Avian Predation in a Declining Outbreak Population of the Spruce Budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Spruce Budworm Biology
2.2. Study Plots
2.3. Observations on the Spruce Budworm
2.3.1. Exclosure Cages
2.3.2. Population Sampling 1986
2.3.3. Implanted Cohorts 1987
2.3.4. Implanted Cohorts 1988
2.3.5. Data Analysis
2.4. Observations on Birds
2.4.1. Population Censuses
2.4.2. Foraging Activity
2.4.3. Nesting Activity
2.4.4. Data Analysis
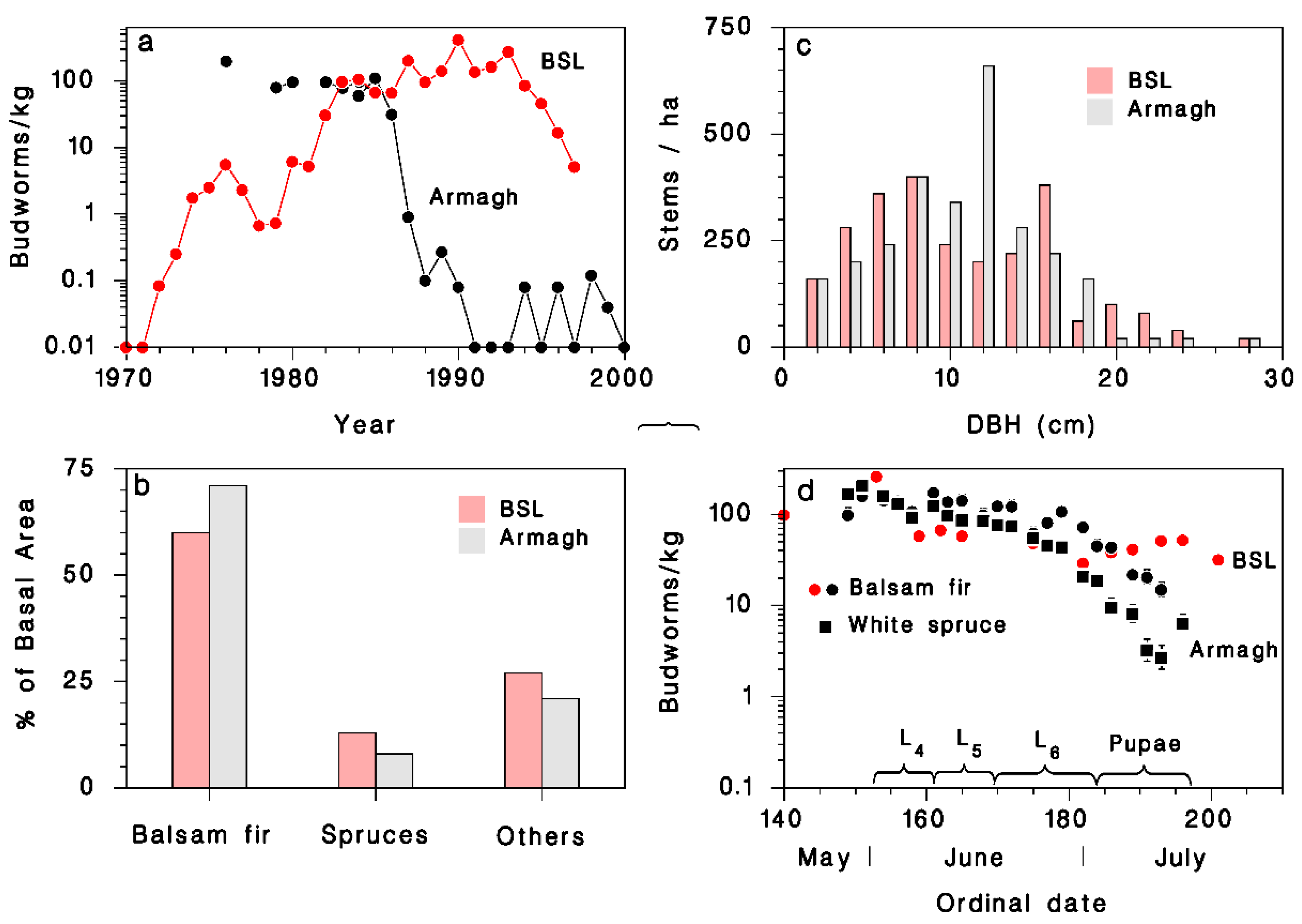
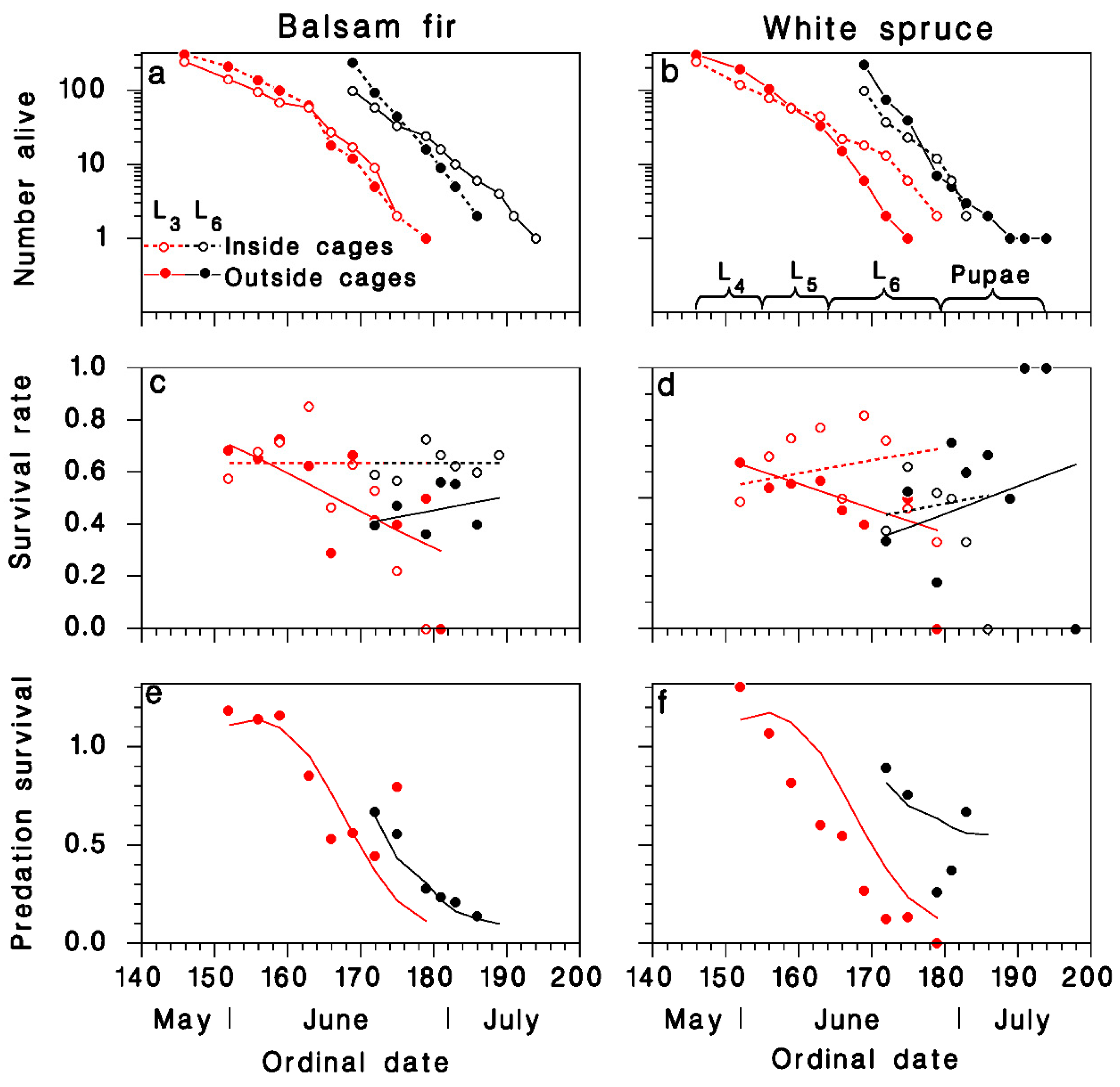
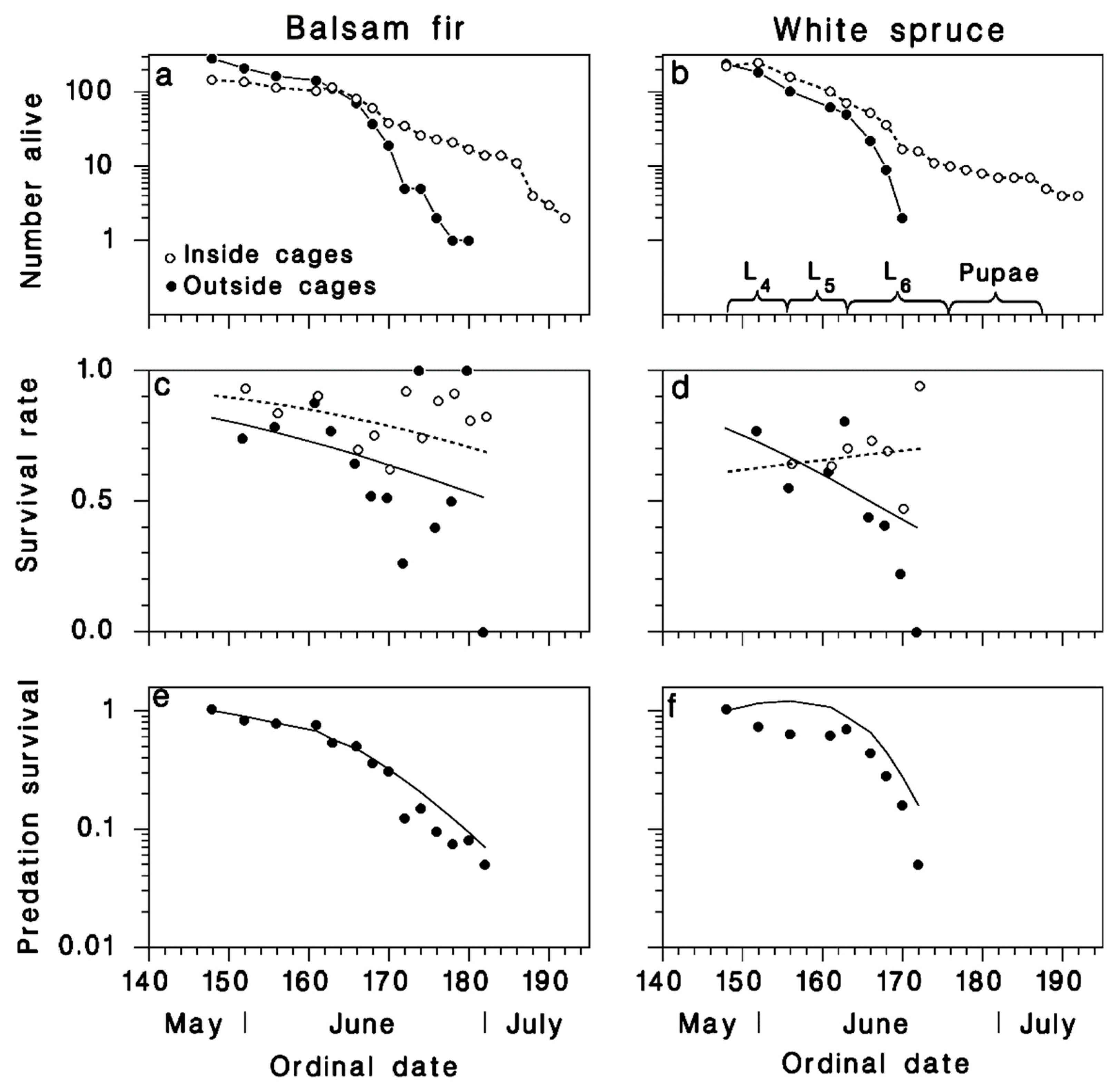
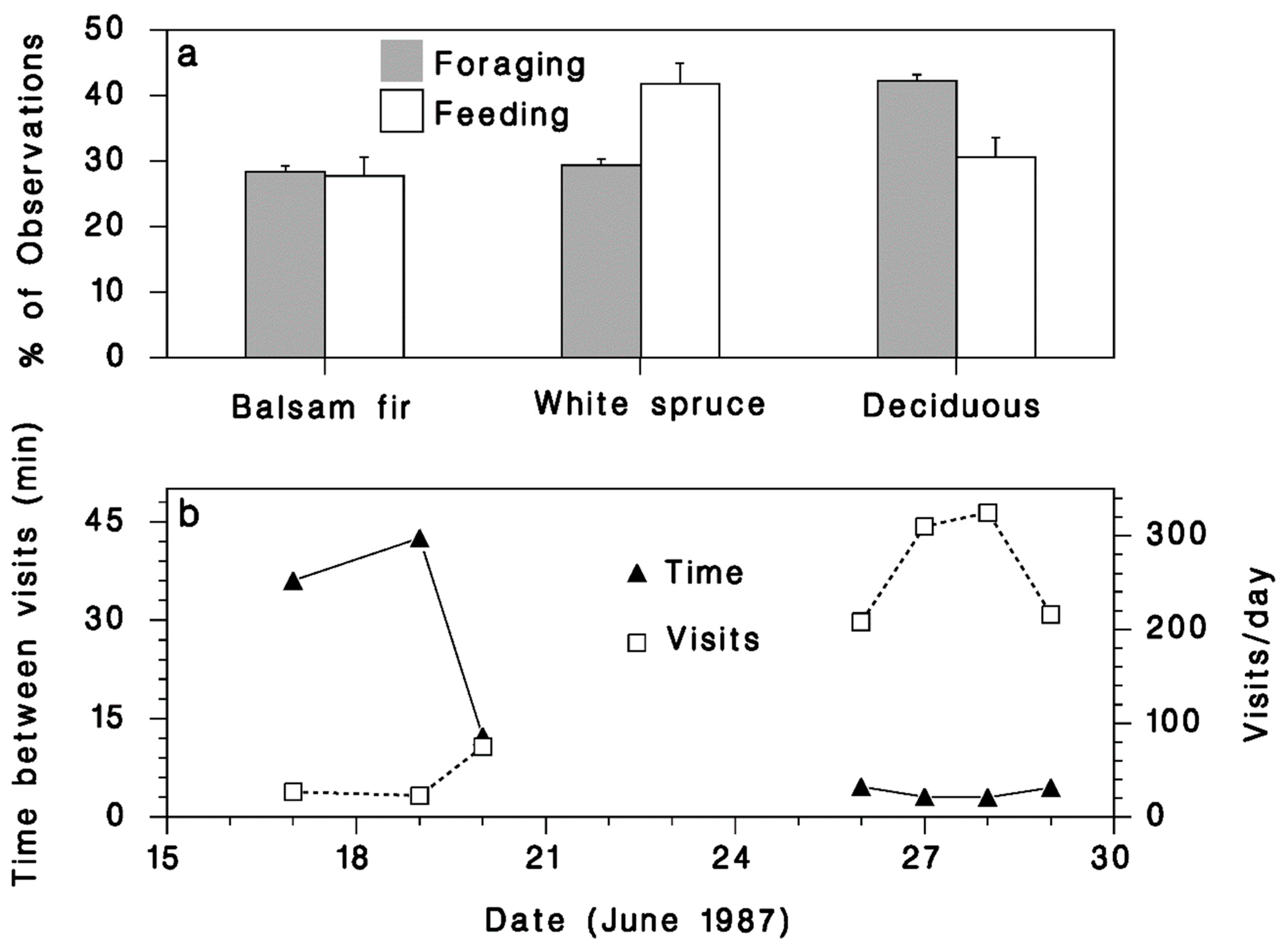
3. Results
3.1. Observations on Spruce Budworm
3.2. Observations on Birds
3.2.1. Population Censuses
3.2.2. Foraging Activity
3.2.3. Nesting Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooke, B.J.; Nealis, V.G.; Régnière, J. Insect defoliators as periodic disturbances in northern forest ecosystems. In Plant Disturbance Ecology: The Process and the Response, 2nd ed.; Johnson, E.A., Miyanishi, K., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands; London, UK, 2020; pp. 423–461. [Google Scholar] [CrossRef]
- Battisti, C.; Poeta, G.; Fanelli, C.G. An Introduction to Disturbance Ecology: A Road Map for Wildlife Management and Conservation; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Johns, C.R.; Bowden, J.B.; Carleton, R.D.; Cooke, B.J.; Edwards, S.; Emilson, E.J.S.; James, P.M.A.; Kneeshaw, D.; MacLean, D.A.; Martel, V.; et al. A conceptual framework for the spruce budworm early intervention strategy: Can outbreaks be stopped? Forests 2019, 10, 910. [Google Scholar] [CrossRef] [Green Version]
- Holling, C.S. Temperate forest insect outbreaks, tropical deforestation and migratory birds. Mem. Entomol. Soc. Can. 1988, 146, 21–32. [Google Scholar] [CrossRef]
- Dowden, P.B.; Jaynes, H.A.; Carolin, V.M. The role of birds in a spruce budworm outbreak in Maine. J. Econ. Entomol. 1953, 46, 307–312. [Google Scholar] [CrossRef]
- Tothill, J.D. Notes on the outbreaks of spruce budworm, forest tent caterpillar, and larch sawfly in New Brunswick. Acadian Entomol. Soc. 1923, 8, 172–182. [Google Scholar]
- Morris, R.F. The analysis of generation survival in relation to age-interval survivals in the unsprayed area. Mem. Entomol. Soc. Can. 1963, 31, 32–37. [Google Scholar] [CrossRef]
- Holling, C.S. Some Characteristics of Simple Types of Predation and Parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Clark, W.C.; Jones, D.D.; Holling, C.S. Lessons for ecological policy design: A case study of ecosystem management. Ecol. Model. 1979, 7, 1–53. [Google Scholar] [CrossRef] [Green Version]
- Pureswaran, D.S.; Johns, R.; Heard, S.B.; Quiring, D. Paradigms in eastern spruce budworm (Lepidoptera: Tortricidae) population ecology: A century of debate. Environ. Entomol. 2016, 45, 1333–1342. [Google Scholar] [CrossRef]
- Royama, T. Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol. Monogr. 1984, 54, 429–462. [Google Scholar] [CrossRef] [Green Version]
- Régnière, J.; Nealis, V. Ecological mechanisms of population change during outbreaks of the spruce budworm. Ecol. Entomol. 2007, 32, 461–477. [Google Scholar] [CrossRef]
- Royama, T.; Eveleigh, E.S.; Miron, J.R.B.; Pollock, S.J.; McCarthy, P.C.; McDougall, G.A.; Lucarotti, C.J. Mechanisms underlying spruce budworm outbreak processes as elucidated by a 14-year study in New Brunswick, Canada. Ecol. Monogr. 2017, 87, 600–631. [Google Scholar] [CrossRef]
- Régnière, K.; Cooke, B.J.; Béchard, A.; Dupont, A.; Therrien, P. Dynamics and management of rising outbreak spruce budworm populations. Forests 2019, 10, 748. [Google Scholar] [CrossRef] [Green Version]
- Royama, T. Factors governing the hunting behaviour and selection of food by the great tit (Parus major L.). J. Anim. Ecol. 1970, 39, 619–668. [Google Scholar] [CrossRef]
- Morse, D.H. The insectivorous bird as an adaptive strategy. Ann. Rev. Ecol. Syst. 1971, 2, 177–200. [Google Scholar] [CrossRef]
- Crawford, H.S.; Jennings, D.T. Predation by birds on spruce budworm Choristoneura fumiferana: Functional, numerical and total responses. Ecology 1989, 70, 152–163. [Google Scholar] [CrossRef]
- Toms, J.D.; Hannon, S.J.; Schniegelow, F.K.A. Population dynamics of songbirds in the boreal mixedwood forests of Alberta, Canada: Estimating minimum and maximum extents of spatial population synchrony. Landsc. Ecol. 2004, 20, 543–553. [Google Scholar] [CrossRef]
- Venier, L.A.; Holmes, S.B. A review of the interactions between forest birds and eastern spruce budworm. Environ. Rev. 2010, 18, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Drever, M.C.; Smith, A.C.; Venier, L.A.; Sleep, D.J.H.; MacLean, D.A. Cross-scale effects of spruce budworm outbreaks on boreal warblers in eastern Canada. Ecol. Evol. 2018, 8, 7334–7345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moisan-Perrier, J.; Kneeshaw, D.; Saint-Laurent, M.-H.; Pyle, P.; Villard, M.-A. Budworm-linked warblers as early indicators of defoliation by spruce budworm: A field study. Ecol. Indic. 2021, 125, 107543. [Google Scholar] [CrossRef]
- Rimmer, C.C.; McFarland, K.P. Tennessee Warbler (Leiothlypis peregrina), Version 1.0. In Birds of the World; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Baltz, M.E.; Latta, S.C. Cape May Warbler (Setophaga tigrina), Version 1.0. In Birds of the World; Rodewald, P.G., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Venier, L.A.; Holmes, S.; Williams, J.M. Bay-breasted Warbler (Setophaga castanea), Version 1.0. In Birds of the World; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca NY, USA, 2020. [Google Scholar]
- Koller, C.N.; Leonard, D.E. Comparison of energy budgets for spruce budworm Choristoneura fumiferana (Clemens) on balsam fir and white spruce. Oecologia 1981, 49, 14–20. [Google Scholar] [CrossRef]
- Sanders, C.J. Monitoring spruce budworm population density with sex pheromone traps. Can. Entomol. 1988, 120, 175–183. [Google Scholar] [CrossRef]
- Régnière, J.; Lysyk, T.J.; Auger, M. Population density estimation of spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae) on balsam fir and white spruce from 45-cm mid-crown branch tips. Can. Entomol. 1989, 121, 267–281. [Google Scholar] [CrossRef]
- Lethiecq, J.-L.; Régnière, J. CFS Spruce Budworm Population Studies: Site Descriptions. Canadian Forest Service Information Report LAU-X-83. 1988. Available online: https://cfs.nrcan.gc.ca/publications/download-pdf/21262 (accessed on 9 August 2021).
- Sanders, C.J. A Summary of Current Techniques Used for Sampling Spruce Budworm Populations and Estimating Defoliation in Eastern Canada. Natural Resources Canada, Canadian Forest Service Info. Rep. O-X-306. 1980. Available online: https://cfs.nrcan.gc.ca/publications/download-pdf/8982 (accessed on 9 August 2021).
- Roe, A.R.; Demidovich, M.; Dedes, J. Origins and history of laboratory insect stocks in a multispecies insect production facility, with the proposal of standardized nomenclature and designation of formal standard names. J. Insect Sci. 2018, 18, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Régnière, J. Temperature-dependent development of eggs and larvae of Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae) and simulation of its seasonal history. Can. Entomol. 1987, 119, 717–728. [Google Scholar] [CrossRef]
- Régnière, J.; Duval, P. Overwintering mortality of spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptrera: Tortricidae), populations under field conditions. Can. Entomol. 1998, 130, 13–26. [Google Scholar] [CrossRef]
- Anderson, T.W.; Darling, D.D. Asymptotic theory of certain “goodness-of-fit” criteria based on stochastic processes. Ann. Math. Stat. 1952, 23, 193–212. [Google Scholar] [CrossRef]
- Patten, M.A.; Burger, J.C. Spruce budworm outbreaks and the incidence of vagrancy in eastern North American wood-warblers. Can. J. Zool. 1998, 76, 433–439. [Google Scholar] [CrossRef]
- Morse, D.H. Variables affecting the density and territory size of breeding spruce-woods warblers. Ecology 1976, 57, 290–301. [Google Scholar] [CrossRef]
- Howe, R.W.; Niemi, G.J.; Lewis, S.J.; Welsh, D.A. A standard method for monitoring songbird populations in the Great Lakes region. Passeng. Pigeon 1997, 59, 183–194. [Google Scholar]
- Mantel, N. Chi-square tests with one degree of freedom, extensions of the Mantel-Haenszel procedure. J. Amer. Stat. Assoc. 1963, 58, 690–700. [Google Scholar] [CrossRef]
- Carpenter, J.W. Appendix II: Energy requirements for growing birds. In Hand-Rearing Birds; Gage, L.J., Duerr, R.S., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef] [Green Version]
- Venier, L.A.; Pearce, J.L.; Fillman, D.R.; McNikol, D.K.; Welsh, D.A. Effects of spruce budworm (Choristoneura fumiferana (Clem.)) outbreaks on boreal mixed-wood bird communities. Avian Conserv. Ecol. 2009, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Holmes, S.B.; Sanders, C.J.; Fillman, D.; Welsh, D.A. Changes in a forest bird community during an outbreak cycle of the spruce budworm in northwestern Ontario. Bird Pop. 2009, 9, 13–28. [Google Scholar]
- Sigiura, S.; Yamakasi, K. The role of silk threads as lifelines for caterpillars: Pattern and significance of lifeline-climbing behaviour. Ecol. Entomol. 2006, 31, 52–57. [Google Scholar] [CrossRef]
- Jactel, H.; Moreira, X.; Castagneyrol, B. Tree diversity and forest resistance to insect pests: Patterns, mechanisms and prospects. Ann. Rev. Entomol. 2021, 66, 277–296. [Google Scholar] [CrossRef]
- Sousa, W.P. The role of disturbance in natural communities. Ann. Rev. Ecol. Syst. 1984, 15, 353–391. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; White, P.S. The Ecology of Disturbance and Patch Dynamics; Academic Press: Orlando, FL, USA, 1985. [Google Scholar] [CrossRef]
- Cappuccino, N.; Lavertu, D.; Bergeron, Y.; Régnière, J. Spruce budworm impact, abundance and parasitism rate in a patchy landscape. Oecologia 1998, 114, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Quayle, D.; Régnière, J.; Cappuccino, N.; Dupont, A. Forest composition, host-population density, and parasitism of spruce budworm Choristoneura fumiferana eggs by Trichogramma minutum. Entomol. Exp. Appl. 2003, 107, 215–227. [Google Scholar] [CrossRef]
- Eveleigh, E.S.; McCann, K.S.; McCarthy, P.C.; Pollock, S.J.; Lucarotti, C.J.; Morin, B.; McDougall, G.A.; Strongman, D.B.; Huber, J.T.; Umbanhowar, J.; et al. Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc. Natl. Acad. Sci. USA 2007, 104, 16976–16981. [Google Scholar] [CrossRef] [Green Version]
- Legault, S.; James, P.M.A. Parasitism rates of spruce budworm larvae: Testing the enemy hypothesis along a gradient forest diversity measured at different spatial scales. Environ. Entomol. 2018, 47, 1083–1095. [Google Scholar] [CrossRef]
- Hardy, Y.; Lafond, A.; Hamel, L. The epidemiology of the current spruce budworm outbreak in Quebec. Forest Sci. 1983, 29, 715–725. [Google Scholar] [CrossRef]
- Régnière, J.; Lysyk, T.J. Population dynamics of the spruce budworm, Choristoneura fumiferana. In Forest Insects Pests in Canada; Armstrong, J.A., Ives, W.G.H., Eds.; Natural Resources Canada, Canadian Forest Service Publication: Ottawa, ON, Canada, 1995; pp. 95–105. Available online: https://publications.gc.ca/collections/collection_2020/rncan-nrcan/Fo42-235-1995-eng.pdf (accessed on 9 August 2021).





| Full Model | Reduced Model | |||||
|---|---|---|---|---|---|---|
| Source | df | F | p | df | F | p |
| s | 1 | 1.48 | 0.519 | |||
| t | 1 | 1.47 | 0.234 | |||
| s × t | 1 | 3.44 | 0.072 | 1 | 41.05 | <0.001 |
| t2 | 1 | 5.50 | 0.025 | 1 | 5.56 | <0.001 |
| s × t2 | 1 | 0.64 | 0.428 | |||
| Error | 36 | 39 | ||||
| Full Model | Reduced Model | |||||
|---|---|---|---|---|---|---|
| Source | df | χ2 | p | df | χ2 | p |
| Regression | 15 | 158.67 | 0.000 | 8 | 156.15 | <0.001 |
| s | 1 | 4.73 | 0.030 | 1 | 11.86 | 0.001 |
| ca | 1 | 6.00 | 0.014 | 1 | 21.95 | <0.001 |
| co | 1 | 0.18 | 0.674 | |||
| t | 1 | 0.28 | 0.597 | |||
| s × ca | 1 | 0.11 | 0.745 | |||
| s × co | 1 | 0.28 | 0.594 | 1 | 14.06 | <0.001 |
| ca × co | 1 | 3.21 | 0.073 | 1 | 11.73 | 0.001 |
| s × t | 1 | 2.79 | 0.095 | 1 | 4.54 | 0.033 |
| ca × t | 1 | 6.84 | 0.009 | 1 | 30.40 | <0.001 |
| co × t | 1 | 0.19 | 0.661 | |||
| s × ca × co | 1 | 0.13 | 0.716 | 1 | 7.00 | 0.008 |
| s × ca × t | 1 | 0.80 | 0.372 | |||
| s × co × t | 1 | 0.04 | 0.850 | |||
| ca × co × t | 1 | 2.64 | 0.104 | 1 | 12.06 | 0.001 |
| AICc | 421.9 | 403.4 | ||||
| Full Model | Reduced Model | |||||
|---|---|---|---|---|---|---|
| Source | df | χ2 | p | df | χ2 | p |
| Regression | 7 | 120.62 | 0.000 | 6 | 117.86 | <0.001 |
| s | 1 | 27.89 | 0.000 | 1 | 38.36 | <0.001 |
| ca | 1 | 3.82 | 0.051 | 1 | 34.83 | <0.001 |
| t | 1 | 11.09 | 0.001 | 1 | 30.81 | <0.001 |
| s × ca | 1 | 11.28 | 0.001 | 1 | 23.29 | <0.001 |
| s × t | 1 | 6.76 | 0.009 | 1 | 10.16 | 0.001 |
| ca × t | 1 | 0.92 | 0.336 | |||
| s × ca × t | 1 | 6.71 | 0.010 | 1 | 14.25 | <0.001 |
| AICc | 320.7 | 318.50 | ||||
| Species | F | p | AD | p | n |
|---|---|---|---|---|---|
| TEWA | 236.1 | <0.001 | 0.998 | 0.01 | 7 |
| CMWA | 227.4 | <0.001 | 0.270 | 0.55 | 7 |
| BLBW | 6.4 | 0.08 | 0.126 | 0.97 | 7 |
| BBWA | 45.2 | <0.001 | 0.129 | 0.97 | 7 |
| Total | 15.3 | <0.001 | 0.151 | 0.93 | 7 |
| Activity | BSL 1983 | Armagh 1987 | χ2 | p |
|---|---|---|---|---|
| Singing and others | 2264 | 1358 | 199.9 | <0.001 |
| Travelling | 509 | 464 | 2.1 | 0.149 |
| Searching | 1737 | 2890 | 932.8 | <0.001 |
| Feeding | 1438 | 252 | 754.1 | <0.001 |
| Overall | 1270 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Régnière, J.; Venier, L.; Welsh, D. Avian Predation in a Declining Outbreak Population of the Spruce Budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). Insects 2021, 12, 720. https://doi.org/10.3390/insects12080720
Régnière J, Venier L, Welsh D. Avian Predation in a Declining Outbreak Population of the Spruce Budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). Insects. 2021; 12(8):720. https://doi.org/10.3390/insects12080720
Chicago/Turabian StyleRégnière, Jacques, Lisa Venier, and Dan Welsh. 2021. "Avian Predation in a Declining Outbreak Population of the Spruce Budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae)" Insects 12, no. 8: 720. https://doi.org/10.3390/insects12080720






