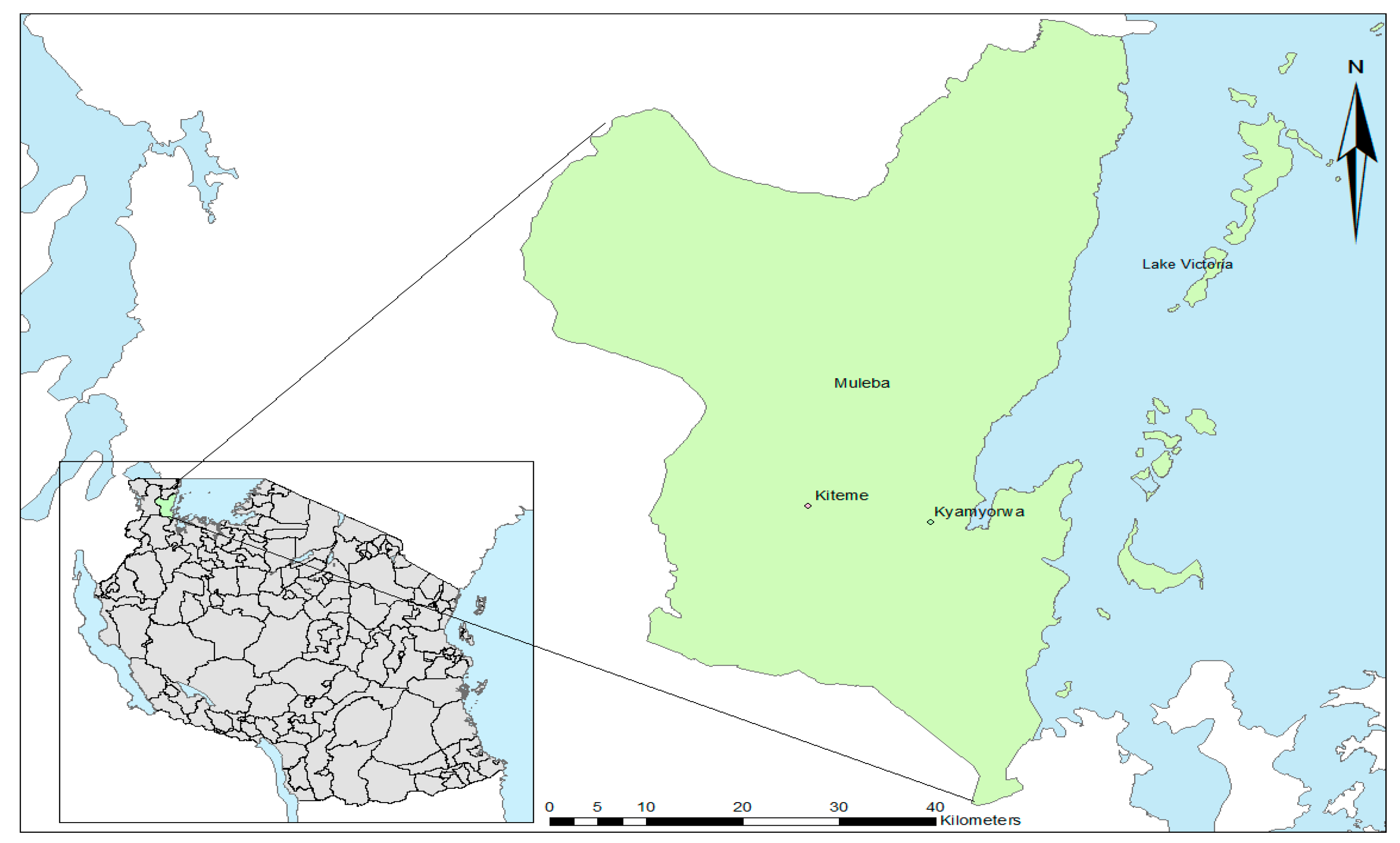
2.1. Study Site
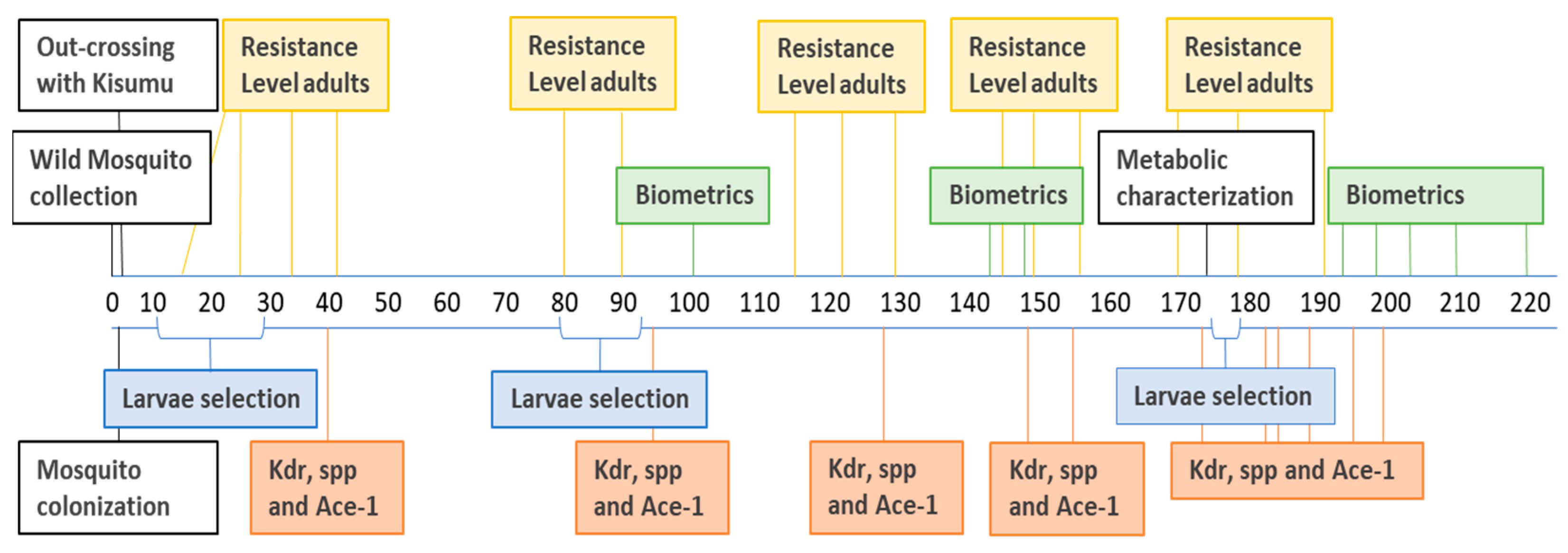
2.2. Timeline
2.3. Collection of Wild Mosquitoes and Introduction into the Insectary
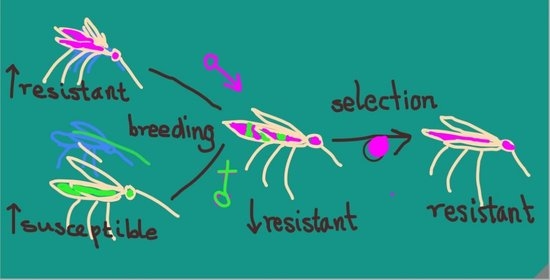
2.4. Crossing for “Insectary Vigor”
2.5. Selection to Maintain Pyrethroid Resistance
2.6. Authentication of the Outcrossed An. Gambiae s.s. Muleba-Kis Strain
2.6.1. Phenotypic Resistance
WHO Susceptibility Test and CDC Bioassay
Synergist-Insecticide Bottle Bioassay
2.6.2. Genotypic Basis of Resistance
Detection of kdr and Ace-1 Mutations
2.6.3. Species Identification and Biometric Measures for Fitness
Species Identification
Biometric Measurements
2.7. Statistical Analysis
3. Results
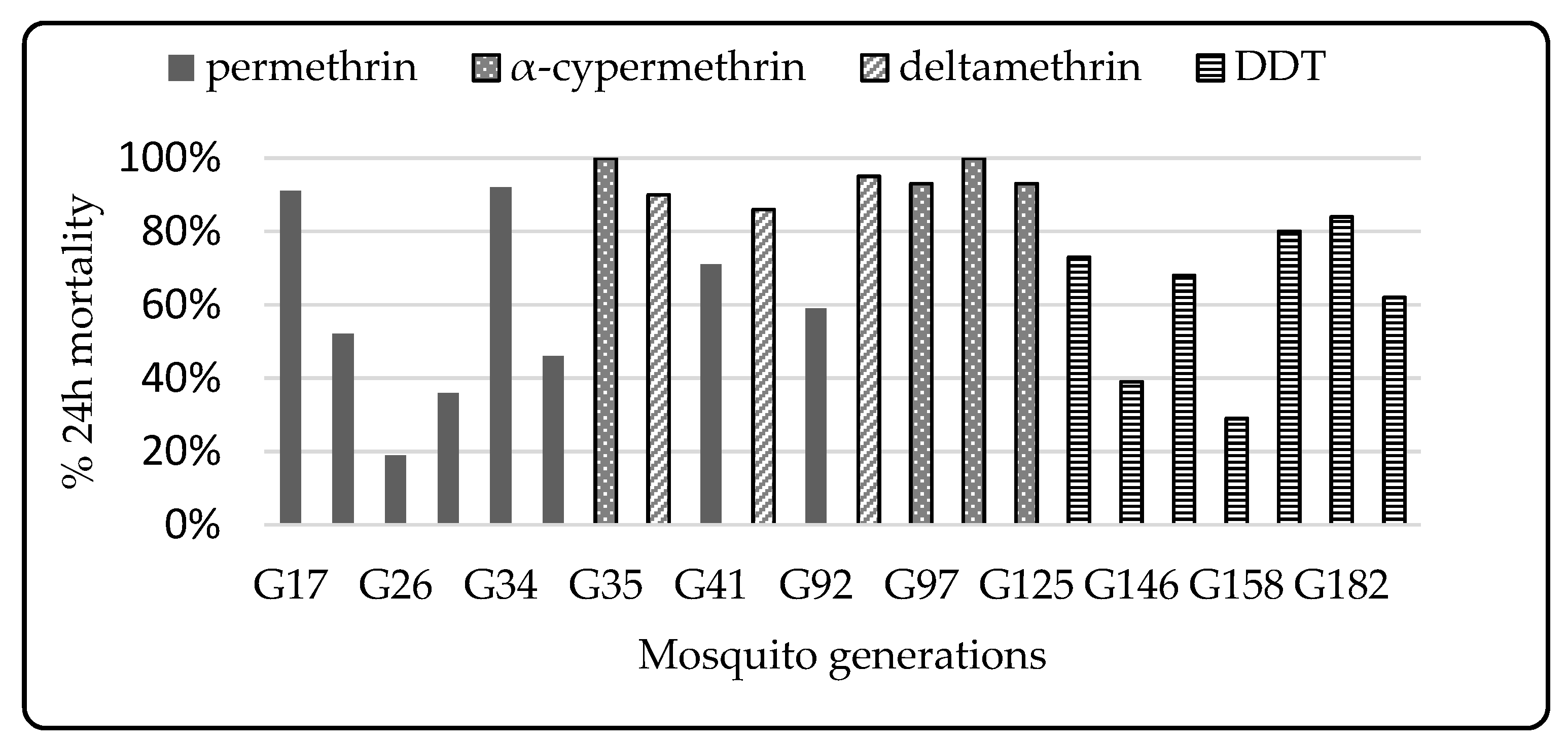
3.1. Colony Selection
3.2. Phenotypic Resistance
3.2.1. WHO Susceptibility
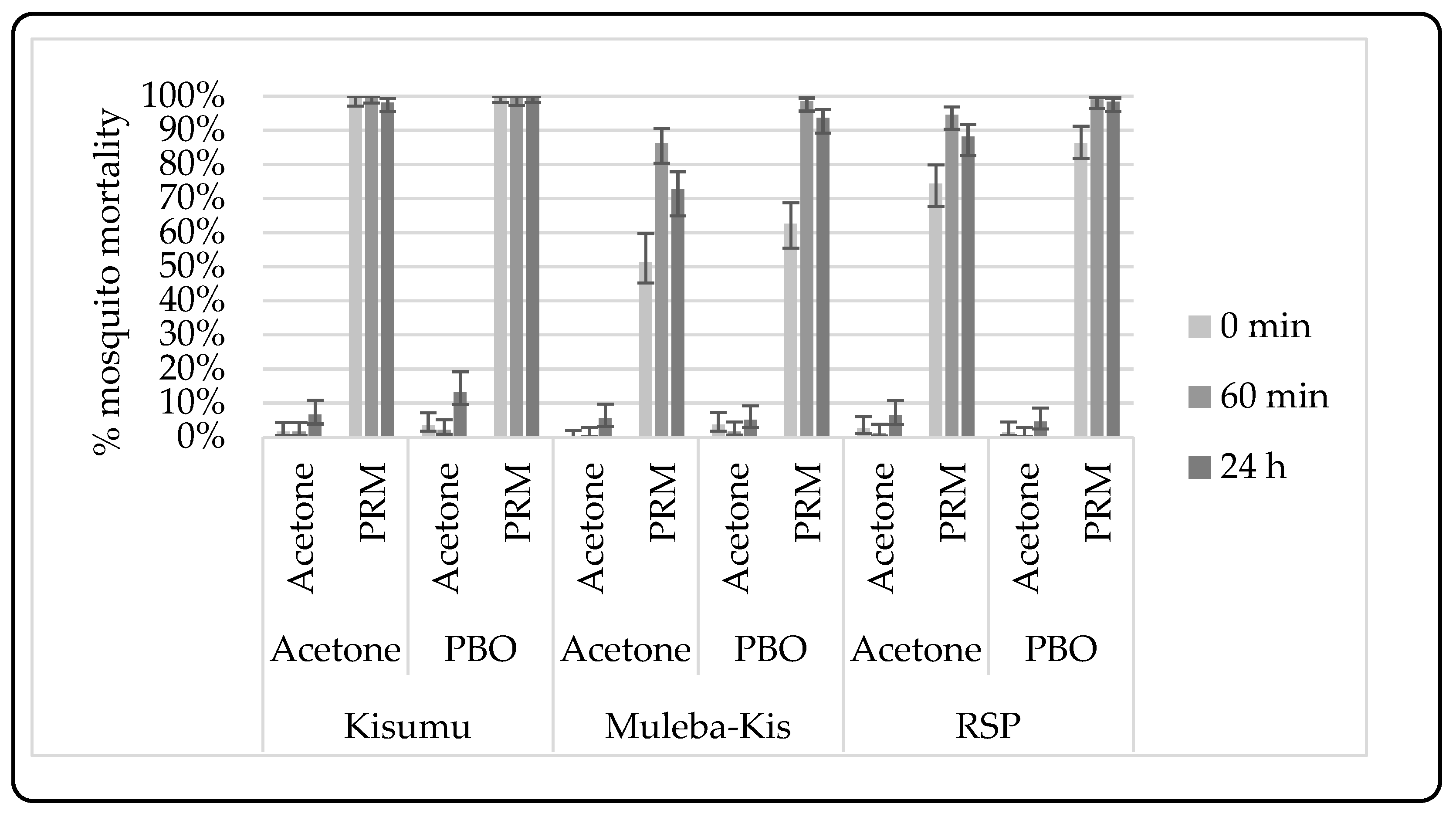
3.2.2. Synergist-Insecticide Bottle Bioassay
3.3. Polymerase Chain Reaction (PCR) for Species Identification and Resistance Status
3.4. Resistance Strength of the Selected Colony: CDC Bottle Bioassay
3.5. Biometric Measures for Fitness
4. Discussion
4.1. Blood-Feeding Challenges with Wild Mosquitoes
4.2. Initial Low Insecticide Resistance Following Cross-Breeding
4.3. Impact of Mosquito Developmental Stage Used for Selection
4.4. Impact of Selection Using Pyrethroids
4.5. Differential Resistance to Type I and Type II Pyrethroids
4.6. Metabolic Resistance
4.7. Intermittent Quality Control Checks and Regular Strain Authentication
4.8. Effect of Mosquito Weight and Wing Length on Phenotypic Resistance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- malERA Consultative Group on Vector Control. A research agenda for malaria eradication: Vector control. PLoS Med. 2011, 8, e1000401. [Google Scholar]
- Miller, J.E.; Lindsay, S.W.; Armstrong, J.R. Experimental hut trials of bednets impregnated with synthetic pyrethroid or organophosphate insecticide for mosquito control in The Gambia. Med. Vet. Entomol. 1991, 5, 465–476. [Google Scholar] [CrossRef]
- Lindsay, S.W.; Adiamah, J.H.; Miller, J.E.; Armstrong, J.R. Pyrethroid-treated bednet effects on mosquitoes of the Anopheles gambiae complex in The Gambia. Med. Vet. Entomol. 1991, 5, 477–483. [Google Scholar] [CrossRef]
- Ranson, H.; N’guessan, R.; Lines, J.; Moiroux, N.; Nkuni, Z.; Corbel, V. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011, 27, 91–98. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Specifications for Public Health Pesticides, Permethrin (25:75 cis:trans, Nonracemic). 2015. Available online: https://www.who.int/whopes/quality/Permethrin_25:75_nonracemic_specs_eval_July_2015.pdf?ua=1 (accessed on 1 December 2020).
- World Health Organization. Deltamethrin Long-Lasting (Incorporated into Filaments) Insecticidal Net. 2011. Available online: https://www.who.int/whopes/quality/Alpha-cypermethrin_incorporated_LN_specs_eval_WHO_October_2014.pdf?ua=1 (accessed on 1 December 2020).
- World Health Organization. Deltamethrin Long-Lasting (Incorporated into Filaments) Insecticidal Net. 2011. Available online: https://www.who.int/whopes/quality/Deltamethrin_LN_incorporated_into_filaments_WHO_spec_eval_Sep_2011.pdf (accessed on 1 December 2020).
- Bradberry, S.M.; Cage, S.A.; Proudfoot, A.T.; Vale, J.A. Poisoning due to pyrethroids. Toxicol Rev. 2005, 24, 93–106. [Google Scholar] [CrossRef]
- Toé, K.H.; Jones, C.M.; N’Fale, S.; Ismail, H.M.; Dabiré, R.K.; Ranson, H. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Infect. Dis. 2014, 20, 1691–1696. [Google Scholar] [CrossRef]
- Zaim, M.; Guillet, P. Alternative insecticides: An urgent need. Trends Parasitol. 2002, 18, 161–163. [Google Scholar] [CrossRef]
- World Health Organization. Global Plan for Insecticide Resistance Management in Malaria Vectors; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Hemingway, J.; Shretta, R.; Wells, T.N.C.; Bell, D.; Djimdé, A.A.; Achee, N.; Qi, G. Tools and strategies for malaria control and elimination: What do we need to achieve a grand convergence in malaria? PLoS Biol. 2016, 14, e1002380. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, R.M.; N’Guessan, R.; Kitau, J.; Tungu, P.K.; Malone, D.; Mosha, F.W.; Rowland, M.W. A new class of insecticide for malaria vector control: Evaluation of mosquito nets treated singly with indoxacarb (oxadiazine) or with a pyrethroid mixture against Anopheles gambiae and Culex quinquefasciatus. Malar. J. 2015, 14, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Insecticide Discovery & Development. 2019. Available online: https://www.ivcc.com/research-development/insecticide-discovery-and-development/ (accessed on 1 December 2020).
- Lees, R.; Praulins, G.; Davies, R.; Brown, F.; Parsons, G.; White, A.; Ranson, H.; Small, G.; Malone, D. A testing cascade to identify repurposed insecticides for next-generation vector control tools: Screening a panel of chemistries with novel modes of action against a malaria vector. Gates Open Res. 2019, 3, 1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guidelines for Laboratory and Field Testing of Long-Lasting Insecticidal Nets; WHO/HTM/NTD/WHOPES/2013.1; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Benedict, M.Q.; Burt, A.; Capurro, M.L.; de Barro, P.; Handler, A.M.; Hayes, K.R.; Marshall, J.M.; Tabachnick, W.J.; Adelman, Z.L. Recommendations for Laboratory Containment. Vector Borne Zoonotic Dis. 2018, 18, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Gloria-Soria, A.; Soghigian, J.; Kellner, D.; Powell, J.R. Genetic diversity of laboratory strains and implications for research: The case of Aedes aegypti. PLoS Negl. Trop. Dis. 2019, 13, e0007930. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Hallas, R.; Sinclair, C.; Partridge, L. Rapid loss of stress resistance in Drosophila melanogaster under adaptation to laboratory culture. Evol. Int. J. Org. Evol. 2001, 55, 436–438. [Google Scholar] [CrossRef]
- Bravo, I.S.J.; Zucoloto, F.S. Performance and feeding behavior of Ceratitis capitata: Comparison of a wild population and a laboratory population. Entomol. Exp. Appl. 1998, 87, 67–72. [Google Scholar] [CrossRef]
- Matos, M.; Rose, M.R.; Rocha Pité, M.T.; Rego, C.; Avelar, T. Adaptation to the laboratory environment in Drosophila subobscura. J. Evol. Biol. 2000, 13, 9–19. [Google Scholar] [CrossRef]
- Diamantidis, A.D.; Carey, J.R.; Nakas, C.T.; Papadopoulos, N.T. Ancestral populations perform better in a novel environment: Domestication of Mediterranean fruit fly populations from five global regions. Biol. J. Linn. Soc. 2011, 102, 334–345. [Google Scholar] [CrossRef]
- Gingerich, P.D. Rates of evolution: Effects of time and temporal scaling. Science 1983, 222, 159–161. [Google Scholar] [CrossRef]
- Hendry, A.P.; Kinnison, M.T. Perspective: The pace of modern life: Measuring rates of contemporary microevolution. Evolution 1999, 53, 1637. [Google Scholar] [CrossRef]
- Baeshen, R.; Ekechukwu, N.E.; Toure, M.; Paton, D.; Coulibaly, M.; Traoré, S.F.; Tripet, F. Differential effects of inbreeding and selection on male reproductive phenotype associated with the colonization and laboratory maintenance of Anopheles gambiae. Malar. J. 2014, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; Ross, P.A. Rates and Patterns of Laboratory Adaptation in (Mostly) Insects. J. Econ. Entomol. 2018, 111, 501–509. [Google Scholar] [CrossRef]
- Matowo, J.; Kulkarni, M.A.; Mosha, F.W.; Oxborough, R.M.; Kitau, J.A.; Tenu, F.; Rowland, M. Biochemical basis of permethrin resistance in Anopheles arabiensis from Lower Moshi, north-eastern Tanzania. Malar. J. 2010, 9, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matowo, J.; Kitau, J.; Kaaya, R.; Kavishe, R.; Wright, A.; Kisinza, W.; Kleinschmidt, I.; Mosha, F.; Rowland, M.; Protopopoff, N. Trends in the selection of insecticide resistance in Anopheles gambiae s.l. mosquitoes in northwest Tanzania during a community randomized trial of longlasting insecticidal nets and indoor residual spraying: Selection of insecticide resistance in An. gambiae. Med. Vet. Entomol. 2015, 29, 51–59. [Google Scholar] [PubMed] [Green Version]
- Kuno, G. Early history of laboratory breeding of Aedes aegypti (Diptera: Culicidae) Focusing on the origins and use of selected strains. J. Med. Entomol. 2010, 47, 957–971. [Google Scholar] [CrossRef] [Green Version]
- Ranson, H.; Jensen, B.; Vulule, J.M.; Wang, X.; Hemingway, J.; Collins, F.H. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Biol. 2000, 9, 491–497. [Google Scholar] [CrossRef]
- Stump, A.D.; Atieli, F.K.; Vulule, J.M.; Besansky, N.J. Dynamics of the pyrethroid knockdown resistance allele in western Kenyan populations of Anopheles gambiae in response to insecticide-treated bed net trials. Am. J. Trop. Med. Hyg. 2004, 70, 591–596. [Google Scholar] [CrossRef]
- Nwane, P.; Etang, J.; Chouaїbou, M.; Toto, J.C.; Mimpfoundi, R.; Simard, F. Kdr-based insecticide resistance in Anopheles gambiae s.s. populations in Cameroon: Spread of the L1014F and L1014S mutations. BMC Res. Notes 2011, 4, 463. [Google Scholar] [CrossRef] [Green Version]
- Namountougou, M.; Diabaté, A.; Etang, J.; Bass, C.; Sawadogo, S.P.; Gnankinié, O.; Baldet, T.; Martin, T.; Chandre, F.; Simard, F.; et al. First report of the L1014S kdr mutation in wild populations of Anopheles gambiae M and S molecular forms in Burkina Faso (West Africa). Acta Trop. 2013, 125, 123–127. [Google Scholar] [CrossRef]
- Etang, J.; Fondjo, E.; Chandre, F.; Morlais, I.; Brengues, C.; Nwane, P.; Chouaibou, M.; Ndjemai, H.; Simard, F. First report of knockdown mutations in the malaria vector Anopheles gambiae from Cameroon. Am. J. Trop. Med. Hyg. 2006, 74, 795–797. [Google Scholar] [CrossRef]
- O’Reilly, A.O.; Khambay, B.P.S.; Williamson, M.S.; Field, L.M.; Wallace, B.A.; Davies, T.G.E. Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem. J. 2006, 396, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Reimer, L.; Fondjo, E.; Patchoké, S.; Diallo, B.; Lee, Y.; Ng, A.; Ndjemai, H.M.; Atangana, J.; Traore, S.F.; Lanzaro, G.; et al. Relationship between kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae. J. Med. Entomol. 2008, 45, 260–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protopopoff, N.; Matowo, J.; Malima, R.; Kavishe, R.; Kaaya, R.; Wright, A.; West, P.A.; Kleinschmidt, I.; Kisinza, W.; Mosha, F.W.; et al. High level of resistance in the mosquito Anopheles gambiae to pyrethroid insecticides and reduced susceptibility to bendiocarb in north-western Tanzania. Malar. J. 2013, 12, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protopopoff, N.; Mosha, J.F.; Lukole, E.; Charlwood, J.D.; Wright, A.; Mwalimu, C.D.; Manjurano, A.; Mosha, F.W.; Kisinza, W.; Kleinschmidt, I.; et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: A cluster, randomised controlled, two-by-two factorial design trial. Lancet 2018, 391, 1577–1588. [Google Scholar] [PubMed] [Green Version]
- Ramphul, U.; Boase, T.; Bass, C.; Okedi, L.M.; Donnelly, M.J.; Müller, P. Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1121–1126. [Google Scholar] [CrossRef]
- Kabula, B.; Kisinza, W.; Tungu, P.; Ndege, C.; Batengana, B.; Kollo, D.; Malima, R.; Kafuko, J.; Mohamed, M.; Magesa, S. Co-occurrence and distribution of East (L1014S) and West (L1014F) African knock-down resistance in Anopheles gambiae sensu lato population of Tanzania. Trop. Med. Int. Health 2014, 19, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Dida, G.O.; Ohashi, K.; Komagata, O.; Kasai, S.; Tomita, T.; Sonye, G.; Maekawa, Y.; Mwatele, C.; Njenga, S.M.; et al. Multimodal pyrethroid resistance in malaria vectors, Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus s.s. in western Kenya. PLoS ONE 2011, 6, e22574. [Google Scholar] [CrossRef] [PubMed]
- Abeku, T.A.; Helinski, M.E.H.; Kirby, M.J.; Ssekitooleko, J.; Bass, C.; Kyomuhangi, I.; Okia, M.; Magumba, G.; Meek, S.R. Insecticide resistance patterns in Uganda and the effect of indoor residual spraying with bendiocarb on kdr L1014S frequencies in Anopheles gambiae s.s. Malar. J. 2017, 16, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munywoki, D.N.; Kokwaro, E.D.; Mwangangi, J.M.; Muturi, E.J.; Mbogo, C.M. Insecticide resistance status in Anopheles gambiae (s.l.) in coastal Kenya. Parasites Vectors 2021, 14, 207. [Google Scholar] [CrossRef]
- Kisinza, W.N.; Nkya, T.E.; Kabula, B.; Overgaard, H.J.; Massue, D.J.; Mageni, Z.; Greer, G.; Kaspar, N.; Mohamed, M.; Moore, S.; et al. Multiple insecticide resistance in Anopheles gambiae from Tanzania: A major concern for malaria vector control. Malar. J. 2017, 16, 439. [Google Scholar] [CrossRef] [Green Version]
- Weill, M.; Luffalla, G.; Mogensen, K.; Chandre, F.; Berthomieu, A.; Berticat, C.; Pasteur, N.; Philips, A.; Fort, P.; Raymond, M. Insecticide resistance in mosquito vectors. Nature 2003, 423, 136–137. [Google Scholar] [CrossRef]
- West, P.A.; Protopopoff, N.; Wright, A.; Kivaju, Z.; Tigererwa, R.; Mosha, F.W.; Kisinza, W.; Rowland, M.; Kleinschmidt, I. Indoor Residual Spraying in combination with Insecticide-Treated Nets compared to Insecticide-Treated Nets alone for protection against malaria: A Cluster Randomised Trial in Tanzania. PLoS Med. 2014, 11, e1001630. [Google Scholar] [CrossRef] [Green Version]
- Bass, C.; Williamson, M.S.; Wilding, C.S.; Donnelly, M.J.; Field, L.M. Identification of the main malaria vectors in the Anopheles gambiae species complex using a TaqMan real-time PCR assay. Malar. J. 2007, 9, 155. [Google Scholar] [CrossRef] [Green Version]
- Ranson, H.; Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Moyes, C.L.; Athinya, D.K.; Seethaler, T.; Battle, K.E.; Sinka, M.; Hadi, M.P.; Hemingway, J.; Coleman, M.; Hancock, P.A. Evaluating insecticide resistance across African districts to aid malaria control decisions. Proc. Natl. Acad. Sci. USA 2020, 117, 22042–22050. [Google Scholar] [CrossRef] [PubMed]
- Shidrawi, G.R. Laboratory tests on mosquito tolerance to insecticides and the development of resistance by Aedes aegypti. Bull. World Health Organ. 1957, 17, 377–411. [Google Scholar] [PubMed]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Brogdon, W.G.; McAllister, J.C. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J. Am. Mosq. Control Assoc. 1998, 14, 159–164. [Google Scholar]
- Bass, C.; Nikou, D.; Vontas, J.; Williamson, M.S.; Field, L.M. Development of high-throughput real-time PCR assays for the identification of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae. Pestic. Biochem. Physiol. 2010, 96, 80–85. [Google Scholar] [CrossRef]
- Nasci, R.S. Relationship of wing length to adult dry weight in several mosquito species (Diptera: Culicidae). J. Med. Entomol. 1990, 27, 716–719. [Google Scholar] [CrossRef]
- Yeap, H.L.; Endersby, N.M.; Johnson, P.H.; Ritchie, S.A.; Hoffmann, A.A. Body size and wing shape measurements as quality indicators of Aedes aegypti mosquitoes destined for field release. Am. J. Trop. Med. Hyg. 2013, 89, 78–92. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- STATA. Base Reference Manual; Stata Press: College Station, TX, USA, 2019; Available online: https://www.stata.com/bookstore/base-reference-manual/ (accessed on 26 February 2020).
- Pasteur, N.; Raymond, M. Insecticide resistance genes in mosquitoes: Their mutations, migration, and selection in field populations. J. Hered. 1996, 87, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Stone, C.; Gross, K. Evolution of host preference in anthropophilic mosquitoes. Malar. J. 2018, 17, 257. [Google Scholar] [CrossRef] [Green Version]
- Main, B.J.; Lee, Y.; Ferguson, H.M.; Kreppel, K.S.; Kihonda, A.; Govella, N.J.; Collier, T.C.; Cornel, A.J.; Eskin, E.; Kang, E.-Y.; et al. The genetic basis of host preference and resting behavior in the major African malaria vector, Anopheles arabiensis. PLoS Genet. 2016, 12, e1006303. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Kramer, L.D.; Jones, M.J.; Marra, P.P.; Daszak, P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006, 4, e82. [Google Scholar] [CrossRef] [PubMed]
- Takken, W.; Verhulst, N.O. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 2013, 58, 433–453. [Google Scholar] [CrossRef] [Green Version]
- Barrett, R.D.H.; Schluter, D. Adaptation from standing genetic variation. Trends Ecol. Evol. 2008, 23, 38–44. [Google Scholar] [CrossRef]
- Fisher, R.A. The Genetical Theory of Natural Selection: A Complete Variorum Edition; Bennett, H., Ed.; Oxford University Press: London, UK, 1999. [Google Scholar]
- Darlington, C.D. New paths in genetics. Nature 1942, 149, 317. [Google Scholar] [CrossRef]
- Koella, J.C.; Lynch, P.A.; Thomas, M.B.; Read, A.F. Towards evolution-proof malaria control with insecticides. Evol. Appl. 2009, 2, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef]
- Nkya, T.E.; Poupardin, R.; Laporte, F.; Akhouayri, I.; Mosha, F.; Magesa, S.; Kisinza, W.; David, J.-P. Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: A multigenerational study in controlled conditions. Parasites Vectors 2014, 7, 480. [Google Scholar]
- Kudom, A.A.; Anane, L.N.; Afoakwah, R.; Adokoh, C.K. Relating high insecticide residues in larval breeding habitats in urban residential areas to the selection of pyrethroid resistance in Anopheles gambiae s.L. (Diptera: Culicidae) in Akim Oda, Ghana. J. Med. Entomol. 2018, 55, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Cheng, P.; Jiao, B.; Song, X.; Wang, H.; Wang, H.; Wang, H.; Huang, X.; Liu, H.; Gong, M. Investigation of mosquito larval habitats and insecticide resistance in an area with a high incidence of mosquito-borne diseases in Jining, Shandong Province. PLoS ONE 2020, 15, e0229764. [Google Scholar] [CrossRef] [Green Version]
- Talom, A.D.; Essoung, M.A.; Gbankoto, A.; Tchigossou, G.; Akoton, R.; Sahabi, B.B.A.; Atoyebi, S.M.; Kuate, A.F.; Verspoor, V.R.; Tamò, M.; et al. A preliminary analysis on the effect of copper on Anopheles coluzzii insecticide resistance in vegetable farms in Benin. Sci. Rep. 2020, 10, 6392. [Google Scholar] [CrossRef] [PubMed]
- Machani, M.G.; Ochomo, E.; Zhong, D.; Zhou, G.; Wang, X.; Githeko, A.K.; Yan, G.; Afrane, Y.A. Phenotypic, genotypic and biochemical changes during pyrethroid resistance selection in Anopheles gambiae mosquitoes. Sci. Rep. 2020, 10, 19063. [Google Scholar] [CrossRef]
- Brown, T.M.; Payne, G.T. Experimental selection for insecticide resistance. J. Econ. Entomol. 1988, 81, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.B.S.; Shad, S.A.; Ejaz, M.; Serrao, J.E. Laboratory selection, cross-resistance, and estimations of realized heritability of indoxacarb resistance in Phenacoccus solenopsis (Homoptera: Pseudococcidae). Pest Manag. Sci. 2020, 76, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Flood, L.; Praulins, G.; Ingham, V.A.; Morgan, J.; Lees, R.S.; Ranson, H. Characterisation of Anopheles strains used for laboratory screening of new vector control products. Parasites Vectors 2019, 12, 522. [Google Scholar] [CrossRef] [Green Version]
- Hunt, R.H.; Brooke, B.D.; Pillay, C.; Koekemoer, L.L.; Coetzee, M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Med. Vet. Entomol. 2005, 19, 271–275. [Google Scholar] [CrossRef]
- Subramaniam, S.; Aris, E.M.; Ahmad, N.W.; Lee, H.L.; Azahari, A.H. The development of resistance and susceptibility of Aedes aegypti larvae and adult mosquitoes against selection pressure to malathion, permethrin and temephos insecticides and its cross-resistance relationship against propoxur. Malays. J. Sci. 2006, 25, 1–13. [Google Scholar]
- Saleh, M.S.; El-Meniawi, F.A.; Kelada, N.L.; Zahran, H.M. Resistance development in mosquito larvae Culex pipiens to the bacterial agent Bacillus thuringiensis var. israelensis. J. Appl. Entomol. 2003, 127, 29–32. [Google Scholar] [CrossRef]
- Viana-Medeiros, P.F.; Bellinato, D.F.; Valle, D. Laboratory selection of Aedes aegypti field populations with the organophosphate malathion: Negative impacts on resistance to deltamethrin and to the organophosphate temephos. PLoS Negl. Trop. Dis. 2018, 12, e0006734. [Google Scholar] [CrossRef]
- Hedrick, P.W. Adaptive introgression in animals: Examples and comparison to new mutation and standing variation as sources of adaptive variation. Mol. Ecol. 2013, 22, 4606–4618. [Google Scholar] [CrossRef]
- Song, Y.; Endepols, S.; Klemann, N.; Richter, D.; Matuschka, F.-R.; Shih, C.-H.; Nachman, M.W.; Kohn, M.H. Adaptive introgression of anticoagulant rodent poison resistance by hybridization between old world mice. Curr. Biol. 2011, 21, 1296–1301. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.J.; Steinberg, E.; Yozzo, A.; Song, Y.; Kohn, M.H.; Nakhleh, L. Interspecific introgressive origin of genomic diversity in the house mouse. Proc. Natl. Acad. Sci. USA 2015, 112, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Trpis, M.; Hausermann, W. Genetics of house-entering behaviour in East African populations of Aedes aegypti (L.) (Diptera: Culicidae) and its relevance to speciation. Bull. Entomol. Res. 1978, 68, 521–532. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Clark, J.M.; Sheets, L.P.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; Stevens, J.T.; Weiner, M.L. Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef]
- Kamgang, B.; Wilson-Bahun, T.A.; Yougang, A.P.; Lenga, A.; Wondji, C.S. Contrasting resistance patterns to type I and II pyrethroids in two major arbovirus vectors Aedes aegypti and Aedes albopictus in the Republic of the Congo, Central Africa. Infect. Dis. Poverty 2020, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Warr, E.; Stevenson, B.J.; Pignatelli, P.M.; Morgan, J.C.; Steven, A.; Yawson, A.E.; Mitchell, S.N.; Ranson, H.; Hemingway, J.; et al. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008, 4, e1000286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, E.E.; Marcet, P.L.; Sutcliffe, A.C. Authentication scheme for routine verification of genetically similar laboratory colonies: A trial with Anopheles gambiae. BMC Biotechnol. 2009, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerio, L.; Collins, C.M.; Lees, R.S.; Benedict, M.Q. Benchmarking vector arthropod culture: An example using the African malaria mosquito, Anopheles gambiae (Diptera: Culicidae). Malar. J. 2016, 15, 262. [Google Scholar] [CrossRef] [Green Version]
- Owusu, H.F.; Chitnis, N.; Müller, P. Insecticide susceptibility of Anopheles mosquitoes’ changes in response to variations in the larval environment. Sci. Rep. 2017, 7, 3667. [Google Scholar] [CrossRef] [Green Version]
- Petersen, V.; Marchi, M.J.; Natal, D.; Marrelli, M.T.; Barbosa, A.C.; Suesdek, L. Assessment of the correlation between wing size and body weight in captive Culex quinquefasciatus. Rev. Soc. Bras. Med. Trop. 2016, 49, 508–511. [Google Scholar] [CrossRef] [Green Version]
- Siegel, J.P.; Novak, R.J.; Lampman, R.L.; Steinly, B.A. Statistical appraisal of the weight-wing length relationship of mosquitoes. J. Med. Entomol. 1992, 29, 711–714. [Google Scholar] [CrossRef]
- Martins, A.J.; Bellinato, D.F.; Peixoto, A.A.; Valle, D.; Lima, J.B.P. Effect of insecticide resistance on development, longevity and reproduction of field or laboratory selected Aedes aegypti populations. PLoS ONE 2012, 7, e31889. [Google Scholar] [CrossRef] [Green Version]

| Generation | Number of Samples | Molecular Assay | ||||||
|---|---|---|---|---|---|---|---|---|
| Species | kdr-E | Ace-1 | ||||||
| %Ar | %Ga | %RRe | %RSe | %SSe | %RRe | %SSe | ||
| G43 | 37 | 0 | 100 | 30 | 27 | 43 | 0 | 100 |
| G99 | 57 | 0 | 100 | 100 | 0 | 0 | 0 | 100 |
| G131 | 50 | 0 | 100 | 100 | 0 | 0 | 0 | 100 |
| G150 | 84 | 0 | 100 | 100 | 0 | 0 | N | N |
| G162 | 100 | 0 | 100 | 100 | 0 | 0 | N | N |
| G168 | 84 | 0 | 100 | 100 | 0 | 0 | N | N |
| G178 | 84 | 0 | 100 | 100 | 0 | 0 | N | N |
| G188 | 84 | 0 | 100 | 100 | 0 | 0 | N | N |
| G190 | 84 | 0 | 100 | 100 | 0 | 0 | 0 | 100 |
| G198 | 88 | 0 | 100 | 100 | 0 | 0 | N | N |
| G204 | 88 | 0 | 100 | 100 | 0 | 0 | N | N |
| Year | Samples (N) | Mean Wing Length | 95% CI | p-Value * |
|---|---|---|---|---|
| 2016 | 50 | 2.9504 | 2.8995-3.0013 | 0.6592 |
| 2017 | 150 | 2.9362 | 2.9035–2.9689 | |
| 2017 | 150 | 2.9362 | 2.9035–2.9689 | <0.0001 |
| 2019 | 149 | 3.0405 | 3.0066–3.0745 | |
| 2019 | 149 | 3.0405 | 3.0066–3.0745 | 0.0025 |
| 2020 | 100 | 2.9532 | 2.9063–3.0001 |
| Year | Samples (N) | Mean Weight | 95% CI | p-Value * |
|---|---|---|---|---|
| 2016 | 50 | 0.0011 | 0.0010–0.0012 | <0.0001 |
| 2017 | 150 | 0.0016 | 0.0015–0.0017 | |
| 2017 | 150 | 0.0016 | 0.0015–0.0017 | <0.0001 |
| 2019 | 149 | 0.0012 | 0.0012–0.0013 | |
| 2019 | 149 | 0.0012 | 0.0012–0.0013 | 0.4281 |
| 2020 | 100 | 0.0012 | 0.0011–0.0012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizi, S.; Snetselaar, J.; Wright, A.; Matowo, J.; Shirima, B.; Kaaya, R.; Athumani, R.; Tenu, F.; Protopopoff, N.; Kirby, M. Colonization and Authentication of the Pyrethroid-Resistant Anopheles gambiae s.s. Muleba-Kis Strain; an Important Test System for Laboratory Screening of New Insecticides. Insects 2021, 12, 710. https://doi.org/10.3390/insects12080710
Azizi S, Snetselaar J, Wright A, Matowo J, Shirima B, Kaaya R, Athumani R, Tenu F, Protopopoff N, Kirby M. Colonization and Authentication of the Pyrethroid-Resistant Anopheles gambiae s.s. Muleba-Kis Strain; an Important Test System for Laboratory Screening of New Insecticides. Insects. 2021; 12(8):710. https://doi.org/10.3390/insects12080710
Chicago/Turabian StyleAzizi, Salum, Janneke Snetselaar, Alexandra Wright, Johnson Matowo, Boniface Shirima, Robert Kaaya, Rashid Athumani, Filemoni Tenu, Natacha Protopopoff, and Matthew Kirby. 2021. "Colonization and Authentication of the Pyrethroid-Resistant Anopheles gambiae s.s. Muleba-Kis Strain; an Important Test System for Laboratory Screening of New Insecticides" Insects 12, no. 8: 710. https://doi.org/10.3390/insects12080710
APA StyleAzizi, S., Snetselaar, J., Wright, A., Matowo, J., Shirima, B., Kaaya, R., Athumani, R., Tenu, F., Protopopoff, N., & Kirby, M. (2021). Colonization and Authentication of the Pyrethroid-Resistant Anopheles gambiae s.s. Muleba-Kis Strain; an Important Test System for Laboratory Screening of New Insecticides. Insects, 12(8), 710. https://doi.org/10.3390/insects12080710