A New Look at Cultivar Preference in Hoplocampa testudinea (Hymenoptera: Tenthredinidae) on Apple in the Annapolis Valley of Nova Scotia, Canada
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Orchard Blocks
2.2. Damage Assessments: 2010–2014 and 2015–2019
2.3. Oviposition Preference
2.4. Bagging Study
2.5. Data Analyses
3. Results
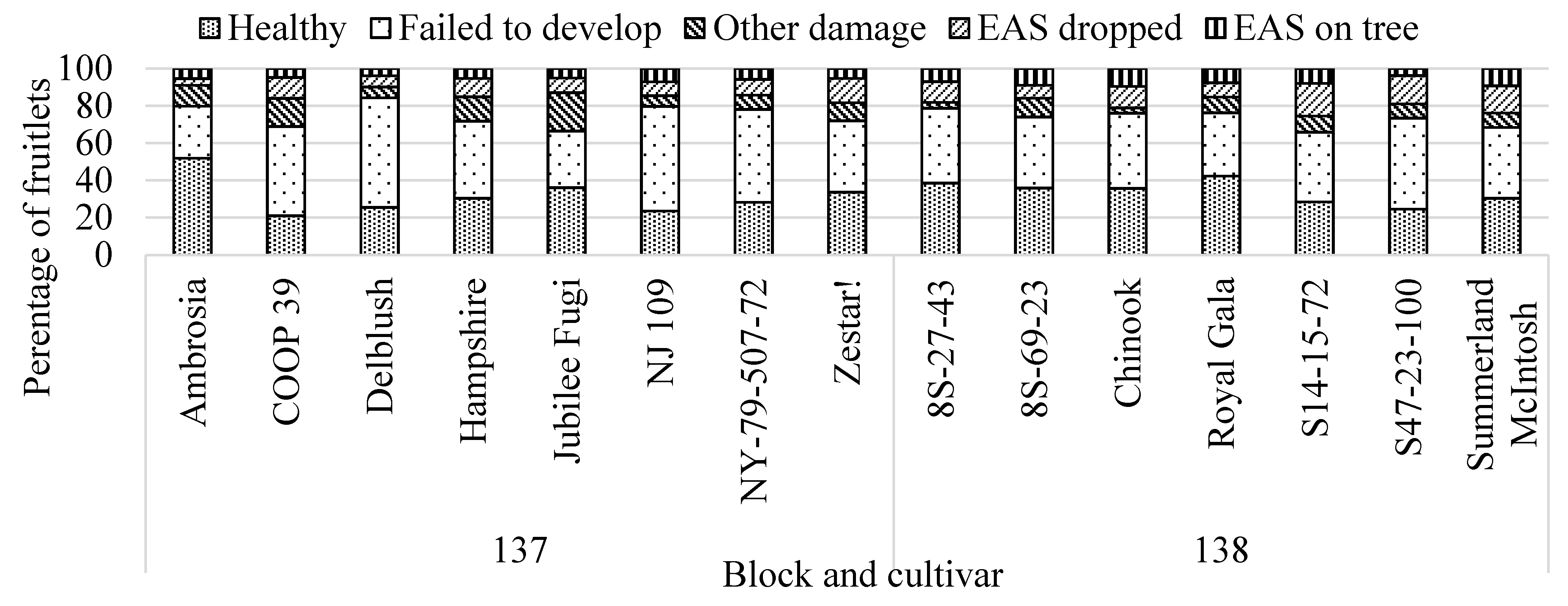
3.1. Damage Assessment at Harvest
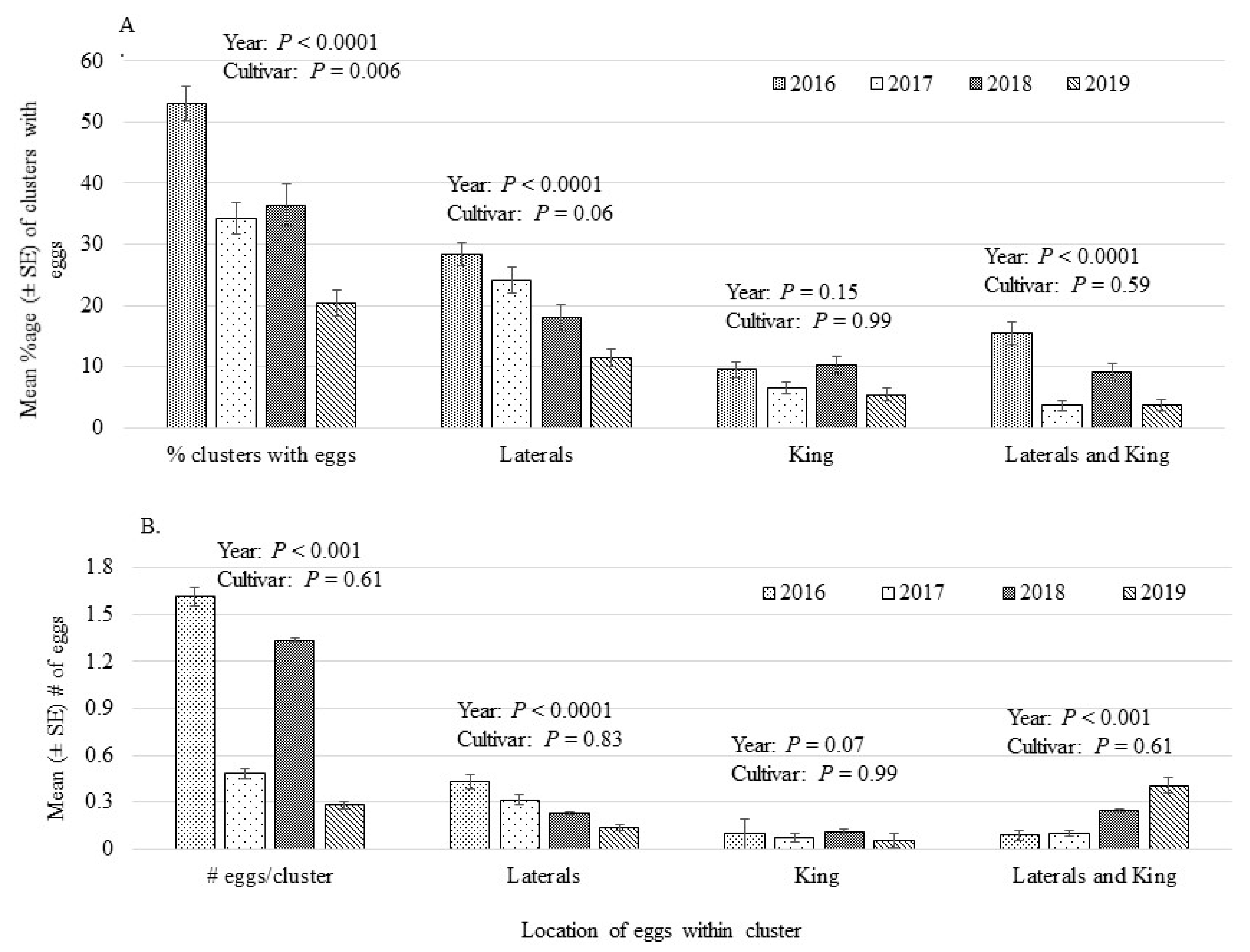
3.2. Oviposition Preference
3.3. Bagging Study
3.3.1. Fruitlet Assessments
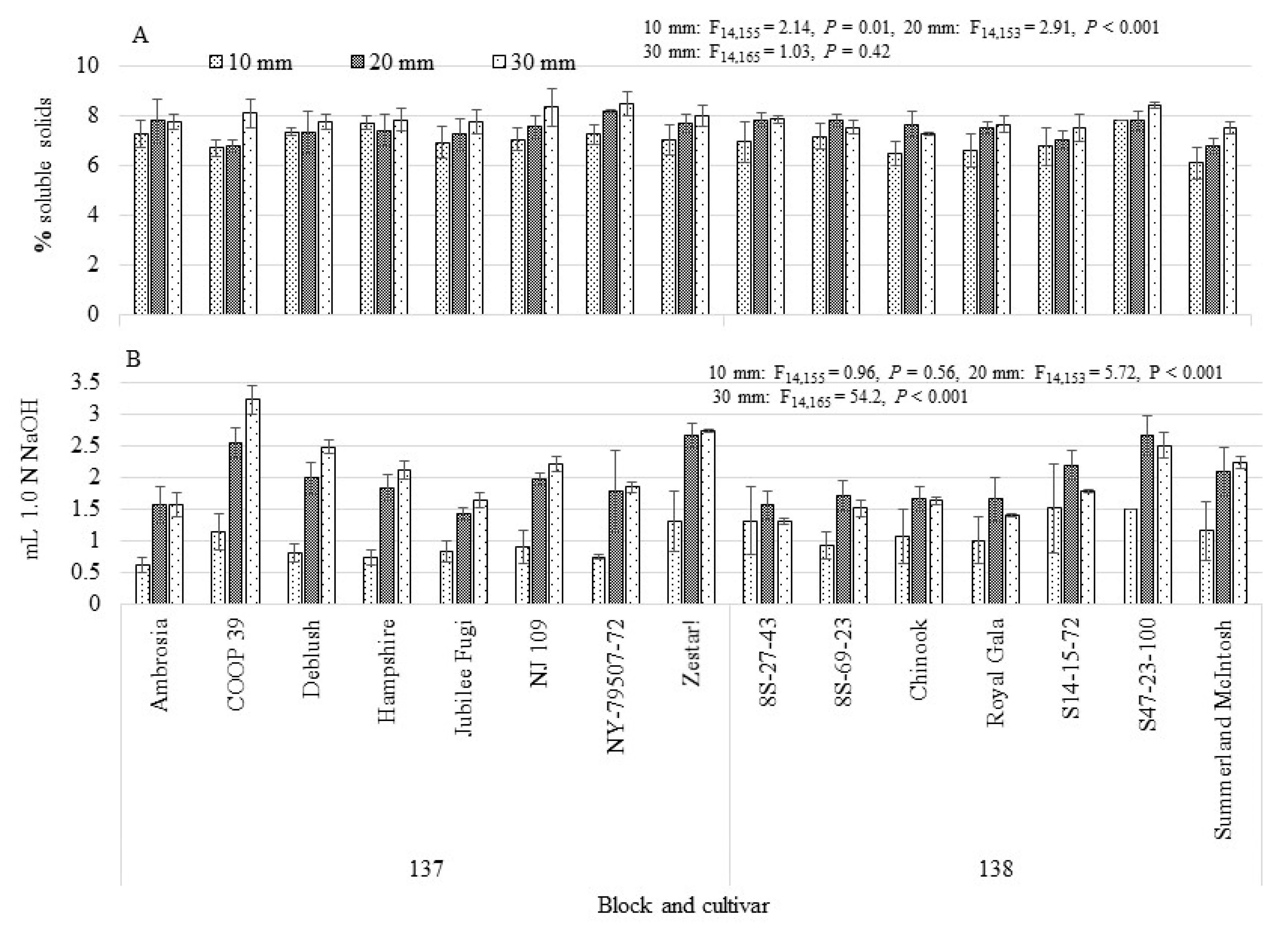
3.3.2. Fruitlet Chemistry
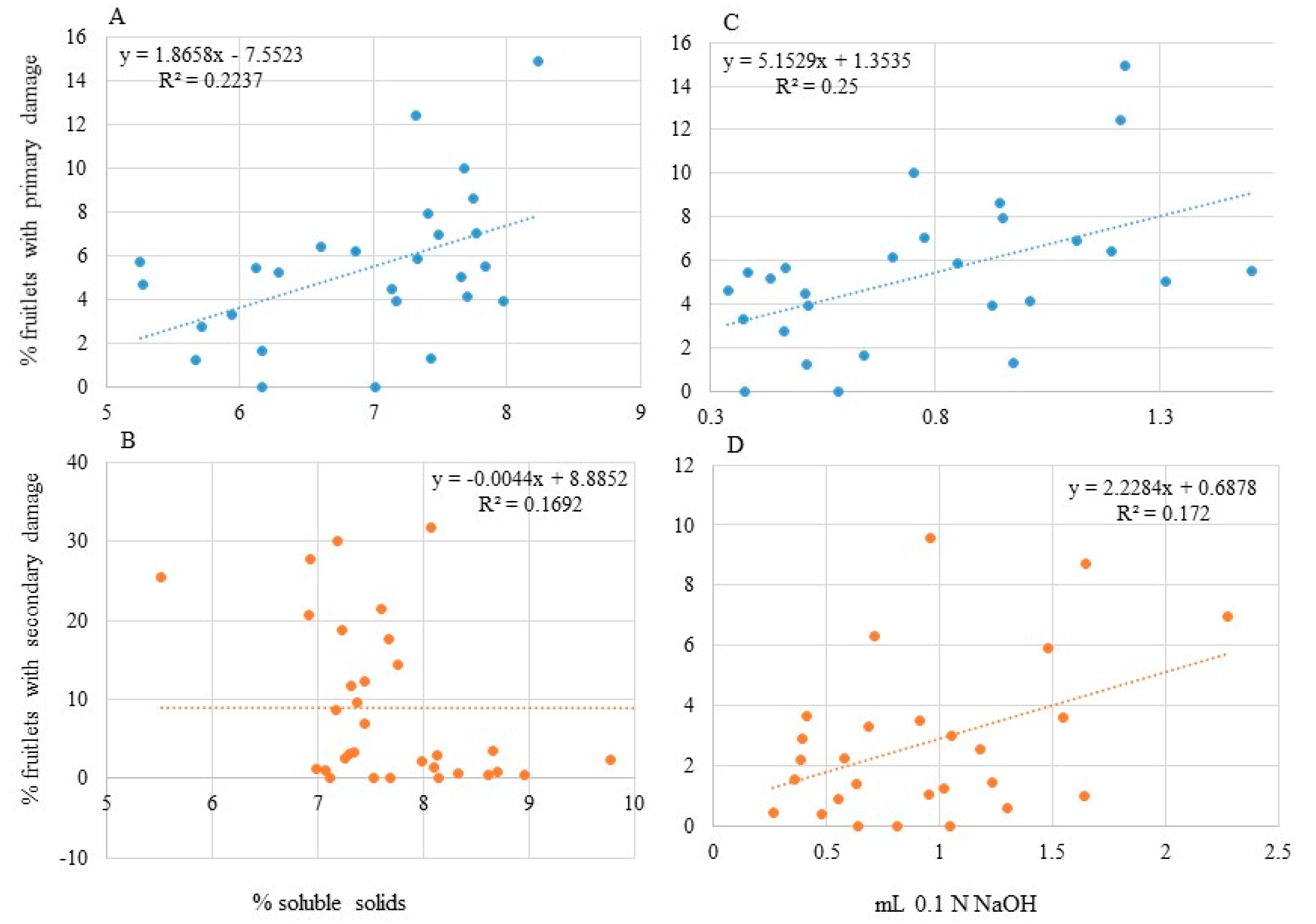
3.4. Correlations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velusamy, R.; Heinrichs, E.A. Tolerance in crop plants to insect pests. Int. J. Trop. Insect Sci. 1986, 7, 689–696. [Google Scholar] [CrossRef]
- Sharma, H.C.; Ortiz, R. Host plant resistance to insects: An eco-friendly approach for pest management and environment conservation. J. Environ. Biol. 2002, 23, 111–135. [Google Scholar] [PubMed]
- Cook, S.M.; Smart, L.E.; Martin, J.L.; Murray, D.A.; Watts, N.P.; Williams, I.H. Exploitation of host plant preferences in pest management strategies for oilseed rape (Brassica napus). Entomol. Exp. App. 2006, 119, 221–229. [Google Scholar] [CrossRef]
- Romeis, J.; Shelton, A.M.; Kennedy, G.G. Progress in Biological Control, Volume 5: Integration of Insect-Resistant Genetically Modified Crops within IPM Programs; Springer Science and Business Media B.V.: Dordrecht, The Netherlands, 2008; pp. 1–27. [Google Scholar]
- Mody, K.; Collatz, J.; Dorn, S. Plant genotype and the preference and performance of herbivores: Cultivar affects apple resistance to the florivorous weevil. Agric. For. Entomol. 2015, 17, 337–346. [Google Scholar] [CrossRef]
- Vincent, C.; Babendreier, D.; Swiergiel, W.; Helsen, H.; Blommers, L.H. A review of the apple sawfly, Hoplocampa testudinea (Hymenoptera Tenthredinidae). Bull. Insectol. 2019, 72, 35–54. [Google Scholar]
- Downes, W.; Andison, H. The apple sawfly Hoplocampa testudinea Klug on Vancouver Island, British Columbia. J. Entomol. Soc. BC 1942, 39, 13–16. [Google Scholar]
- Downes, W. Recent experimental work on the control of the apple sawfly, Hoplocampa testudinea (Hymenoptera: Tenthredinidae). Entomol. Soc. BC Proc. 1944, 41, 29–30. [Google Scholar]
- Pyenson, L. A destructive apple sawfly new to North America. J. Econ. Entomol. 1943, 36, 218–221. [Google Scholar] [CrossRef]
- Paradis, R.O. L’hoplocampe des pommes, Hoplocampa testudinea (Klug) (Hymenoptera, Tenthredinidae) au Québec. Phytoprotection 1980, 61, 26–29. [Google Scholar]
- Weires, R.W. European Apple Sawfly. 1991. Available online: http://nysipm.cornell.edu/factsheets/treefruit/pests/eas/eas.asp (accessed on 18 July 2012).
- Vincent, C.; Babendreier, D.; Kuhlmann, U. “Hoplocampa Testudinea (Klug)” Biological Control Programmes in Canada, 1981–2000; CABI Publishing: Wallingford, UK, 2002. [Google Scholar]
- Vincent, C. Management of the European Apple Sawfly (Hoplocampa Testudinea) Using A Parasitic Wasp (Lathrolestes Ensator). 2011. Available online: http://www4.agr.gc.ca/AAFC-AAC/display-afficher.do?id=1296664889042&lang=eng (accessed on 29 September 2012).
- Boevé, J. Chemecology of larvae of the European apple sawfly. Entomol. Exp. Appl. 1996, 80, 286–288. [Google Scholar] [CrossRef]
- Ontario Ministry of Agrciulture, Food and Rural Affairs. European Apple Sawfly. In Integrated Pest Management for Apples; Ontario Ministry of Agriculture, Food and Rural Affairs, Service, Ontario Publications: Toronto, ON, Canada, 2011. [Google Scholar]
- Miles, H.W. On the biology of the apple sawfly Hoplocampa testudinea Klug. Ann. App. Biol. 1932, 19, 420–431. [Google Scholar] [CrossRef]
- Hull, L.A.; Pfeiffer, D.G.; Biddinger, D.J. Insects and mites: Apple direct pests. In Mid-Atlantic Orchard Monitoring Guide; Hogmire, H., Ed.; Plants and Life Science Publishing: Ithaca, NY, USA, 1995; pp. 14–15. [Google Scholar]
- Tamošiūnas, R.; Valiuškaitė, A. The study of temperature sum model for predicting apple sawfly spring emergence and flight intensity in Lithuania. Sodininkystėir Daržininkystė; 2013, 32, 23–37. [Google Scholar]
- Briggs, J.B.; Alston, F.H. Sources of pest resistance in apple cultivars. East Malling Res. Sta. Annu. Rep. 1969, 56, 159–162. [Google Scholar]
- Alford, D.V. Apple sawfly [revised]. Ministry of Agriculture, Fisheries and Food (Great Britain). Advis. Leafl. 1973, 13, 1–5. [Google Scholar]
- Hogmire, H.W.; Miller, S.S. Relative Susceptibility of New Apple Cultivars to Arthropod Pests. HortScience 2005, 40, 2071–2075. [Google Scholar] [CrossRef] [Green Version]
- Burgart, C.; Hillier, N.K.; Blatt, S. Apple cultivar preference by Hoplocampa testudinea in the Annapolis Valley of Nova Scotia. Can. Entomol. 2016, 148, 724–735. [Google Scholar] [CrossRef]
- Dicker, J.H.L. Some notes on the biology of the apple sawfly (Hoplocampa testudinea Klug). J. Hort. Sci. 1954, 28, 238–245. [Google Scholar] [CrossRef]
- Stoeckli, S.; Mody, K.; Dorn, S.; Kellerhals, M. Association between Herbivore Resistance and Fruit Quality in Apple. HortScience 2011, 46, 12–15. [Google Scholar] [CrossRef]
- Levins, R.; MacArthur, R. An Hypothesis to Explain the Incidence of Monophagy. Ecology 1969, 50, 910–911. [Google Scholar] [CrossRef]
- Jaenike, J. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 1978, 14, 350–356. [Google Scholar] [CrossRef]
- Jafarian, F.; Jafari, S.; Fathipour, Y. Evaluation of antibiosis resistance in seven apple cultivars to Eotetranychus frosti (Tetranychidae). Syst. Appl. Acarol. 2020, 25, 525–537. [Google Scholar] [CrossRef]
- Nugnes, F.; Gualtieri, L.; Bonsignore, C.P.; Parillo, R.; Annarumma, R.; Griffo, R.; Bernardo, U. Resistance of a Local Ecotype of Castanea sativa to Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in Southern Italy. Forests 2018, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Venables, W.N.; Ripley, B.D. Package MASS. Available online: http://www.r-project.org (accessed on 17 October 2012).
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]
- Mphosi, M.S.; Foster, S.P. Female preference and larval performance of sunflower moth, Homoeosoma electellum, on sunflower pre-breeding lines. Èntomol. Exp. Appl. 2010, 134, 182–190. [Google Scholar] [CrossRef]
- Balagawi, S.; Drew, R.A.I.; Clarke, A.R. Simultaneous tests of the preference-performance and phylogenetic conservatism hypotheses: Is either theory useful? Arthropod Plant Interact. 2013, 7, 299–313. [Google Scholar] [CrossRef]
- Cantelo, W.W.; Sanford, L.L. Insect Population Response to Mixed and Uniform Plantings of Resistant and Susceptible Plant Material. Environ. Èntomol. 1984, 13, 1443–1445. [Google Scholar] [CrossRef]
- Butler, G.D., Jr.; Henneberry, T.J.; Wilson, F.D. Bemisia tabaci (Homoptera: Aleyrodidae) on cotton: Adult activity and cultivar oviposition preference. J. Econ. Entomol. 1986, 79, 350–354. [Google Scholar] [CrossRef]
- Cohen, M.B.; Alam, S.N.; Medina, E.B.; Bernal, C.C. Brown planthopper, Nilaparvata lugens, resistance in rice cultivar IR64: Mechanism and role in successful N. lugens management in Central Luzon, Philippines. Èntomol. Exp. Appl. 1997, 85, 221–229. [Google Scholar] [CrossRef]
- Smith, C.M.; Chuang, W.-P. Plant resistance to aphid feeding: Behavioral, physiological, genetic and molecular cues regulate aphid host selection and feeding. Pest Manag. Sci. 2014, 70, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Aluga, M.; Arredondo, J.; Diaz-Fleischer, F.; Birke, A.; Rull, J.; Niogret, J.; Epsky, N. Susceptibility of 15 mango (Sapindales: Anacardiaceae) cultivars to the attack by Anastrepha ludens and Anastrepha obliqua (Diptera: Tephritidae) and the role of underdeveloped fruit as pest reservoirs: Management implications. J. Econ. Entomol. 2014, 107, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akköprü, E.P.; Atlihan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphidididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef]
- Li, T.; Blande, J.; Holopainen, J. Atmospheric transformation of plant volatiles disrupts host plant finding. Sci. Rep. 2016, 6, srep33851. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. Available online: www.ajevonline.org/node/5320.full.print (accessed on 24 June 2021).
- Balagawi, S.; Vijaysegaran, S.; Drew, R.A.; Raghu, S. Influence of fruit traits on oviposition preference and offspring performance of Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) on three tomato (Lycopersicon lycopersicum) cultivars. Aust. J. Èntomol. 2005, 44, 97–103. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, J.; Yan, Z.; Ma, Y.; Yang, M.; Zhang, M.; Zhang, Z.; Qin, L.; Cao, Q. Host Preference and Performance of the Yellow Peach Moth (Conogethes punctiferalis) on Chestnut Cultivars. PLoS ONE 2016, 11, e0157609. [Google Scholar] [CrossRef] [PubMed]
- Kalinová, B.; Stránský, K.; Harmatha, J.; Čtvrtečka, R.; Žd’árek, J. Can chemical cues from blossom buds influence cultivar preference in the apple blossom weevil (Anthonomus pomorum)? Entomol. Exp. Appl. 2000, 95, 47–52. [Google Scholar] [CrossRef]
- Owens, E.D.; Prokopy, R.J. Visual Monitoring Trap for European Apple Sawfly. J. Econ. Èntomol. 1978, 71, 576–578. [Google Scholar] [CrossRef]
- Roitberg, B.D.; Prokopy, R.J. Oviposition behavior and egg distribution of the European apple sawfly. J. N. Y. Entomol. Soc. 1979, 88, 69. [Google Scholar]
- Craig, T.P.; Itami, J.K.; Price, P.W. Plant wound compounds from oviposition scars used in host discrimination by a stem-galling sawfly. J. Insect Behav. 1988, 1, 343–356. [Google Scholar] [CrossRef]
- Roitberg, B.; Prokopy, R.J. Host discrimination by adult and larval European apple sawflies Hoplocampa testudinea (Klug) (Hymenoptera: Tenthredinidae). Environ. Entomol. 1984, 13, 1000–1003. [Google Scholar] [CrossRef]
- Kasap, I. Effect of apple cultivar and of temperature on the biology and life table parameters of the two spotted spider mite Tetranychus urticae. Phytoparasitica 2004, 32, 73–82. [Google Scholar] [CrossRef]
- Altman, P.L.; Dittmer, D.S. Metabolism; Federal American Society of Experimental Biology: Bethesda, MD, USA, 1968; pp. 148–167. [Google Scholar]
- Heron, R.J. The role of chemotactic stimuli in the feeding behaviour of spruce budworm on white spruce. Can. J. Zool. 1965, 43, 247–269. [Google Scholar] [CrossRef]
- Harvey, G.T. Nutritional studies of eastern spruce budworm (lepidoptera: Tortricidae): I. soluble sugars. Can. Èntomol. 1974, 106, 353–365. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Papadopoulos, N.T.; Nanos, G.D. Survival and development of immature stages of the Mediterranean fruit fly (Diptera: Tephritidae) in citrus fruit. J. Econ. Entomol. 2008, 101, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Leszczyǹski, B.; Warchoł, J.; Niraz, S. The influence of phenolic compounds on the preference of winter wheat cultivars by cereal aphids. Insect Sci. Appl. 1985, 6, 157–158. [Google Scholar] [CrossRef]
- Nottingham, S.F.; Son, K.-C.; Wilson, D.D.; Severson, R.F.; Kays, S.J. Feeding and oviposition preferences of sweet potato weevil, Cylas formicarius elegantulus (Summers), on storage roots of sweet potato cultivars with differing surface chemistries. J. Chem. Ecol. 1989, 15, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef]
- Kinjo, H.; Kunimi, Y.; Ban, T.; Nakai, M. Oviposition Efficacy of Drosophila suzukii (Diptera: Drosophilidae) on Different Cultivars of Blueberry. J. Econ. Èntomol. 2013, 106, 1767–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, A.E.; Gleadow, R.M.; Zacarias, A.M.; Cuambe, C.E.; Miller, R.E.; Cavagnaro, T.R. Variations in the chemical composition of cassava (Manihot esculenta Crantz) leaves and roots as affected by genotypic and environmental variation. J. Agric. Food Chem. 2012, 60, 4946–4956. [Google Scholar] [CrossRef]
- Vandegeer, R.; Miller, R.E.; Bain, M.; Gleadow, R.M.; Cavagnaro, T.R. Drought adversely affects tuber development and nutritional quality of the staple crop cassava (Manihot esculenta Crantz). Funct. Plant Biol. 2013, 40, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chagné, D.; Dayatilake, D.; Diack, R.; Oliver, M.; Ireland, H.; Watson, A.; Gardiner, S.E.; Johnston, J.W.; Schaffer, R.J.; Tustin, S. Genetic and environmental control of fruit maturation, dry matter and firmness in apple (Malus × domestica Borkh). Hortic. Res. 2014, 1, 14046. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Block | Cultivar | 2010–2014 a | 2015–2019 b |
|---|---|---|---|
| 137 | 8S-26-50 | 5.73 (1.92) | |
| Ambrosia | 2.12 (0.78) | 4.41 (1.95) | |
| Autumn Gold | 9.06 (1.92) | ||
| Chinook | 10.8 (3.38) | ||
| COOP 29 | 2.71 (0.60) | ||
| COOP 39 | 8.57 (3.11) | 6.37 (0.65) | |
| COR10T-17 | 5.65 (1.06) | ||
| Delblush | 3.23 (1.18) | 5.31 (2.24) | |
| Golden Delicious | 8.69 (2.53) | ||
| Hampshire | 7.76 (2.05) | 5.75 (1.41) | |
| Jubilee Fugi | 9.33 (1.79) | 3.19 (0.76) | |
| NJ 109 | 2.26 (1.11) | 6.74 (1.72) | |
| NJ 90 | 11.1 (6.16) | ||
| NY-65-707-19 | 4.39 (0.69) | ||
| NY-79-507-49 | 6.01 (1.37) | ||
| NY-79-507-72 | 10.6 (0.31) | 8.07 (3.47) | |
| Pinova | 4.07 (0.67) | ||
| Rogers’ McIntosh | 6.02 (1.81) | ||
| Runkel | 8.12 (1.89) | ||
| Zestar! | 1.94 (0.80) | 3.63 (2.01) | |
| 138 | 8NE-07-72 | 5.82 (1.40) | |
| 8S-26-50 | 7.50 (2.51) | ||
| 8S-27-43 | 8.36 (1.78) | 3.53 (1.29) | |
| 8S-69-23 | 6.80 (0.94) | 5.43 (1.48) | |
| Chinook | 12.8 (2.68) | 8.93 (1.40) | |
| Royal Gala | 4.93 (1.29) | 6.32 (1.01) | |
| S14-15-72 | 11.7 (2.31) | 7.91 (2.69) | |
| S23-06-153 | 4.68 (0.61) | ||
| S43-43-79 | 6.29 (1.65) | ||
| S47-23-100 | 3.25 (1.13) | 4.83 (2.29) | |
| Silken | 5.23 (0.81) | ||
| Summerland McIntosh | 5.58 (0.92) | 5.91 (2.10) | |
| Zestar! | 4.87 (3.16) |
| Mean Percentage (±SE) of Clusters with Eggs | ||||||
|---|---|---|---|---|---|---|
| Block | Cultivar | 2016 * | 2017 | 2018 | 2019 | Across Years |
| 137 | Ambrosia | 61.1 (8.4) | 10.0 (4.1) b | 5.6 (3.3) bc | 12.2 (1.9) ab | 22.2 (13.0) ab |
| COOP 39 | 73.3 (6.9) | 56.0 (7.5) a | 70.0 (10.0) a | 10.4 (4.7) ab | 52.4 (14.5) a | |
| Delblush | 34.0 (5.1) | 14.0 (5.1) b | 29.6 (10.7) bc | 2.0 (2.0) bc | 19.9 (7.3) b | |
| Hampshire | 67.8 (15.8) | 26.5 (5.7) a | 46.0 (17.2) bc | 16.5 (9.2) ab | 39.2 (11.3) ab | |
| Jubilee Fugi | 43.3 (13.3) | 33.7 (10.4) ab | 13.3 (8.8) bc | 16.7 (6.7) ab | 26.7 (7.1) ab | |
| NJ 109 | 54.0 (14.0) | 38.0 (8.0) ab | 40.0 (8.4) bc | 24.9 (7.9) ab | 39.2 (5.9) ab | |
| NY79-507-72 | 43.7 (14.6) | 34.0 (6.8) ab | 23.5 (7.2) ab | 24.0 (7.5) ab | 31.3 (4.8) ab | |
| Zestar! | 56.0 (11.7) | 22.0 (7.3) b | 34.0 (5.1) bc | 33.6 (5.4) a | 36.4 (7.1) ab | |
| 138 | 8S-27-43 | 60.8 (10.8) | 26.0 (9.3) | 10.8 (7.8) c | 26.0 (6.0) ab | 30.9 (10.6) ab |
| 8S-69-23 | 21.5 (7.1) | 48.0 (12.8) | 36.2 (14.9) bc | 22.0 (3.7) ab | 31.9 (6.4) ab | |
| Chinook | 65.6 (4.9) | 46.0 (8.7) | 55.2 (8.7) bc | 10.4 (4.7) bc | 44.3 (11.9) ab | |
| Royal Gala | 62.5 (8.5) | 33.3 (8.4) | 33.6 (11.4) bc | 16.7 (3.3) ab | 36.5 (9.5) ab | |
| S14-15-72 | 59.7 (9.9) | 39.0 (10.9) | 42.5 (14.4) bc | 47.3 (6.1) a | 47.1 (4.5) ab | |
| S47-23-100 | 45.8 (10.1) | ---- | --- | 6.0 (6.0) bc | 25.9 (19.9) ab | |
| Summerland McIntosh | 36.4 (7.3) | 58.0 (5.8) | 64.0 (8.1) bc | 36.0 (9.3) ab | 48.6 (7.3) a | |
| Mean Number (±SE) Eggs/Cluster * | ||||||
|---|---|---|---|---|---|---|
| Block | Cultivar | 2016 | 2017 | 2018 | 2019 | Across Years |
| 137 | Ambrosia | 1.56 (0.2) ab | 0.87 (0.3) | 0.50 (0.3) | 1.10 (0.1) abc | 1.01 (0.1) |
| COOP 39 | 1.78 (0.2) ab | 1.31 (0.1) | 1.21 (0.1) | 0.70 (0.3) bc | 1.25 (0.1) | |
| Delblush | 1.36 (0.1) ab | 0.80 (0.2) | 1.63 (0.4) | 0.40 (0.4) bc | 1.03 (0.2) | |
| Hampshire | 1.57 (0.2) ab | 1.05 (0.1) | 1.36 (0.4) | 0.70 (0.3) bc | 1.15 (0.1) | |
| Jubilee Fugi | 1.47 (0.3) ab | 0.97 (0.3) | 0.77 (0.4) | 1.00 (0.0) abc | 1.04 (0.1) | |
| NJ 109 | 1.72 (0.3) ab | 1.28 (0.2) | 1.49 (0.2) | 0.90 (0.2) bc | 1.35 (0.1) | |
| NY79-507-72 | 1.81 (0.3) ab | 1.22 (0.1) | 1.50 (0.5) | 1.39 (0.2) abc | 1.48 (0.2) | |
| Zestar! | 1.66 (0.2) ab | 1.00 (0.3) | 1.50 (0.2) | 1.67 (0.2) ab | 1.44 (0.1) | |
| 138 | 8S-27-43 | 2.21 (0.2) a | 1.08 (0.3) | 0.50 (0.3) | 1.05 (0.1) bc | 1.25 (0.2) |
| 8S-69-23 | 1.00 (0.3) b | 1.68 (0.2) | 1.45 (0.4) | 1.10 (0.1) abc | 1.31 (0.1) | |
| Chinook | 1.91 (0.1) ab | 1.47 (0.2) | 1.76 (0.4) | 0.70 (0.3) bc | 1.49 (0.1) | |
| Royal Gala | 1.94 (0.2) ab | 1.17 (0.3) | 1.46 (0.2) | 1.00 (0.0) abc | 1.39 (0.1) | |
| S14-15-72 | 1.59 (0.2) ab | 1.52 (0.1) | 1.20 (0.4) | 1.81 (0.2) a | 1.55 (0.1) | |
| S47-23-100 | 1.47 (0.2) ab | ---- | ---- | 0.20 (0.2) bcd | 0.83 (0.2) | |
| Summerland McIntosh | 1.45 (0.2) ab | 1.54 (0.2) | 1.49 (0.2) | 1.57 (0.2) ab | 1.51 (0.1) | |
| Total Damage | Primary Damage | Secondary Damage | |
| Percentage of clusters with eggs | 0.34, <0.0001 | 0.01, 0.43 | 0.40, <0.0001 |
| Total eggs/cluster | 0.35, <0.0001 | 0.03, 0.29 | 0.22, <0.001 |
| Percentage of eggs/cluster (on King) | 0.09, 0.04 | 0.03, 0.27 | 0.07, 0.07 |
| Percentage of eggs/cluster (on Laterals) | 0.04, 0.22 | 0.007, 0.58 | 0.03, 0.27 |
| Percentage of eggs/cluster (on King + Laterals) | 0.26, <0.0001 | 0.03, 0.29 | 0.22, <0.001 |
| % Soluble solids (at 10 mm) | 0.02, 0.48 | 0.22, 0.01 | 0.13, 0.06 |
| % Soluble solids (at 20 mm) | 0.009, 0.63 | 0.13, 0.06 | 0.09, 0.13 |
| % Soluble solids (at 30 mm) | 0.18, 0.005 | 0.03, 0.27 | 0.22, 0.002 |
| Change in soluble solids (10 to 20 mm) | 0.007, 0.67 | 0.01, 0.54 | <0.001, 0.99 |
| Change in soluble solids (20 to 30 mm) | 0.0008, 0.88 | 0.04, 0.76 | 0.02, 0.49 |
| Acidity a (at 10 mm) | 0.05, 0.26 | 0.25, 0.008 | 0.06, 0.22 |
| Acidity (at 20 mm) | 0.03, 0.35 | 0.003, 0.79 | 0.07, 0.15 |
| Acidity (at 30 mm) | 0.02, 0.36 | 0.001, 0.83 | 0.04, 0.20 |
| Change in acidity (10 to 20 mm) | <0.001, 0.98 | 0.09, 0.12 | 0.17, 0.03 |
| Change in acidity (20 to 30 mm) | 0.02, 0.47 | 0.03, 0.35 | <0.001, 0.90 |
| Force (at 20 mm) | 0.007, 0.67 | 0.11, 0.07 | 0.08, 0.13 |
| Force (at 30 mm) | 0.02, 0.42 | 0.02, 0.34 | 0.009, 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blatt, S.; Hiltz, K. A New Look at Cultivar Preference in Hoplocampa testudinea (Hymenoptera: Tenthredinidae) on Apple in the Annapolis Valley of Nova Scotia, Canada. Insects 2021, 12, 769. https://doi.org/10.3390/insects12090769
Blatt S, Hiltz K. A New Look at Cultivar Preference in Hoplocampa testudinea (Hymenoptera: Tenthredinidae) on Apple in the Annapolis Valley of Nova Scotia, Canada. Insects. 2021; 12(9):769. https://doi.org/10.3390/insects12090769
Chicago/Turabian StyleBlatt, Suzanne, and Kim Hiltz. 2021. "A New Look at Cultivar Preference in Hoplocampa testudinea (Hymenoptera: Tenthredinidae) on Apple in the Annapolis Valley of Nova Scotia, Canada" Insects 12, no. 9: 769. https://doi.org/10.3390/insects12090769







