Bioactivity of Cereal- and Legume-Based Macaroni Pasta Volatiles to Adult Sitophilus granarius (L.)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Pasta Materials
2.3. Multiple-Choice Behavioural Bioassays
2.4. Two-Choice Behavioural Bioassays
2.5. Susceptibility of Pasta Samples
2.6. Extraction of Pasta Volatiles
2.7. Gas Chromatography-Mass Spectrometry (GC-MS)
2.8. Data Analysis
3. Results
3.1. Multiple-Choice Behavioural Bioassays
3.2. Two-Choice Behavioural Bioassays
3.3. Susceptibility of Pasta Samples
3.4. Characterisation of Pasta Volatiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Süss, L.; Locatelli, D.P. Activity of Sitophilus oryzae (L.), Rhyzopertha dominica (F.), Tribolium confusum Duval and Plodia interpunctella (Hbn.) on alimentary pastas. Tec. Molit. 1999, 50, 516–524. [Google Scholar]
- Riudavets, J.; Lucas, E.; Pons, M.J. Insects and mites of stored products in the northeast of Spain. IOBC Bull. 2002, 25, 41–44. [Google Scholar]
- Barros, G.; Maia, A.; Rodrigues, A.; Mexia, A. Stored product insect pests in pasta residues in Portugal. In Proceedings of the 3rd Meeting of COST Action 842, WG-IV, Berlin, Germany, 4–5 December 2003; p. 48. [Google Scholar]
- Trematerra, P. Integrated Management of insects infesting pasta factories in Italy. In Proceedings of the 5th Meeting COST Action 842, WG-IV: “Bio-Control of Arthropod Pests in Stored Products”, Barcelona, Spain, 28–29 October 2004; pp. 31–34. [Google Scholar]
- Trematerra, P. Preferences of Sitophilus zeamais to different types of Italian commercial rice and cereal pasta. Bull. Insectol. 2009, 62, 103–106. [Google Scholar]
- Stejskal, V.; Kucerova, Z.; Lukas, J. Evidence and symptoms of pasta infestation by Sitophilus oryzae (Curculionidae; Coleoptera) in the Czech Republic. Plant Prot. Sci. 2004, 40, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Trematerra, P.; Süss, L. Integrated pest management in Italian pasta factories. In Proceedings of the 9th International Working Conference of Stored-Product Protection, Brazilian Post-Harvest Association, Campinas, San Paolo, Brazil, 28–29 October 2006; Brazilian Post-Harvest Association: Campinas, San Paolo, Brazil, 2006; pp. 747–753. [Google Scholar]
- Athanassiou, C.G.; Riudavets, J.; Kavallieratos, G. Preventing stored-product insect infestations in packaged-food products. Stewart Postharvest Rev. 2011, 3, 1–5. [Google Scholar] [CrossRef]
- Longstaff, B.C. Biology of the grain pest species of the genus Sitophilus (Coleoptera: Curculionidae): A critical review. Prot. Ecol. 1981, 3, 83–130. [Google Scholar]
- Suss, L.; Savoldelli, S. Egg mortality of pasta pests during pasta making. Tec. Molit. 2011, 62, 60–65. [Google Scholar]
- Stejskal, V.; Bostlova, M.; Nesvorna, M.; Volek, V.; Dolezal, V.; Hubert, J. Comparison of the resistance of mono and mul-tilayer packaging films to stored product insects in a laboratory test. Food Control 2017, 73, 566–573. [Google Scholar] [CrossRef]
- Riudavets, J.; Salas, I.; Pons, M.J. Damage characteristics produced by insect pests in packaging film. J. Stored Prod. Res. 2007, 43, 564–570. [Google Scholar] [CrossRef]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Behavioral responses of adult Sitophilus granarius to individual cereal volatiles. J. Chem. Ecol. 2008, 34, 523–529. [Google Scholar] [CrossRef]
- Murata, M.; Imamura, T.; Miyanoshita, A. Infestation and development of Sitophilus spp. in pouch-packaged spaghetti in Japan. J. Econ. Entomol. 2008, 101, 1006–1010. [Google Scholar] [CrossRef]
- Trematerra, P.; Savoldelli, S. Pasta preference and ability to penetrate through packaging of Sitophilus zeamais Motschulsky (Coleoptera: Dryophthoridae). J. Stored Prod. Res. 2014, 59, 126–132. [Google Scholar] [CrossRef]
- Schöller, M.; Prozell, S.; Suma, P.; Russo, A. Biological control of stored product insects. In Recent Advances in Stored Product Protection; Athanassiou, C.G., Arthur, F.H., Eds.; Springer: Berlin, Germany, 2018; pp. 183–209. [Google Scholar]
- Hou, X.; Fields, P.; Taylor, W. The effect of repellents on penetration into packaging by stored-product insects. J. Stored Prod. Res. 2004, 40, 47–54. [Google Scholar] [CrossRef]
- Scheff, D.S.; Sehgal, B.; Subramanyam, B. Evaluating penetration ability of Plodia interpunctella (Hubner) (Lepidoptera: Pyralidae) larvae into multilayer polypropylene packages. Insects 2018, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Vrabič Brodnjak, U.; Jordan, J.; Trematerra, P. Resistance of packaging against infestation by Sitophilus zeamais. J. Food Sci. Technol. 2020, 55, 2970–2980. [Google Scholar] [CrossRef]
- Vrabič Brodnjak, U.; Jordan, J.; Trematerra, P. Durable pasta packaging with bio-based barrier to prevent insect infestation. In Proceedings of the 11th International Conference on Simulation and Modelling in the Food and Bio-Industry, FOODSIM, Ghent, Belgium, 6–10 September 2020; pp. 102–105. [Google Scholar]
- Germinara, G.S.; Conte, A.; Lecce, L.; Di Palma, A.; Del Nobile, M.A. Propionic acid in bio-based packaging to prevent Sitophilus granarius (L.) (Coleoptera, Dryophthoridae) infestation in cereal products. Innov. Food Sci. Emerg. Technol. 2010, 11, 498–502. [Google Scholar] [CrossRef]
- Mullen, M.A.; Vardeman, J.M.; Bagwell, J. Insect Resistant Packaging. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KS, USA, 2012; pp. 135–141. [Google Scholar]
- Heeps, J. Insect Management for Food Storage and Processing; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–231. [Google Scholar]
- Riudavets, J.; Pons, M.J.; Messeguer, J.; Gabarra, R. Effect of CO2 modified atmosphere packaging on aflatoxin production in maize infested with Sitophilus zeamais. J. Stored Prod. Res. 2018, 77, 89–91. [Google Scholar] [CrossRef] [Green Version]
- Fields, P.; Xie, Y.; Hou, X. Repellent effect of pea (Pisum sativum) fractions against stored-product insects. J. Stored Prod. Res. 2001, 37, 359–370. [Google Scholar] [CrossRef]
- Fields, P. Effect of Pisum sativum fractions on the mortality and progeny production of nine stored-grain beetles. J. Stored Prod. Res. 2006, 42, 86–96. [Google Scholar] [CrossRef]
- CABI. Compendium: Status as Determined by CABI Editor. Available online: https://www.cabi.org/isc/datasheet/10850 (accessed on 14 June 2021).
- Harborne, J.B.; Boulter, D.; Turner, B.L. Chemotaxonomy of the Leguminosae; Academic Press: London, UK, 1971; pp. 1–612. [Google Scholar]
- Bell, E.A. Toxins in seeds. In Biochemical Aspects of Plant and Animal Coevolution; Harborne, J.B., Ed.; Academic Press: New York, NY, USA, 1978; pp. 143–161. [Google Scholar]
- Coombs, C.W.; Billings, C.J.; Porter, J.E. The effect of yellow split-peas (Pisum sativum L.) and other pulses on the productivity of certain strains of Sitophilus oryzae (L.) (Col. Curculionidae) and the ability of other strains to breed thereon. J. Stored Prod. Res. 1977, 13, 53–58. [Google Scholar] [CrossRef]
- Holloway, G.J. The potency and effect of phytotoxins within yellow split-pea (Pisum sativum) and adzuki bean (Vigna angularis) on survival and reproductive potential of Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Bull. Ent. Res. 1986, 76, 287–295. [Google Scholar] [CrossRef]
- Bodnaryk, R.; Fields, P.G.; Xie, Y.; Fulcher, K. Insecticidal Factors from Field Pea. U.S. Patent 5,955,082, 21 September 1999. [Google Scholar]
- Delobel, B.; Grenier, A.; Gueguen, J.; Ferrasson, E.; Mbailao, M. Utilisation d’un Polypeptide Dérivé d’une Albumine PA1b de légumineuse Comme Insecticide. French Patent 98,05877, 11 May 1998. [Google Scholar]
- Beleggia, R.; Platani, C.; Spano, G.; Monteleone, M.; Cattivelli, L. Metabolic profiling and analysis of volatile composition of durum wheat semolina and pasta. J. Cereal Sci. 2009, 49, 301–309. [Google Scholar] [CrossRef]
- Germinara, G.S.; Beleggia, R.; Fragasso, M.; Pistillo, M.O.; De Vita, P. Kernel volatiles of some pigmented wheats do not elicit a preferential orientation in Sitophilus granarius adults. J. Pest. Sci. 2018, 92, 653–664. [Google Scholar] [CrossRef]
- Vandendool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Co.: New York, NY, USA, 1995; pp. 1–896. [Google Scholar]
- Najar-Rodriguez, A.J.; Galizia, C.G.; Stierle, J.; Dorn, S. Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. J. Exp. Biol. 2010, 213, 3388–3397. [Google Scholar] [CrossRef] [Green Version]
- Webster, B.; Gezan, S.; Bruce, T.; Hardie, J.; Pickett, J. Between plant and diurnal variation in quantities and ratios of volatile compounds emitted by Vicia faba plants. Phytochemistry 2010, 71, 81–89. [Google Scholar] [CrossRef]
- Cha, D.H.; Linn, C.E.; Teal, P.E.A.; Zhang, A.; Roelofs, W.L.; Loeb, G.M. Eavesdropping on plant volatiles by a specialist moth: Significance of ratio and concentration. PLoS ONE 2011, 6, e17033. [Google Scholar] [CrossRef] [Green Version]
- Bruce, T.J.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects-finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Germinara, G.S.; Rotundo, G.; De Cristofaro, A.; Giacometti, R. Risposte elettroantennografiche di Sitophilus granarius (L.) e S. zeamais Motschulsky a sostanze volatili dei cereali. Tec. Molit. 2002, 53, 27–34. [Google Scholar]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Ann. Rev. Plant. Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Hubert, J.; Munzbergova, Z.; Santino, A. Plant volatile aldehydes as natural insecticides against stored-product beetles. Pest Manag. Sci. 2008, 64, 57–64. [Google Scholar] [CrossRef]
- Azarnia, S.; Boye, J.I. (Eds.) Flavour Compounds in Legumes: Chemical and Sensory Aspects. In Progress in Food Science and Technology; Nova Science Publishers: New York, NY, USA, 2011. [Google Scholar]
- Khrisanapant, P.; Kebede, B.; Leong, S.Y.; Oey, I. A Comprehensive Characterisation of Volatile and Fatty Acid Profiles of Legume Seeds. Foods 2019, 8, 651. [Google Scholar] [CrossRef] [Green Version]
- Pasqualone, A.; Paradiso, V.M.; Summo, C.; Caponio, F.; Gomes, T. Influence of Drying Conditions on Volatile Compounds of Pasta. Food Bioprocess Technol. 2014, 7, 719–731. [Google Scholar] [CrossRef]
- Germinara, G.S.; Conte, A.; De Cristofaro, A.; Lecce, L.; Di Palma, A.; Rotundo, G.; Del Nobile, M.A. Electrophysiological and behavioural activity of (E)-2-hexenal in the granary weevil and its application in food packaging. J. Food Prot. 2011, 75, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Pretheep-Kumar, P.; Mohan, S.; Ramaraju, K. Protein-enriched pea flour extract protects stored milled rice against the rice weevil, Sitophilus oryzae. J. Insect Sci. 2004, 4, 26. [Google Scholar] [CrossRef]
- Gressent, F.; Rahioui, I.; Rahbé, Y. Characterization of a high-affinity binding site for the pea albumin 1b entomotoxin in the weevil Sitophilus. Eur. J. Biochem. 2003, 30, 2429–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
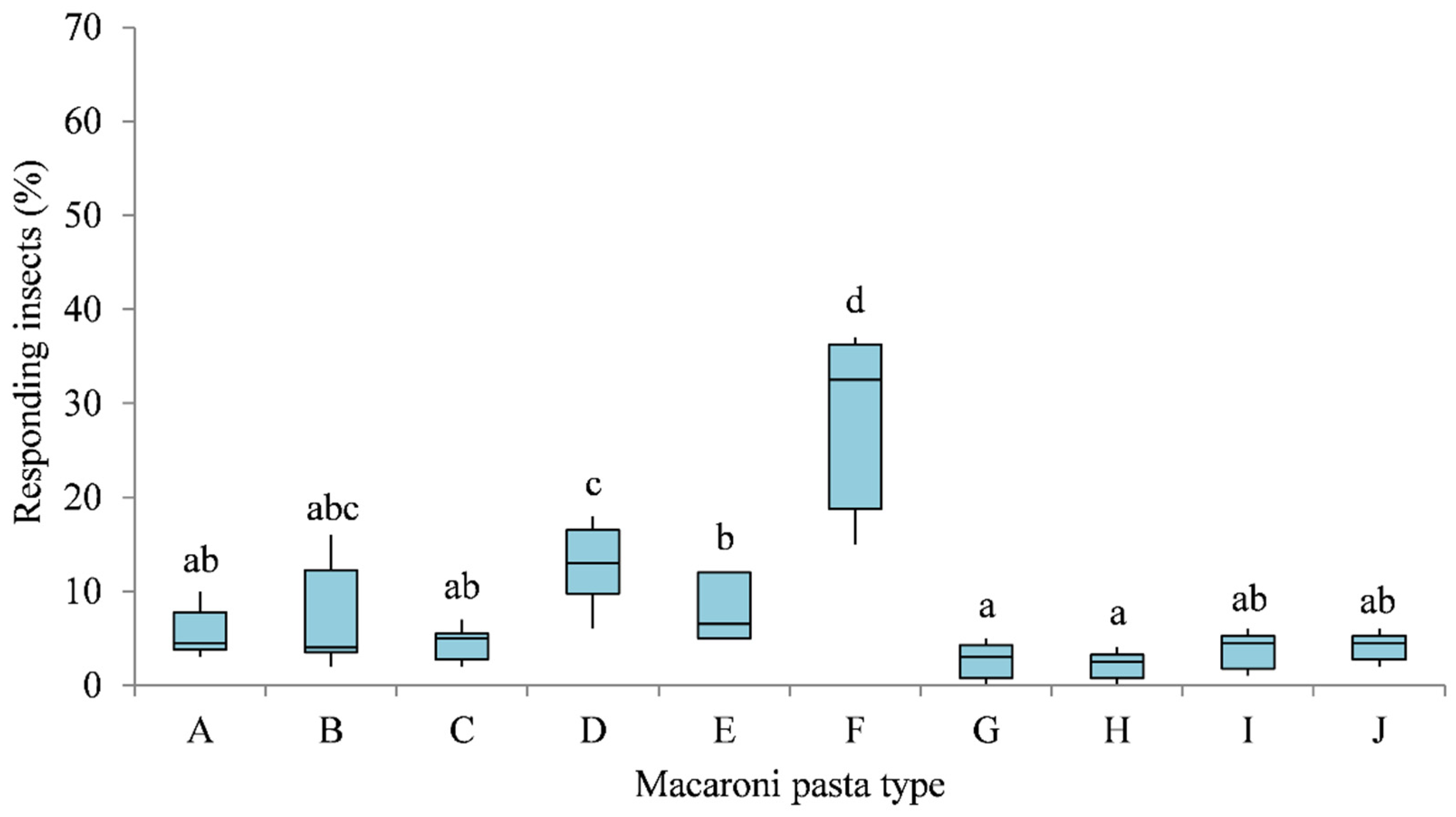
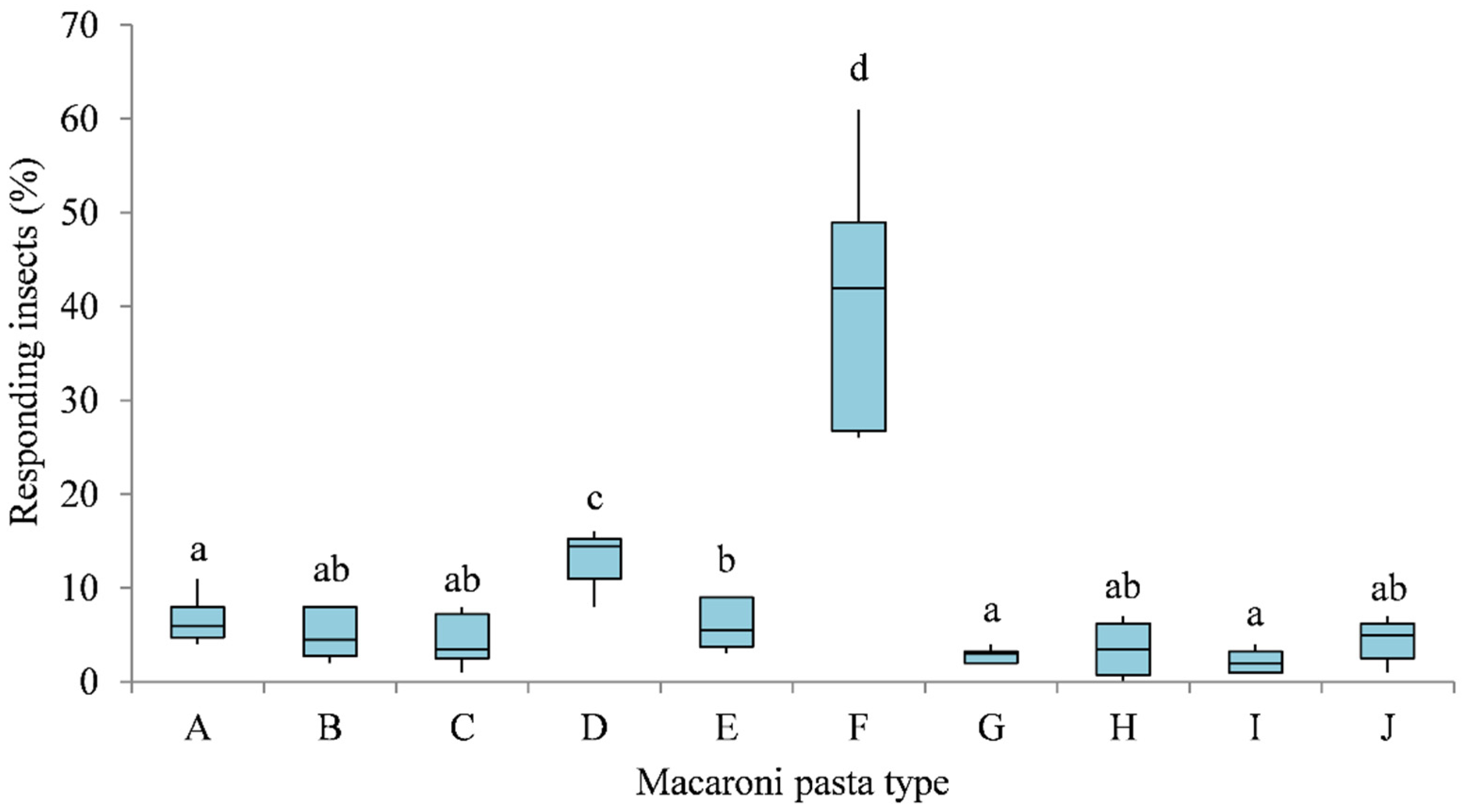
| Samples | Pasta Type | Cereal/Legume | Energy Value | Fats g/100 g | Carboydrates g/100 g | Fibers g/100 g | Proteins g/100 g | Salt g/100 g | |
|---|---|---|---|---|---|---|---|---|---|
| Cereal pasta | A | Durum wheat pasta | Triticum durum | 359 kcal 1525 kj | 1 0.3 satur. | 74.0 3.5 sugars | 3.0 | 13.5 | 0.03 |
| B | Durum whole wheat pasta | Triticum durum | 347 kcal 1468 kj | 2.20 0.5 satur. | 64.5 2.5 sugars | 6.5 | 14.0 | 0.004 | |
| C | Spelt pasta | Triticum dicoccum | 351 kcal 1485 kj | 3.1 0.5 g satur. | 65.0 sugars 3.2 | 7.0 | 12.0 | 0.06 | |
| D | Five cereals pasta | wheat, spelt, barley, maize, rye | 352 kcal 1484 kj | 2.2 0.5 satur. | 67.0 sugars 3.0 | 6.8 | 12.5 | 0.007 | |
| E | Kamut pasta | Triticum turgidum | 350 kcal 1483 kj | 1.3 0.3 satur. | 69.0 sugars 4.3 | 3.0 | 14.0 | 0.00 | |
| F | Khorasan pasta | Triticum turanicum | 346 kcal 1453 kj | 1.4 0.3 satur. | 68.0 sugars 2.5 | 4.8 | 13.0 | 0.03 | |
| Legume pasta | G | Lens pasta | Lens culinaris | 325 kcal 1375 kj | 0.3 0.2 satur. | 48.0 sugars 1.2 | 13.6 | 25.8 | 0.05 |
| H | Chickpea pasta | Cicer arietinum | 345 kcal 1457 kj | 3.1 1.6 satur. | 53.8 sugars 1.8 | 11.1 | 20.0 | 0.06 | |
| I | Pea pasta | Pisum sativum | 334 kcal 1415 kj | 0.5 0.3 satur. | 57.5 sugars 7.2 | 6.7 | 21.4 | 0.05 | |
| J | Faba bean pasta | Vicia faba | 334 kcal 1412 kj | 0.5 0.3 satur. | 54.3 sugars 2.6 | 6.3 | 24.7 | 0.05 |
| Two-Choice Bioassay | First Choice (±SE) | Second Choice (±SE) | Student’s t-Test | Response Index (±SE) | |
|---|---|---|---|---|---|
| First vs. Second Choice | t-Value | p-Value | |||
| F vs. Control | 64.50 ± 2.25 | 1.25 ± 0.25 | <0.001 | 28.111 | 63.25 ± 2.25 |
| H vs. Control | 20.00 ± 1.08 | 3.50 ± 0.65 | 0.001 | 13.863 | 16.50 ± 1.19 |
| F+H (1:1) vs. Control | 80.00 ± 1.47 | 2.00 ± 0.41 | <0.001 | 63.687 | 78.00 ± 1.22 |
| F vs. H | 73.75 ± 1.55 | 4.75 ± 0.48 | <0.001 | 35.242 | 69.00 ± 1.96 |
| F+H (1:1) vs. F+H (3:1) | 40.75 ± 2.84 | 39.25 ± 4.33 | 0.828 | 0.236 | 1.50 ± 6.34 |
| F+H (1:1) vs. F+H (1:3) | 39.00 ± 1.78 | 41.50 ± 1.32 | 0.464 | –0.837 | −2.5 ± 2.99 |
| Samples | Pasta | Percentage of Dead Adults (±SE) * | Number of Progeny Emergence |
|---|---|---|---|
| A | Durum wheat pasta | 70.00 ± 5.27 b | 0 |
| B | Durum whole wheat pasta | 76.67 ± 3.60 bc | 1 |
| C | Spelt pasta | 85.83 ± 2.50 bc | 0 |
| D | Five cereals pasta (wheat, spelt, barley, maize rye) | 68.33 ± 5.00 b | 0 |
| E | Kamut pasta | 73.33 ± 4.30 bc | 0 |
| F | Khorasan pasta | 27.50 ± 4.97 a | 10 |
| G | Lens pasta | 100.00 c | 0 |
| H | Chickpea pasta | 100.00 c | 0 |
| I | Pea pasta | 100.00 c | 0 |
| J | Faba bean pasta | 100.00 c | 0 |
| Area (%) ± S.E. 1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak No. | Compound | R.T | RIcal 2 | RIref 3 | A | B | C | D | E | F | G | H | I | J |
| Aldehydes | ||||||||||||||
| 3 | 3-Methylbutanal | 1.70 | 665 | 668 | 0.69 ± 0.01 | - | 2.11 ± 0.03 | 0.74 ± 0.01 | - | - | - | - | - | - |
| 4 | 2-Methylbutanal | 1.75 | 669 | 668 | - | 1.35 ± 0.02 | 1.08 ± 0.01 | 0.72 ± 0.01 | - | - | - | - | - | - |
| 5 | Pentanal | 1.91 | 709 | 706 | 1.07 ± 0.03 | 0.66 ± 0.03 | 1.61 ± 0.03 | 1.89 ± 0.04 | 1.43 ± 0.02 | 1.82 ± 0.07 | 0.37 ± 0.03 | 0.22 ± 0.02 | 0.51 ± 0.01 | - |
| 8 | Hexanal | 2.85 | 803 | 801 | 33.31 ± 0.64 | 32.66 ± 0.36 | 23.24 ± 0.07 | 35.4 ± 0.26 | 38.01 ± 0.08 | 38.66 ± 0.36 | 8.33 ± 0.10 | 6.00 ± 0.07 | 10.03 ± 0.08 | 8.59 ± 0.21 |
| 13 | Heptanal | 4.92 | 901 | 902 | 2.81 ± 0.03 | - | 1.14 ± 0.02 | 2.24 ± 0.05 | 1.05 ± 0.04 | 1.44 ± 0.07 | - | 0.13 ± 0.01 | 0.58 ± 0.06 | - |
| 15 | (E)-2-Heptenal | 6.45 | 957 | 954 | 3.32 ± 0.05 | - | 2.14 ± 0.02 | 2.47 ± 0.07 | 3.2 ± 0.09 | 1.82 ± 0.04 | 0.91 ± 0.01 | 0.54 ± 0.03 | 1.15 ± 0.03 | 0.65 ± 0.02 |
| 23 | Octanal | 7.87 | 996 | 998 | 1.53 ± 0.01 | 0.82 ± 0.01 | 0.66 ± 0.01 | 0.87 ± 0.02 | 1.13 ± 0.07 | 0.76 ± 0.06 | 0.41 ± 0.01 | 1.06 ± 0.03 | 1.24 ± 0.05 | - |
| 30 | (E)-2-Octenal | 9.58 | 1051 | 1054 | 1.42 ± 0.02 | 1.08 ± 0.03 | 2.93 ± 0.04 | 1.18 ± 0.05 | 1.18 ± 0.08 | 1.36 ± 0.10 | 1.65 ± 0.04 | 1.58 ± 0.03 | 0.41 ± 0.01 | 1.66 ± 0.04 |
| 34 | Nonanal | 11.00 | 1096 | 1100 | 11.64 ± 0.38 | 3.09 ± 0.05 | 2.15 ± 0.02 | 6.22 ± 0.07 | 8.87 ± 0.18 | 3.48 ± 0.02 | 1.26 ± 0.03 | 2.54 ± 0.12 | 3.70 ± 0.10 | 1.93 ± 0.03 |
| 36 | (E)-2-Nonenal | 12.75 | 1160 | 1157 | - | 1.82 ± 0.03 | 1.22 ± 0.06 | 2.77 ± 0.05 | 3.13 ± 0.07 | 1.12 ± 0.07 | 0.63 ± 0.02 | 0.69 ± 0.01 | 0.89 ± 0.02 | 0.88 ± 0.03 |
| 39 | Decanal | 14.07 | 1202 | 1201 | 2.83 ± 0.01 | 0.98 ± 0.07 | 0.71 ± 0.01 | 1.86 ± 0.04 | 2.81 ± 0.12 | 1.04 ± 0.10 | 0.27 ± 0.03 | 0.67 ± 0.03 | 0.59 ± 0.02 | 0.71 ± 0.01 |
| 40 | 2,4 Nonadienal | 14.30 | 1215 | 1217 | - | 0.48 ± 0.01 | 0.34 ± 0.01 | 0.16 ± 0.01 | 0.22 ± 0.01 | 0.18 ± 0.05 | 0.07 ± 0.01 | 0.16 ± 0.02 | - | - |
| 46 | 2-Butyl-2-octenal | 18.72 | 1353 | 1360 | 0.47 ± 0.01 | 0.91 ± 0.02 | 0.81 ± 0.06 | 0.64 ± 0.02 | 0.73 ± 0.02 | 2.50 ± 0.09 | - | 0.21 ± 0.01 | - | - |
| Total aldehydes | 59.09 ± 1.15 | 43.85 ± 0.58 | 40.14 ± 0.69 | 57.16 ± 0.62 | 61.76 ± 0.71 | 54.18 ± 1.01 | 13.9 ± 0.25 | 13.8 ± 0.31 | 19.1 ± 0.35 | 14.42 ± 0.30 | ||||
| Alcohols | ||||||||||||||
| 6 | 3-Methylbutanol | 2.15 | 638 | 740 | - | - | 0.25 ± 0.02 | - | - | - | - | 0.15 ± 0.03 | 0.53 ± 0.03 | 0.60 ± 0.02 |
| 7 | 1-Pentanol | 2.44 | 765 | 771 | 2.02 ± 0.08 | 1.95 ± 0.02 | 2.15 ± 0.03 | 2.47 ± 0.05 | 3.07 ± 0.04 | 4.85 ± 0.05 | 3.28 ± 0.11 | 4.08 ± 0.04 | 4.24 ± 0.07 | 2.43 ± 0.11 |
| 9 | 1-Hexanol | 4.11 | 867 | 870 | 1.89 ± 0.02 | 3.3 ± 0.05 | 3.92 ± 0.04 | 1.25 ± 0.05 | 1.99 ± 0.07 | 2.57 ± 0.21 | 42.09 ± 0.51 | 38.22 ± 0.24 | 45.06 ± 0.24 | 39.97 ± 0.22 |
| 12 | 2-Heptanol | 4.87 | 891 | 896 | 2.23 ± 0.05 | 1.53 ± 0.02 | 0.76 ± 0.01 | - | 0.71 ± 0.29 | 0.78 ± 0.02 | 2.27 ± 0.08 | 0.86 ± 0.03 | 1.83 ± 0.03 | 0.86 ± 0.03 |
| 17 | 1-Heptanol | 6.86 | 961 | 966 | - | 0.18 ± 0.01 | 0.36 ± 0.01 | - | - | 0.73 ± 0.04 | 1.93 ± 0.03 | 3.00 ± 0.07 | 2.30 ± 0.10 | 0.75 ± 0.02 |
| 18 | 1-Octen-3-ol | 7.15 | 969 | 971 | 3.81 ± 0.04 | 2.39 ± 0.02 | 3.48 ± 0.02 | 4.13 ± 0.07 | 3.42 ± 0.09 | 3.71 ± 0.02 | 6.81 ± 0.01 | 2.47 ± 0.14 | 5.19 ± 0.10 | 5.24 ± 0.05 |
| 21 | 3-Octanol | 7.65 | 987 | 991 | - | - | - | - | - | - | 0.52 ± 0.02 | - | 0.62 ± 0.02 | 0.25 ± 0.02 |
| 22 | 2-Octanol | 7.80 | 984 | 994 | 1.83 ± 0.03 | 0.66 ± 0.02 | 0.52 ± 0.01 | 0.86 ± 0.02 | 1.43 ± 0.06 | 1.45 ± 0.03 | 1.15 ± 0.06 | 0.70 ± 0.02 | 0.87 ± 0.03 | 0.69 ± 0.02 |
| 26 | 2-Ethylhexanol | 8.68 | 1036 | 1038 | 0.62 ± 0.01 | 0.75 ± 0.02 | 1.21 ± 0.05 | - | - | - | - | 0.12 ± 0.00 | - | 0.59 ± 0.02 |
| 31 | (E)-2-Octenol | 9.91 | 1057 | 1060 | 0.22 ± 0.01 | - | - | 0.31 ± 0.01 | - | - | 0.56 ± 0.03 | 0.55 ± 0.03 | 0.93 ± 0.03 | 0.42 ± 0.02 |
| 32 | 1-Octanol | 9.99 | 1059 | 1063 | - | 0.42 ± 0.01 | - | 0.43 ± 0.02 | 0.65 ± 0.03 | 0.94 ± 0.03 | 1.33 ± 0.02 | 3.86 ± 0.05 | 2.82 ± 0.08 | 0.68 ± 0.03 |
| 33 | 2,5-Dimethylcyclohexanol | 10.68 | 1084 | 1099 | - | - | - | - | 0.52 ± 0.06 | 2.03 ± 0.08 | 0.23 ± 0.02 | 0.85 ± 0.03 | 0.23 ± 0.01 | - |
| 37 | 1-Nonanol | 13.05 | 1158 | 1165 | - | - | - | - | - | - | 0.98 ± 0.07 | 2.28 ± 0.09 | 1.21 ± 0.05 | 1.00 ± 0.06 |
| Total alcohols | 12.62 ± 0.19 | 11.18 ± 0.15 | 12.65 ± 0.16 | 9.45 ± 0.20 | 11.79 ± 0.58 | 17.06 ± 0.41 | 61.15 ± 0.93 | 57.14 ± 0.72 | 65.83 ± 0.77 | 53.48 ± 0.58 | ||||
| Ketones | ||||||||||||||
| 1 | 2-Propanone | 1.33 | 481 | 487 | - | 2.43 ± 0.07 | 0.76 ± 0.01 | 1.42 ± 0.02 | 1.72 ± 0.01 | 0.52 ± 0.02 | 0.26 ± 0.03 | - | - | 0.48 ± 0.01 |
| 10 | 2-Heptanone | 4.63 | 881 | 889 | - | - | 2.09 ± 0.05 | 0.92 ± 0.03 | - | - | - | - | 0.49 ± 0.02 | - |
| 28 | 3-Octen-2-one | 8.99 | 1027 | 1030 | - | 0.15 ± 0.01 | 0.42 ± 0.01 | - | 0.33 ± 0.01 | 1.77 ± 0.07 | 0.55 ± 0.03 | 1.53 ± 0.67 | 1.08 ± 0.08 | 0.41 ± 0.30 |
| Total ketones | 0 | 2.58 ± 0.05 | 3.27 ± 0.04 | 2.34 ± 0.04 | 2.05 ± 0.01 | 2.29 ± 0.07 | 0.81 ± 0.05 | 1.53 ± 0.67 | 1.57 ± 0.06 | 0.89 ± 0.26 | ||||
| Terpenes | ||||||||||||||
| 14 | α-Pinene | 5.80 | 931 | 939 | - | 0.41 ± 0.01 | 0.23 ± 0.01 | 0.54 ± 0.03 | - | - | 0.27 ± 0.01 | 0.20 ± 0.01 | 0.50 ± 0.03 | 1.69 ± 0.03 |
| 19 | Sulcatone | 7.38 | 973 | 974 | 2.20 ± 0.06 | 2.08 ± 0.04 | 1.39 ± 0.01 | 1.53 ± 0.02 | 0.54 ± 0.06 | 0.80 ± 0.04 | 0.63 ± 0.03 | - | 0.21 ± 0.01 | 0.34 ± 0.01 |
| 25 | Limonene | 8.66 | 1028 | 1031 | - | 0.95 ± 0.02 | 0.34 ± 0.02 | 1.09 ± 0.03 | 0.54 ± 0.06 | 0.46 ± 0.05 | 0.43 ± 0.03 | 0.13 ± 0.01 | - | 0.72 ± 0.01 |
| 49 | Geranyl acetone | 20.71 | 1451 | 1455 | 0.65 ± 0.02 | 0.5 ± 0.03 | 0.52 ± 0.01 | 0.64 ± 0.02 | 1.06 ± 0.04 | - | 0.13 ± 0.01 | - | - | 0.08 ± 0.01 |
| Total terpenes | 2.85 ± 0.05 | 3.94 ± 0.09 | 2.48 ± 0.04 | 3.80 ± 0.08 | 2.14 ± 0.11 | 1.26 ± 0.06 | 1.46 ± 0.06 | 0.33 ± 0.49 | 0.71 ± 0.07 | 2.83 ± 0.29 | ||||
| Aromatics | ||||||||||||||
| 11 | Styrene | 4.67 | 874 | 890 | 2.86 ± 0.04 | 3.25 ± 0.06 | 4.36 ± 0.04 | 1.17 ± 0.05 | 1.97 ± 0.07 | 1.20 ± 0.11 | 3.4 ± 0.04 | 4.40 ± 0.12 | - | 9.51 ± 0.14 |
| 16 | Benzaldehyde | 6.57 | 958 | 960 | 9.24 ± 0.24 | 3.51 ± 0.05 | 3.6 ± 0.02 | 6.04 ± 0.11 | 7.78 ± 0.07 | 4.62 ± 0.09 | 0.24 ± 0.01 | 0.23 ± 0.03 | 0.47 ± 0.02 | 0.49 ± 0.02 |
| 27 | Benzyl alcohol | 8.83 | 1027 | 1031 | 0.41 ± 0.01 | - | 0.76 ± 0.02 | - | 0.23 ± 0.02 | 2.45 ± 0.07 | 0.55 ± 0.02 | 0.35 ± 0.02 | 0.34 ± 0.02 | 1.20 ± 0.06 |
| 35 | Phenethyl alcohol | 11.29 | 1112 | 1116 | - | - | - | - | - | - | 0.22 ± 0.01 | 0.41 ± 0.01 | 0.31 ± 0.01 | 1.63 ± 0.04 |
| Total aromatics | 12.51 ± 0.22 | 6.76 ± 0.08 | 8.72 ± 0.07 | 7.21 ± 0.13 | 9.98 ± 0.10 | 8.27 ± 0.21 | 4.41 ± 0.07 | 5.39 ± 0.15 | 1.12 ± 0.03 | 12.83 ± 0.21 | ||||
| Lactones | ||||||||||||||
| 29 | Gamma-Hexalactone | 9.44 | 1048 | 1056 | - | 0.26 ± 0.01 | 0.42 ± 0.01 | - | - | - | 0.85 ± 0.03 | 0.72 ± 0.01 | 0.64 ± 0.03 | 0.24 ± 0.02 |
| 41 | Gamma-octalactone | 15.56 | 1243 | 1250 | - | - | - | - | - | - | 0.55 ± 0.01 | 0.49± 0.04 | 0.47 ± 0.05 | - |
| 45 | Gamma-nonalactone | 18.44 | 1358 | 1361 | - | - | - | - | - | - | 1.12 ± 0.07 | 4.23 ± 0.04 | 2.34 ± 0.13 | 0.47 ± 0.03 |
| Total lactones | 0 | 0.26 ± 0.01 | 0.42 ± 0.01 | 0.00 | 0.00 | 0.00 | 7.12 ± 0.08 | 5.44 ± 0.06 | 3.45 ± 0.15 | 0.71 ± 0.03 | ||||
| Furans | ||||||||||||||
| 20 | 2-Pentylfuran | 7.52 | 981 | 988 | 3.54 ± 0.08 | 11.88 ± 0.46 | 12.20 ± 0.07 | 9.20 ± 0.13 | 2.37 ± 0.12 | 6.10 ± 0.12 | 7.41 ± 0.08 | 9.49 ± 0.08 | 3.27 ± 0.09 | 1.63 ± 0.05 |
| Hydrocarbons | ||||||||||||||
| 38 | Dodecane | 13.88 | 1192 | 1200 | - | 1.17 ± 0.01 | 0.82 ± 0.02 | 0.48 ± 0.03 | 0.22 ± 0.02 | 0.41 ± 0.04 | 0.24 ± 0.03 | 0.13 ± 0.01 | 0.07 ± 0.01 | 0.53 ± 0.01 |
| 42 | 2,6,11-Trimethyldodecane | 16.18 | 1269 | 1275 | 0.74 ± 0.02 | 1.62 ± 0.02 | 2.25 ± 0.07 | 0.81 ± 0.02 | 0.84 ± 0.03 | 0.54 ± 0.07 | 0.99 ± 0.06 | 0.26 ± 0.02 | 0.04 ± 0.01 | 1.85 ± 0.04 |
| 44 | Tridecane | 16.73 | 1287 | 1300 | 1.55 ± 0.02 | 7.37 ± 0.34 | 5.68 ± 0.1 | 2.15 ± 0.07 | 0.35 ± 0.03 | 1.04 ± 0.12 | 0.25 ± 0.03 | 0.66 ± 0.03 | 0.20 ± 0.01 | 0.72 ± 0.01 |
| 48 | Tetradecane | 19.37 | 1396 | 1400 | - | 0.67 ± 0.02 | 0.62 ± 0.01 | 0.49 ± 0.04 | 0.39 ± 0.02 | 0.36 ± 0.06 | 0.22 ± 0.02 | 0.14 ± 0.03 | 0.21 ± 0.02 | 0.89 ± 0.04 |
| 50 | Pentadecene | 21.71 | 1488 | 1492 | - | 1.07 ± 0.01 | 1.12 ± 0.03 | 1.45 ± 0.04 | 0.87 ± 0.03 | 0.76 ± 0.04 | 0.22 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.65 ± 0.02 |
| Total hydrocarbons | 2.29 ± 0.08 | 11.90 ± 0.69 | 10.49 ± 0.15 | 5.38 ± 0.18 | 2.67 ± 0.11 | 3.11 ± 0.21 | 1.92 ± 0.13 | 1.23 ± 0.08 | 0.58 ± 0.05 | 4.64 ± 0.09 | ||||
| Others | ||||||||||||||
| 2 | Acetic acid | 1.48 | 623 | 633 | - | - | 2.02 ± 0.01 | - | - | - | - | 0.52 ± 0.01 | - | 1.43 ± 0.02 |
| 24 | Hexyl acetate | 8.22 | 1001 | 1007 | - | - | - | - | - | 0.78 ± 0.05 | 0.33 ± 0.03 | 1.57 ± 0.06 | 0.44 ± 0.03 | 0.13 ± 0.01 |
| 43 | Hexanoic acid, pentyl ester | 16.42 | 1274 | 1282 | 0.51 ± 0.01 | 0.77 ± 0.03 | 0.62 ± 0.01 | 0.47 ± 0.03 | 0.33 ± 0.02 | 0.47 ± 0.05 | 0.35 ± 0.02 | 0.48 ± 0.01 | 0.17 ± 0.01 | 0.54 ± 0.03 |
| 47 | Hexanoic acid, hexyl ester | 19.04 | 1374 | 1383 | 0.5 ± 0.01 | - | - | - | - | - | 0.13 ± 0.01 | 0.72 ± 0.01 | - | - |
| Total others | 1.01 ± 0.01 | 0.77 ± 0.03 | 2.64 ± 0.01 | 0.47± 0.03 | 0.33 ± 0.02 | 1.25 ± 0.07 | 0.81 ± 0.08 | 3.29 ± 0.04 | 0.61 ± 0.05 | 2.10 ±0.02 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trematerra, P.; Pistillo, O.M.; Germinara, G.S.; Colacci, M. Bioactivity of Cereal- and Legume-Based Macaroni Pasta Volatiles to Adult Sitophilus granarius (L.). Insects 2021, 12, 765. https://doi.org/10.3390/insects12090765
Trematerra P, Pistillo OM, Germinara GS, Colacci M. Bioactivity of Cereal- and Legume-Based Macaroni Pasta Volatiles to Adult Sitophilus granarius (L.). Insects. 2021; 12(9):765. https://doi.org/10.3390/insects12090765
Chicago/Turabian StyleTrematerra, Pasquale, Onofrio Marco Pistillo, Giacinto Salvatore Germinara, and Marco Colacci. 2021. "Bioactivity of Cereal- and Legume-Based Macaroni Pasta Volatiles to Adult Sitophilus granarius (L.)" Insects 12, no. 9: 765. https://doi.org/10.3390/insects12090765
APA StyleTrematerra, P., Pistillo, O. M., Germinara, G. S., & Colacci, M. (2021). Bioactivity of Cereal- and Legume-Based Macaroni Pasta Volatiles to Adult Sitophilus granarius (L.). Insects, 12(9), 765. https://doi.org/10.3390/insects12090765








