Acorn Crop, Seed Size and Chemical Defenses Determine the Performance of Specialized Insect Predators and Reproductive Output in a Mediterranean Oak
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Organisms
2.2. Sampling Design
2.3. Numerical Analyses
2.3.1. Between-Year Variations
2.3.2. Acorn Predation by Insects
2.3.3. Number, Weight and Mortality of Insect Immatures
2.3.4. Acorn Tannin Content and Predation by Insects
2.3.5. Seed Output and Insect Productivity
3. Results
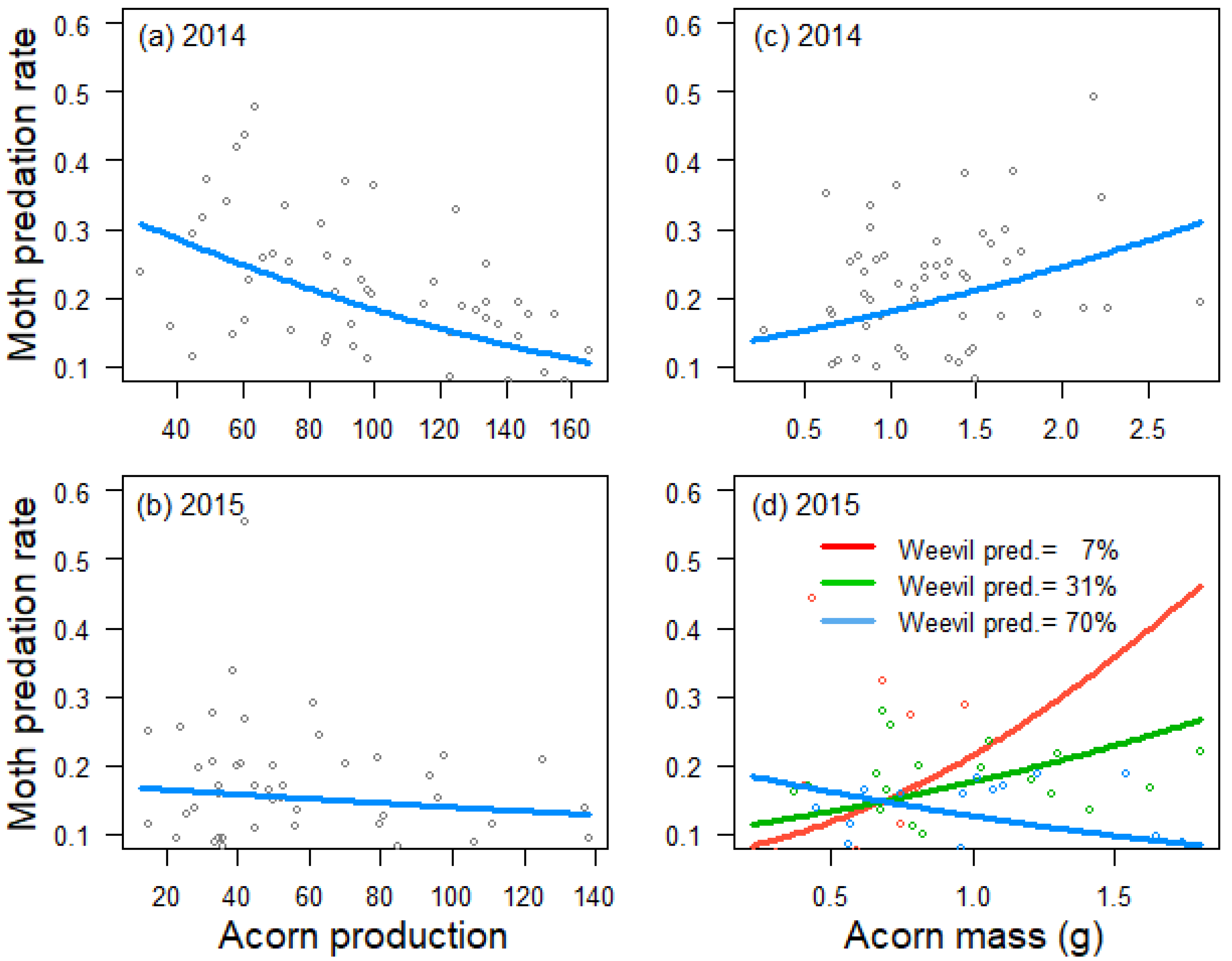
3.1. Crop Size and Acorn Predation by Insects
3.2. Determinants of Seed Predation by Insects
3.3. Number of Insect Immatures Developing in Acorns
3.4. Determinants of Insect Immature Size
3.5. Insect Larval Mortality
3.6. Tannin Content in Acorns and Seed Predation by Insects
3.7. Seed Output and Insect Productivity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janzen, D.H. Seed predation by animals. Annu. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar] [CrossRef]
- Hulme, P.E.; Benkman, C.W. Granivory. In Plant–Animal Interactions: An Evolutionary Approach; Herrera, C.M., Pellmyr, O., Eds.; Blackwell Science: New York, NY, USA, 2002; pp. 185–208. [Google Scholar]
- Crawley, M.J.; Long, C.R. Alternate bearing, predator satiation and seedling recruitment in Quercus robur L. J. Ecol. 1995, 83, 683–696. [Google Scholar] [CrossRef]
- Crawley, M.J. Seed predators and plant population dynamics. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: New York, NY, USA, 2000; pp. 167–182. [Google Scholar]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Maron, J.L.; Crone, E. Herbivory: Effects on plant abundance, distribution and population growth. Proc. R. Soc. B Biol. Sci. 2006, 273, 2575–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, A.N. How important is seed predation to recruitment in stable populations of long-lived perennials? Oecologia 1989, 81, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A.; Ehrlen, J.; Eriksson, O. Ecological and evolutionary consequences of spatial and temporal variation in pre-dispersal seed predation. Perspect. Plant Ecol. Evol. Syst. 2007, 9, 79–100. [Google Scholar] [CrossRef]
- Louda, S.M. Distribution ecology: Variation in plant recruitment over a gradient in relation to insect seed predation. Ecol. Monogr. 1982, 52, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Louda, S.M.; Potvin, M.A. Effect of inflorescence-feeding insects on the demography and lifetime of a native plant. Ecology 1995, 76, 229–245. [Google Scholar] [CrossRef]
- Maron, J.L.; Combs, J.K.; Louda, S.M. Convergent demographic effects of insect attack on related thistles in coastal vs. continental dunes. Ecology 2002, 83, 3382–3392. [Google Scholar] [CrossRef]
- Fröborg, H.; Eriksson, O. Predispersal seed predation and population dynamics in the perennial understorey herb Actaea spicata. Can. J. Bot. 2003, 81, 1058–1069. [Google Scholar] [CrossRef]
- Jordano, P. Angiosperm fleshy fruits and seed dispersers: A comparative analysis of adaptation and constraints in plant-animal interactions. Am. Nat. 1995, 145, 163–191. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Pulido, R.; Albores-Barajas, Y.V.; Díaz, S.A. Fruit removal efficiency and success: Influence of crop size in a neotropical treelet. Plant Ecol. 2007, 189, 147–154. [Google Scholar] [CrossRef]
- Herrera, C.M. Vertebrate-dispersed plants: Why they don’t behave the way they should. In Frugivores and Seed Dispersal; Estrada, A., Fleming, T.H., Eds.; Junk: Dordrecht, The Netherlands, 1986; pp. 5–18. [Google Scholar]
- Sallabanks, R.; Courtney, S.P. Frugivory, seed predation, and insect-vertebrate interactions. Annu. Rev. Entomol. 1992, 37, 377–400. [Google Scholar] [CrossRef]
- Jordano, P. Avian fruit removal: Effects of fruit variation, crop size, and insect damage. Ecology 1987, 68, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Mezquida, E.T.; Olano, J.M. What makes a good neighborhood? Interaction of spatial scale and fruit density in the predator satiation dynamics of a masting juniper tree. Oecologia 2013, 173, 483–492. [Google Scholar] [CrossRef]
- Linhart, Y.B.; Moreira, X.; Snyder, M.A.; Mooney, K.A. Variability in seed cone production and functional response of seed predators to seed cone availability: Support for the predator satiation hypothesis. J. Ecol. 2014, 102, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Vander Wall, S.B. The evolutionary ecology of nut dispersal. Bot. Rev. 2001, 67, 74–117. [Google Scholar] [CrossRef]
- Gómez, J.M.; Schupp, E.W.; Jordano, P. Synzoochory: The ecological and evolutionary relevance of a dual interaction. Biol. Rev. 2019, 94, 874–902. [Google Scholar] [CrossRef]
- Branco, M.; Branco, C.; Merouani, H.; Almeida, M.H. Germination success, survival and seedling vigour of Quercus suber acorns in relation to insect damage. For. Ecol. Manag. 2002, 166, 159–164. [Google Scholar] [CrossRef]
- Perea, R.; Fernandes, G.W.; Dirzo, R. Early plant development depends on embryo damage location: The role of seed size in partial seed predation. Oikos 2020, 129, 320–330. [Google Scholar] [CrossRef]
- Bell, D.M.; Clark, J.S. Seed predation and climate impacts on reproductive variation in temperate forests of the southeastern USA. Oecologia 2016, 180, 1223–1234. [Google Scholar] [CrossRef]
- Bartlow, A.W.; Agosta, S.J.; Curtis, R.; Yi, X.; Steele, M.A. Acorn size and tolerance to seed predators: The multiple roles of acorns as food for seed predators, fruit for dispersal and fuel for growth. Integr. Zool. 2018, 13, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.D.; Mumme, R.L.; Carmen, W.J.; Stanback, M.T. Acorn production by oaks in central coastal California: Variation within and among years. Ecology 1994, 75, 99–109. [Google Scholar] [CrossRef]
- Herrera, C.M.; Jordano, P.; Guitian, J.; Traveset, A. Annual variability in seed production by woody plants and the masting concept: Reassessment of principles and relationship to pollination and seed dispersal. Am. Nat. 1998, 152, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Espelta, J.M.; Cortés, P.; Molowny-Horas, R.; Sánchez-Humanes, B.; Retana, J. Masting mediated by summer drought reduces acorn predation in Mediterranean oak forests. Ecology 2008, 89, 805–817. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.; Sork, V.L. Mast seeding in perennial plants: Why, how, where? Annu. Rev. Ecol. Syst. 2002, 33, 427–447. [Google Scholar] [CrossRef] [Green Version]
- Satake, A.; Bjørnstad, O.N.; Kobro, S. Masting and trophic cascades: Interplay between rowan trees, apple fruit moth, and their parasitoid in southern Norway. Oikos 2004, 104, 540–550. [Google Scholar] [CrossRef]
- Bonal, R.; Muñoz, A.; Díaz, M. Satiation of predispersal seed predators: The importance of considering both plant and seed levels. Evol. Ecol. 2007, 21, 367–380. [Google Scholar] [CrossRef]
- Lombardo, J.A.; McCarthy, B.C. Seed germination and seedling vigor of weevil-damaged acorns of red oak. Can. J. For. Res. 2009, 39, 1600–1605. [Google Scholar] [CrossRef]
- Desouhant, E.; Debouzie, D.; Ploye, H.; Menu, F. Clutch size manipulations in the chestnut weevil, Curculio elephas: Fitness of oviposition strategies. Oecologia 2000, 122, 493–499. [Google Scholar] [CrossRef]
- Espelta, J.M.; Bonal, R.; Sánchez-Humanes, B. Pre-dispersal acorn predation in mixed oak forests: Interspecific differences are driven by the interplay among seed phenology, seed size and predator size. J. Ecol. 2009, 97, 1416–1423. [Google Scholar] [CrossRef]
- Bonal, R.; Muñoz, A. Seed weevils living on the edge: Pressures and conflicts over body size in the endoparasitic Curculio larvae. Ecol. Entomol. 2009, 34, 304–309. [Google Scholar] [CrossRef]
- Yi, X.F.; Yang, Y.Q. Large acorns benefit seedling recruitment by satiating weevil larvae in Quercus aliena. Plant Ecol. 2010, 209, 291–300. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Marino, S.; Bonal, R.; Zwolak, R.; Steele, M.A. Rapid aggregative and reproductive responses of weevils to masting of North American oaks counteract predator satiation. Ecology 2018, 99, 2575–2582. [Google Scholar] [CrossRef]
- Montserrat-Martí, G.; Camarero, J.J.; Palacio, S.; Pérez-Rontomé, C.; Milla, R.; Albuixech, J.; Maestro, M. Summer-drought constrains the phenology and growth of two coexisting Mediterranean oaks with contrasting leaf habit: Implications for their persistence and reproduction. Trees 2009, 23, 787–799. [Google Scholar] [CrossRef] [Green Version]
- Pons, J.; Pausas, J.G. The coexistence of acorns with different maturation patterns explains acorn production variability in cork oak. Oecologia 2012, 169, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, A.; Bonal, R.; Espelta, J.M. Acorn–Weevil interactions in a mixed-oak forest: Outcomes for larval growth and plant recruitment. For. Ecol. Manag. 2014, 322, 98–105. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; García-De La Cruz, Y.; Gómez-Aparicio, L. Contrasting responses of insects and vertebrates as seed consumers of two neotropical oak species: The interactive effects of individual crop size and seed mass. For. Ecol. Manag. 2017, 401, 99–106. [Google Scholar] [CrossRef]
- Debouzie, D.; Heizmann, A.; Desouhant, E.; Menu, F. Interference at several temporal and spatial scales between two chestnut insects. Oecologia 1996, 108, 151–158. [Google Scholar] [CrossRef]
- Steele, M.A.; Knowles, T.; Bridle, K.; Simms, E.L. Tannins and partial consumption of acorns: Implications for dispersal of oaks by seed predators. Am. Midl. Nat. 1993, 130, 229–238. [Google Scholar] [CrossRef]
- Smallwood, P.D.; Steele, M.A.; Faeth, S.H. The ultimate basis of the caching preferences of rodents, and the oak-dispersal syndrome: Tannins, insects, and seed germination. Am. Zool. 2001, 41, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Weckerly, F.W.; Sugg, D.W.; Semlitsch, R.D. Germination success of acorns (Quercus): Insect predation and tannins. Can. J. For. Res. 1989, 19, 811–815. [Google Scholar] [CrossRef]
- Xiao, Z.; Harris, M.K.; Zhang, Z. Acorn defenses to herbivory from insects: Implications for the joint evolution of resistance, tolerance and escape. For. Ecol. Manag. 2007, 238, 302–308. [Google Scholar] [CrossRef]
- Blanco, E.; Casado, M.A.; Costa, M.; Escribano, R.; García, M.; Génova, M.; Gómez, A.; Gómez, F.; Moreno, J.C.; Morla, C. Los Bosques Ibéricos. Una interpretación Geobotánica; Planeta: Barcelona, Spain, 1997. [Google Scholar]
- Gómez-de-Aizpúrua, C. Cydia fagiglandana (Zeller, 1841). Lep. Tortricidae, en España. Bol. Sanid. Veg. Plagas 1993, 19, 389–400. [Google Scholar]
- Leiva, M.J.; Fernández-Alés, R. Holm-oak (Quercus ilex subsp. ballota) acorns infestation by insects in Mediterranean dehesas and shrublands: Its effect on acorn germination and seedling emergence. For. Ecol. Manag. 2005, 212, 221–229. [Google Scholar] [CrossRef]
- Bonal, R.; Muñoz, A. Seed growth suppression constrains the growth of seed parasites: Premature acorn abscission reduces Curculio elephas larval size. Ecol. Entomol. 2008, 33, 31–36. [Google Scholar] [CrossRef]
- Pulido, F.J.; Díaz, M. Regeneration of a Mediterranean oak: A whole-cycle approach. Ecoscience 2005, 12, 92–102. [Google Scholar] [CrossRef]
- Gómez, J.M.; Puerta-Piñero, C.; Schupp, E.W. Effectiveness of rodents as local seed dispersers of Holm oaks. Oecologia 2008, 155, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.M. Spatial patterns in long-distance dispersal of Quercus ilex acorns by jays in a heterogeneous landscape. Ecography 2003, 26, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Pons, J.; Pausas, J.G. Not only size matters: Acorn selection by the European jay (Garrulus glandarius). Acta Oecologica 2007, 31, 353–360. [Google Scholar] [CrossRef]
- Koenig, W.D.; Knops, J.M.H.; Carmen, W.J.; Stanback, M.T.; Mumme, R.L. Estimating acorn crops using visual surveys. Can. J. For. Res. 1994, 24, 2105–2112. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Tejerina, D.; García-Torres, S.; de Vaca, M.C.; Vázquez, F.; Cava, R. Acorns (Quercus rotundifolia Lam.) and grass as natural sources of antioxidants and fatty acids in the “montanera” feeding of Iberian pig: Intra-and inter-annual variations. Food Chem. 2011, 124, 997–1004. [Google Scholar] [CrossRef]
- Harrison, X.A. A comparison of observation-level random effect and Beta-Binomial models for modelling overdispersion in Binomial data in ecology & evolution. PeerJ 2015, 3, e1114. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Koenig, W.D.; Knops, J.M.H.; Carmen, W.J.; Sage, R.D. No trade-off between seed size and number in the valley oak Quercus lobata. Am. Nat. 2009, 173, 682–688. [Google Scholar] [CrossRef]
- Turgeon, J.J.; Roques, A.; Groot, P.D. Insect fauna of coniferous seed cones: Diversity, host plant interactions, and management. Annu. Rev. Entomol. 1994, 39, 179–212. [Google Scholar] [CrossRef]
- Koenig, W.D. The effects of tannins and lipids on digestion of acorns by acorn woodpeckers. Auk 1991, 108, 79–88. [Google Scholar]
- Espelta, J.M.; Cortés, P.; Molowny-Horas, R.; Retana, J. Acorn crop size and pre-dispersal predation determine inter-specific differences in the recruitment of co-occurring oaks. Oecologia 2009, 161, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Perea, R.; López, D.; San Miguel, A.; Gil, L. Incorporating insect infestation into rodent seed dispersal: Better if the larva is still inside. Oecologia 2012, 170, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Chang, G.; Yi, X.; Lu, J.; Xiao, Z.; Zhang, H.; Cao, L.; Wang, F.; Li, H.; et al. Trade-off between seed defensive traits and impacts on interaction patterns between seeds and rodents in forest ecosystems. Plant Ecol. 2016, 217, 253–265. [Google Scholar] [CrossRef]
- Menu, F.; Debouzie, D. Coin-flipping plasticity and prolonged diapause in insects: Example of the chestnut weevil Curculio elephas (Coleoptera: Curculionidae). Oecologia 1993, 93, 367–373. [Google Scholar] [CrossRef]
- Pélisson, P.F.; Bernstein, C.; Francois, D.; Menu, F.; Venner, S. Dispersal and dormancy strategies among insect species competing for a pulsed resource. Ecol. Entomol. 2013, 38, 470–477. [Google Scholar] [CrossRef]
- Espelta, J.M.; Arias-Leclaire, H.; Fernández-Martínez, M.; Doblas-Miranda, E.; Muñoz, A.; Bonal, R. Beyond predator satiation: Masting but also the effects of rainfall stochasticity on weevils drive acorn predation. Ecosphere 2017, 8, e01836. [Google Scholar] [CrossRef] [Green Version]


| Variables | 2014 | 2015 | Z or t | p |
|---|---|---|---|---|
| Acorn production | 98.8 (0.6) | 55.3 (4.9) | −6.3 | <0.001 |
| Acorn mass (g) | 1.22 (0.07) | 0.84 (0.06) | −4.3 | <0.001 |
| Overall acorn predation (%) | 51.4 (4.2) | 46.8 (3.4) | −1.2 | 0.231 |
| Acorn predation by weevils (%) | 35.3 (3.8) | 34.5 (3.3) | −0.2 | 0.828 |
| Acorn predation by moths (%) | 20.6 (1.7) | 15.4 (1.6) | −2.0 | 0.046 |
| Acorn predation by both weevils and moths (%) | 4.1 (0.6) | 3.1 (0.6) | −1.6 | 0.115 |
| Number of weevil larvae per acorn | 1.3 (0.1) | 2.0 (0.1) | 5.6 | <0.001 |
| Number of moth larvae per acorn | 1.0 (0.0) | 1.4 (0.1) | 3.9 | <0.001 |
| Weevil larval weight (g) | 0.0463 (0.0029) 49 | 0.0483 (0.0022) 51 | 0.5 | 0.584 |
| Moth larval weight (g) | 0.0210 (0.0011) 51 | 0.0210 (0.0011) 47 | −0.003 | 0.997 |
| Variables | 2014 | 2015 | ||||
|---|---|---|---|---|---|---|
| Estimate (SE) | t | p | Estimate (SE) | t | p | |
| (a) Weevil larval weight | ||||||
| Intercept | 0.060 (0.003) | 20.4 | <0.001 | 0.056 (0.003) | 20.6 | <0.001 |
| Acorn mass | 0.016 (0.002) | 6.7 | <0.001 | 0.023 (0.002) | 9.3 | <0.001 |
| Number of weevil larvae | −0.010 (0.002) | −5.8 | <0.001 | <−0.001 (0.001) | −0.1 | 0.904 |
| Julian date | −0.007 (0.001) | −5.6 | <0.001 | |||
| Acorn mass x weevil larvae | −0.003 (0.001) | −2.5 | 0.012 | −0.004 (0.001) | −5.0 | <0.001 |
| (b) Moth larval weight | ||||||
| Intercept | 0.022 (0.001) | 31.8 | <0.001 | 0.022 (0.001) | 24.8 | <0.001 |
| Acorn mass | 0.004 (0.001) | 6.1 | <0.001 | 0.004 (0.001) | 5.0 | <0.001 |
| Number of weevil larvae | −0.004 (0.001) | −3.5 | <0.001 | −0.004 (0.001) | −3.7 | <0.001 |
| Julian date | −0.004 (0.001) | −4.4 | <0.001 | |||
| Insect Productivity | 2014 | 2015 | Z | p |
|---|---|---|---|---|
| Number of immature weevils per tree | 40.9 ± 5.2 (59) | 47.7 ± 7.7 (51) | 0.7 | 0.477 |
| Weevil biomass per tree | 2.00 ± 0.25 (53) | 2.76 ± 0.52 (51) | 1.4 | 0.156 |
| Number of immature moths per tree | 20.0 ± 1.9 (59) | 12.5 ± 1.6 (51) | −2.7 | 0.006 |
| Moth biomass per tree | 0.44 ± 0.05 (55) | 0.27 ± 0.04 (51) | −1.9 | 0.053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezquida, E.T.; Caputo, P.; Acebes, P. Acorn Crop, Seed Size and Chemical Defenses Determine the Performance of Specialized Insect Predators and Reproductive Output in a Mediterranean Oak. Insects 2021, 12, 721. https://doi.org/10.3390/insects12080721
Mezquida ET, Caputo P, Acebes P. Acorn Crop, Seed Size and Chemical Defenses Determine the Performance of Specialized Insect Predators and Reproductive Output in a Mediterranean Oak. Insects. 2021; 12(8):721. https://doi.org/10.3390/insects12080721
Chicago/Turabian StyleMezquida, Eduardo T., Paula Caputo, and Pablo Acebes. 2021. "Acorn Crop, Seed Size and Chemical Defenses Determine the Performance of Specialized Insect Predators and Reproductive Output in a Mediterranean Oak" Insects 12, no. 8: 721. https://doi.org/10.3390/insects12080721
APA StyleMezquida, E. T., Caputo, P., & Acebes, P. (2021). Acorn Crop, Seed Size and Chemical Defenses Determine the Performance of Specialized Insect Predators and Reproductive Output in a Mediterranean Oak. Insects, 12(8), 721. https://doi.org/10.3390/insects12080721





