Fatty Acids Present in Wheat Kernels Influence the Development of the Grain Weevil (Sitophilus granarius L.)
Abstract
Simple Summary
Abstract
1. Introduction
- determination of the qualitative and quantitative composition of fatty acids in kernels of ten winter wheat cultivars,
- assessment of the number of offspring of S. granarius, mass of produced dust, and loss of grain mass on the tested wheat cultivars,
- identification of the presence and character of a relationship (positive or negative) between the content of determined fatty acids in wheat grain and the parameters describing the development of S. granarius listed above.
2. Materials and Methods
2.1. Materials
2.2. Analysis of the Composition of Fatty Acids (FA)
2.3. Bioassays
2.4. Statistical Analysis
3. Results
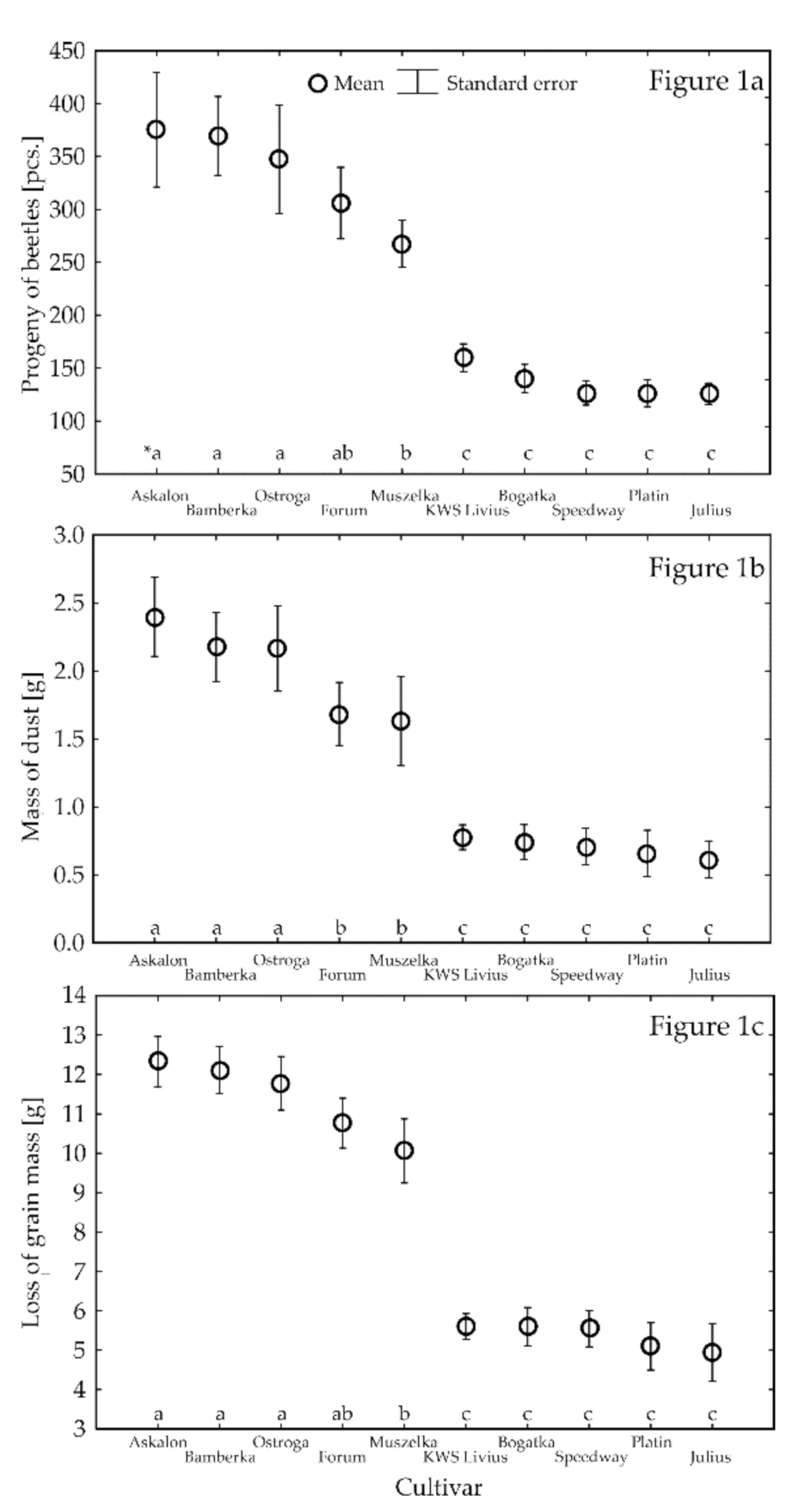
3.1. Parameters of the Development of S. granarius
3.2. Content of Fatty Acids in Wheat Kernels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mebarkia, A.; Rahbe, Y.; Guechi, A.; Bouras, A. Susceptibility of twelve soft wheat varieties (Triticum aestivum) to Sitophilus granarius (L.) (Coleoptera: Curculionidae). Agric. Biol. J. N. Am. 2010, 1, 571–578. [Google Scholar]
- Wawrzyniak, M.; Wrzesińska, D.; Lamparski, R.; Piesik, D. Wpływ suszu z mięty pieprzowej (Mentha piperita L.) na rozwój i płodność wołka zbożowego (Sitophilus granarius (L.). Zesz. Probl. Postęp. Nauk Rol. 2015, 580, 141–148. [Google Scholar]
- Laskowski, W.; Górska-Warsewicz, H.; Rejman, K.; Creuzot, M.; Zwolińska, J. How Important are Cereals and Cereal Products in the Average Polish Diet? Nutriens 2019, 11, 679. [Google Scholar] [CrossRef]
- Acevedo, M.; Zurn, J.; Molero, G.; Singh, P. The role of wheat in global food security. In Agricultural Development and Sustainable Intensification: Technology and Policy Challenges in the Face of Climate Change; Udaya, S., Ed.; Routledge: London, UK, 2018; pp. 81–110. [Google Scholar]
- Fornal, J.; Jeliński, T.; Sadowska, J.; Grudas, S.; Nawrot, J.; Niewiada, A.; Warchalewski, J.R.; Błaszczak, W. Detection of granary weevil Sitophilus granarius (L.) eggs and internal stages in wheat grain using soft X-ray and image analysis. J. Stored Prod. Res. 2007, 43, 142–148. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Lagogiannis, I.; Mpekiri, M.; Pettas, I.; Eliopoulos, P.A. Insecticidal Action of Several Isolates of Entomopathogenic Fungi against The Granary Weevil Sitophilus granarius. Agriculture 2019, 9, 222. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, A. Control of insect pests in crop plants and stored food grains using plant saponins: A review. LWT—Food Sci. Technol. 2018, 87, 93–101. [Google Scholar] [CrossRef]
- Singh, S. Natural plant products—As protectant during grain storage: A review. J. Entomol. Zool. Stud. 2017, 5, 1873–1885. [Google Scholar]
- Nietupski, M.; Ciepielewska, D.; Kordan, B.; Kozirok, W. Czy twardość ziarniaków pszenicy nowych odmian ma związek z rozwojem Sitophilus granarius L.? Prog. Plant Prot. Post. w Ochr. Rośl. 2003, 43, 833–835. [Google Scholar]
- Kordan, B.; Laszczak-Dawid, A.; Nietupski, M.; Żuk-Gołaszewska, K.; Tyburski, J. Wpływ formy przechowywania pszenicy orkisz (Triticum spelta L.) na rozwój wołka zbożowego (S. granarius L.). Prog. in Plant Prot./Post. w Ochr. Rośl. 2007, 47, 263–266. [Google Scholar]
- Kučerová, Z.; Aulický, R.; Stejskal, V. Sitophilus granarius (Curculionidae): Outdoor occurrence in vicinity of a grain store. J. Stored Prod. Res. 2007, 30, 167–171. [Google Scholar]
- Boniecki, P.; Koszela, K.; Świerczyński, K.; Skwarcz, J.; Zaborowicz, M.; Przybył, J. Neural Visual Detection of Grain Weevil (Sitophilus granarius L.). Agriculture 2020, 10, 25. [Google Scholar] [CrossRef]
- Stejskal, V.; Kucerova, Z. The effect of grain size on the biology of Sitophilus granarius L. (Col., Curculionidae). I. Oviposition, distribution of eggs and adult emergence. J. Appl. Entomol. 1996, 120, 143–146. [Google Scholar] [CrossRef]
- Opit, G.; Collins, P.J.; Daglish, G.J. Resistance management. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KS, USA, 2012; pp. 143–155. [Google Scholar]
- Wakil, W.; Kavallieratos, N.G.; Usman, M.; Gulzar, S.; El-Shafie, H.A.F. Detection of Phosphine Resistance in Field Populations of Four Key Stored-Grain Insect Pests in Pakistan. Insects 2021, 12, 288. [Google Scholar] [CrossRef]
- Gołębiowska, Z. The feeding and fecundity of Sitophilus granarius (L.), Sitophilus oryzae (L.), and Rhyzopertha dominica (F.) in wheat grain. J. Stored Prod. Res. 1969, 5, 143–155. [Google Scholar] [CrossRef]
- Kordan, B.; Skrajda-Brdak, M.; Tańska, M.; Konopka, I.; Cabaj, R.; Załuski, D. Phenolic and lipophilic compounds of wheat grain as factors affecting susceptibility to infestation by granary weevil (Sitophilus granarius L.). J. Appl. Bot. Food Qual. 2019, 92, 64–72. [Google Scholar] [CrossRef]
- Nawrot, J. The susceptibility of grain various wheat varietes and cultivars to the post harvest infestation by granary weevil (Sitophilus granarius L.). Pr. Nauk. Inst. Ochr. Rośl. 1981, 23, 133–140. [Google Scholar]
- Nawrot, J.; Warchalewski, J.R.; Piasecka-Kwiatkowska, D.; Niewiada, A.; Gawlak, M.; Grundas, S.; Fornal, J. The effect of some biochemical and technological properties of wheat grain on granary weevil (Sitophilus granarius L.) (Coleoptera Curculionidae) development. In Proceedings of the Ninth International Working Conferenceon Stored Product Protection, Campinas, São Paulo, Brazil, 15–18 October 2006; Lorini, I., Bacaltchuk, B., Beckel, H., Deckers, D., Sundfeld, E., dos Santos, J.P., Biagi, J.D., Celaro, J.C., Faroni, L.R.D.A., de Bartolini, L.O.F., et al., Eds.; ABRAPOS: Rodovia, Brazil, 2006; p. 400. [Google Scholar]
- Nietupski, M.; Ciepielewska, D.; Fornal, L. Wpływ zróżnicowania chemicznego białek w ziarnie wybranych odmian pszenicy na rozwój szkodników magazynowych. Prog. Plant Prot./Post. w Ochr. Rośl. 2006, 2, 420–423. [Google Scholar]
- Nawrot, J.; Gawlak, M.; Szafranek, J.; Szafranek, B.; Synak, E.; Warchalewski, J.R.; Piasecka-Kwiatkowska, D.; Baszcza, W.; Jelinski, T.; Fornal, J. The effect of wheat grain composition, cuticular lipids and kernel surface microstructure on feeding, egg-laying, and the development of the granary weevil, Sitophilus granarius (L.). J. Stored Prod. Res. 2010, 46, 133–141. [Google Scholar] [CrossRef]
- Keskin, S.; Ozkaya, H. Effect of storage and insect infestation on the technological properties of wheat. CYTA J. Food. 2015, 13, 134–139. [Google Scholar] [CrossRef]
- Gowda, G.B.; Patil, N.B.; Adak, T.; Pandi, G.P.; Basak, N.; Dhali, K.; Annamalai, M.; Prasanthi, G.; Mohapatra, S.D.; Jena, M.; et al. Physico-chemical characteristics of rice (Oryza sativa L.) grain imparting resistance and their association with development of rice weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Environ. Sustain. 2019, 2, 369–379. [Google Scholar] [CrossRef]
- Nwosu, L.C. Chemical bases for maize grain resistance to infestation and damage by the maize weevil, Sitophilus zeamais Motschulsky. J. Stored Prod. Res. 2016, 69, 41–50. [Google Scholar] [CrossRef]
- Lemic, D.; Mikac, K.M.; Genda, M.; Jukić, Ž.; Živković, I.P. Durum Wheat Cultivars Express Different Level of Resistance to Granary Weevil, Sitophilus granarius (Coleoptera; Curculionidae) Infestation. Insects 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Niewiada, A.; Nawrot, J.; Szafranek, J.; Szafranek, B.; Synak, E.; Jeleń, H.; Wąsowicz, E. Some factors affecting egg-laying of the granary weevil (Sitophilus granarius L.). J. Stored Prod. Res. 2005, 41, 544–555. [Google Scholar] [CrossRef]
- Liu, K.S. Comparison of Lipid Content and Fatty Acid Composition and Their Distribution within Seeds of 5 Small Grain Specie. J. Food Sci. 2011, 76, 334–342. [Google Scholar] [CrossRef]
- Łoźna, K.; Kita, A.; Styczyńska, M.; Biernat, A. Skład kwasów tłuszczowych olejów zalecanych w profilaktyce chorób cywilizacyjnych. Problemy Higieny i Epidemiologii. 2012, 93, 871–875. [Google Scholar]
- Mebarkia, A.; Guechi, A.; Mekhalif, S.; Makhlouf, M. Biochemical composition effect of the some cereal species’ on the behaviour of Sitophilus granarius L. and Rhyzopertha dominica F. species in semi-arid zone of Setif, Algeria. J. Agron. 2009, 8, 60–66. [Google Scholar] [CrossRef]
- Edwards, M.A. Morphological Features of Wheat Grain and Genotype Affecting Flour Yield. Ph.D. Thesis, Southern Cross University, Lismore, NSW, Australia, 2010; p. 252. [Google Scholar]
- Narducci, V.; Finotti, E.; Galli, V.; Carcea, M. Lipids and Fatty Acids in Italian Durum Wheat (Triticum durum Desf.) Cultivars. Foods 2019, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Żegarska, Z.; Jaworski, J.; Borejszo, Z. Ocena zmodyfikowanej metody Peiskera otrzymania estrów metylowych kwasów tłuszczowych. Acta Acad. Agricult. Technol. Aliment. 1991, 24, 25–33. [Google Scholar]
- Marciniak-Łukasiak, K. Rola i znaczenie kwasów tłuszczowych omega-3. Żywność Nauka Technologia Jakość. 2011, 6, 18. [Google Scholar]
- Halstead, D.G.H. External sex differences in stored-product Coleoptera. Bull. Entomol. Res. 1963, 54, 19–34. [Google Scholar] [CrossRef]
- Nietupski, M.; Kwiatkowski, J.; Kosewska, A. Physicochemical properties of achenes of different buckwheat genotypes affecting the development of grain weevil (Sitophilus granarius L.) and lesser grain borer (Rhyzopertha dominica F.). Zemdirbyste. 2017, 104, 311–320. [Google Scholar] [CrossRef][Green Version]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4); Microcomputer Power: Ithaca, NY, USA, 1998; p. 352. [Google Scholar]
- Piłat, B.; Zaderkowski, R. Substancje bioaktywne, pozytywne i negatywne skutki dodawania do żywności. Technika-Technologia. 2017, 71, 19–22. [Google Scholar] [CrossRef]
- Mihálik, D.; Klčová, L.; Ondreičková, K.; Hudcovicová, M.; Gubišová, M.; Klempová, T.; Čertík, M.; Pauk, J.; Kraic, J. Biosynthesis of Essential Polyunsaturated Fatty Acids in Wheat Triggered by Expression of Artificial Gene. Int. J. Mol. Sci. 2015, 16, 30046–30060. [Google Scholar] [CrossRef] [PubMed]
- Alnajim, I.; Du, X.; Lee, B.; Agarwal, M.; Liu, T.; Ren, Y. New Method of Analysis of Lipids in Tribolium castaneum (Herbst) and Rhyzopertha dominica (Fabricius) Insects by Direct Immersion Solid-Phase Microextraction (DI-SPME) Coupled with GC–MS. Insects 2019, 10, 363. [Google Scholar] [CrossRef]
- Mitra, S.; Sarkar, N.; Barik, A. Long-chain alkanes and fatty acids from Ludwigia octovalvis weed leaf surface waxes as short-range attractant and ovipositional stimulant to Altica cyanea (Weber) (Coleoptera: Chrysomelidae). Bull. Entomol. Res. 2017, 107, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, P.; Mukherjee, A.; Barik, A. Free fatty acids from Lathyrus sativus seed coats acting as short-range attractants to Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2016, 67, 56–62. [Google Scholar] [CrossRef]
- Krzyżowski, M.; Francikowski, J.; Baran, B.; Babczyńska, A. The short-chain fatty acids as potential protective agents against Callosobruchus maculatus infestation. J. Stored Prod. Res. 2020, 86, 101570. [Google Scholar] [CrossRef]
- González-Thuillier, I.; Salt, L.; Chope, G.; Penson, S.; Skeggs, P.; Tosi, P.; Powers, S.J.; Ward, J.L.; Wilde, P.; Shewry, P.R.; et al. Distribution of Lipids in the Grain of Wheat (cv. Hereward) Determined by Lipidomic Analysis of Milling and Pearling Fractions. Agric. Food Chem. 2015, 63, 10705–10716. [Google Scholar] [CrossRef]
- Nawrot, J. Podstawy do zwalczania wołka zbożowego (Sitophilus granarius L.) (Coleoptera: Curculionidae) przy użyciu naturalnych związków chemicznych wpływających na zachowanie się chrząszczy. Prace Nauk. Inst. Ochr. Rośl. 1983, 24, 174–197. [Google Scholar]
- Nawrot, J.; Winiecki, Z.; Szafranek, J.; Malinski, E.; Harmatha, J. Search for natural antifeedants and attractants for stored product pests. In Proceedings of the International Forum on Stored Product Protection and Post-Harvest Treatment of Plant Products, Strasbourg, France, 7–8 November 1995; Council of Europe, Ed.; pp. 149–164. [Google Scholar]

| df | Wald’s Statistic | p | |
|---|---|---|---|
| Progeny of beetles | 9 | 4227.4 | 0.00 |
| Mass of dust | 9 | 31.82 | 0.00 |
| Loss of grain mass | 9 | 108.53 | 0.00 |
| Fatty Acids | df | Wald’s Statistic | ANOVA | p |
|---|---|---|---|---|
| F Value | ||||
| C 12:0 | 9 | 4835.5 | - | 0.00 |
| C 14:0 | 9 | - | 24.60 | 0.00 |
| C 15:0 | 9 | - | 57.14 | 0.00 |
| C 16:0 | 9 | - | 193.84 | 0.00 |
| C 16:1 | 9 | - | 91.86 | 0.00 |
| C 17:0 | 9 | - | 22.56 | 0.00 |
| C 17:1 | 9 | - | 14.87 | 0.00 |
| C 18:0 | 9 | - | 64.94 | 0.00 |
| C 18:1 | 9 | - | 228.10 | 0.00 |
| C 18:2 | 9 | - | 290.11 | 0.00 |
| C 18:3 | 9 | - | 357.57 | 0.00 |
| C 20:0 | 9 | - | 49.08 | 0.00 |
| C 20:1 | 9 | - | 87.70 | 0.00 |
| C 22:0 | 9 | 2835.0 | - | 0.00 |
| Fatty Acids | Cultivar | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bogatka | Muszelka | Ostroga | Askalon | Bamberka | Forum | Speedway | Platin | KWS Livius | Julius | |
| C 12:0 | 566.3 * bc ** | 551.3 bc | 650.0 b | 507.7 bc | 1569.3 a | 522.3 bc | 485.0 bc | 519.3 bc | 415.0 bc | 305.3 c |
| % | 0.0029 | 0.0034 | 0.0029 | 0.0081 | 0.0026 | 0.0027 | 0.0027 | 0.0025 | 0.0022 | 0.0016 |
| C 14:0 | 3496.3 a | 2880.3 b | 2793.3 bc | 2320.3 cd | 3024.7 ab | 2149.3 d | 2490.0 c | 2578.7 c | 1810.0 de | 1703.0 e |
| % | 0.0181 | 0.0145 | 0.0149 | 0.0157 | 0.0120 | 0.0134 | 0.0111 | 0.0129 | 0.0094 | 0.0088 |
| C 15:0 | 2447.0 b | 2950.3 a | 2756.7 ab | 2453.7 b | 2522.3 b | 1817.0 d | 2281.7 c | 2365.3 c | 1802.7 d | 1290.0 e |
| % | 0.0127 | 0.0143 | 0.0153 | 0.0131 | 0.0127 | 0.0123 | 0.0094 | 0.0118 | 0.0093 | 0.0067 |
| C 16:0 | 437,622.7 ab | 458,877.7 a | 448,480.0 a | 379,471.0 c | 416,400.3 b | 329,163.7 d | 371,442.0 c | 384,799.3 c | 282,869.0 e | 227,370.7 f |
| % | 2.2696 | 2.3259 | 2.3798 | 2.1595 | 1.9680 | 1.9956 | 1.7071 | 1.9264 | 1.4670 | 1.1792 |
| C 16:1 | 3540.7 cd | 5198.0 a | 3921.3 bc | 3952.7 b | 3709.3 c | 2933.7 f | 3444.3 d | 3421.0 d | 3039.0 e | 2263.3 g |
| % | 0.0184 | 0.0203 | 0.0270 | 0.0192 | 0.0205 | 0.0177 | 0.0152 | 0.0179 | 0.0158 | 0.0117 |
| C 17:0 | 4400.0 b | 3728.3 d | 3571.3 d | 3569.3 d | 4849.0 a | 3911.3 cd | 3998.0 c | 4594.3 ab | 4199.0 bc | 2667.3 e |
| % | 0.0228 | 0.0185 | 0.0193 | 0.0251 | 0.0185 | 0.0238 | 0.0203 | 0.0207 | 0.0218 | 0.0138 |
| C 17:1 | 3316.3 b | 4004.3 ab | 4288.3 a | 3276.0 b | 3513.0 b | 1747.3 d | 2742.7 bc | 1710.3 d | 2349.7 c | 1071.3 e |
| % | 0.0172 | 0.0222 | 0.0208 | 0.0182 | 0.0170 | 0.0089 | 0.0091 | 0.0142 | 0.0122 | 0.0056 |
| C 18:0 | 31,142.3 ab | 28,317.7 b | 31,775.7 a | 25,101.3 bc | 30,424.0 ab | 22,978.0 d | 30,079.7 ab | 22,330.3 de | 19,327.0 e | 16,289.0 f |
| % | 0.1615 | 0.1648 | 0.1469 | 0.1578 | 0.1302 | 0.1158 | 0.1192 | 0.1560 | 0.1002 | 0.0845 |
| C 18:1 | 334,102.7 a | 274,529.7 c | 336,239.7 a | 259,780.7 c | 332,219.3 a | 221,245.3 d | 311,575.0 b | 215,814.0 d | 230,121.3 d | 162,116.0 e |
| % | 1.7327 | 1.7438 | 1.4238 | 1.7229 | 1.3473 | 1.1193 | 1.1474 | 1.6159 | 1.1935 | 0.8408 |
| C 18:2 | 1,546,602.0 a | 1,315,830.0 c | 1,436,471.0 b | 1,189,117.0 d | 1,300,090.0 c | 977,029.0 f | 1,065,706.0 e | 1,053,818.0 ef | 830,340.0 g | 670,930.0 h |
| % | 8.0210 | 7.4498 | 6.8241 | 6.7425 | 6.1670 | 5.4653 | 5.0671 | 5.5269 | 4.3063 | 3.4796 |
| C 18:3 | 98,222.7 c | 111,380.0 a | 100,923.7 bc | 106,641.7 b | 86,550.3 d | 63,154.0 g | 73,362.7 f | 79,455.7 e | 57,588.0 g | 47,080.0 h |
| % | 0.5094 | 0.5234 | 0.5776 | 0.4489 | 0.5531 | 0.4121 | 0.3275 | 0.3805 | 0.2987 | 0.2442 |
| C 20:0 | 3915.3 a | 3821.3 a | 4137.7 a | 4165.7 a | 4010.7 a | 3101.3 b | 4229.7 a | 3018.3 b | 2769.3 b | 2219.7 c |
| % | 0.0203 | 0.0215 | 0.0198 | 0.0208 | 0.0216 | 0.0157 | 0.0161 | 0.0219 | 0.0144 | 0.0115 |
| C 20:1 | 17,525.7 b | 15,021.7 c | 17,286.7 b | 19,066.7 a | 18,333.7 ab | 12,165.0 d | 14,939.3 c | 12,325.7 d | 15,115.3 c | 9016.0 e |
| % | 0.0909 | 0.0897 | 0.0779 | 0.0951 | 0.0989 | 0.0639 | 0.0631 | 0.0775 | 0.0784 | 0.0468 |
| C 22:0 | 6259.3 ab | 5814.3 ab | 5912.7 ab | 5944.3 ab | 6747.3 a | 5253.7 ab | 5674.3 ab | 6098.3 ab | 4835.3 b | 4031.0 c |
| % | 0.0325 | 0.0307 | 0.0302 | 0.0350 | 0.0308 | 0.0316 | 0.0272 | 0.0294 | 0.0251 | 0.0209 |
| Adults | Mass of Dust | Mass Loss | ||||
|---|---|---|---|---|---|---|
| r | p * | r | p | r | p | |
| Adults | - | - | ||||
| Mass of dust | 0.99 | 0.00 | - | - | ||
| Mass loss | 0.98 | 0.00 | 0.99 | 0.00 | - | - |
| C 12:0 | 0.55 | 0.1 | 0.51 | 0.14 | 0.53 | 0.12 |
| C 14:0 | 0.19 | 0.60 | 0.18 | 0.63 | 0.26 | 0.48 |
| C 15:0 | 0.45 | 0.19 | 0.49 | 0.15 | 0.55 | 0.1 |
| C 16:0 | 0.41 | 0.23 | 0.43 | 0.21 | 0.50 | 0.14 |
| C 16:1 | 0.44 | 0.2 | 0.49 | 0.16 | 0.57 | 0.09 |
| C 17:0 | 0.3 | 0.93 | −0.02 | 0.96 | 0.01 | 0.98 |
| C 17:1 | 0.57 | 0.09 | 0.59 | 0.07 | 0.65 | 0.04 |
| C 18:0 | 0.38 | 0.28 | 0.39 | 0.27 | 0.45 | 0.19 |
| C 18:1 | 0.36 | 0.31 | 0.35 | 0.32 | 0.40 | 0.25 |
| C 18:2 | 0.41 | 0.24 | 0.41 | 0.25 | 0.47 | 0.17 |
| C 18:3 | 0.64 | 0.06 | 0.56 | 0.1 | 0.61 | 0.06 |
| C 20:0 | 0.60 | 0.09 | 0.53 | 0.11 | 0.56 | 0.09 |
| C 20:1 | 0.72 | 0.03 | 0.58 | 0.08 | 0.58 | 0.08 |
| C 22:0 | 0.56 | 0.12 | 0.43 | 0.22 | 0.46 | 0.18 |
| Fatty acids (total) | 0.67 | 0.05 | 0.43 | 0.22 | 0.49 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nietupski, M.; Ludwiczak, E.; Cabaj, R.; Purwin, C.; Kordan, B. Fatty Acids Present in Wheat Kernels Influence the Development of the Grain Weevil (Sitophilus granarius L.). Insects 2021, 12, 806. https://doi.org/10.3390/insects12090806
Nietupski M, Ludwiczak E, Cabaj R, Purwin C, Kordan B. Fatty Acids Present in Wheat Kernels Influence the Development of the Grain Weevil (Sitophilus granarius L.). Insects. 2021; 12(9):806. https://doi.org/10.3390/insects12090806
Chicago/Turabian StyleNietupski, Mariusz, Emilia Ludwiczak, Robert Cabaj, Cezary Purwin, and Bożena Kordan. 2021. "Fatty Acids Present in Wheat Kernels Influence the Development of the Grain Weevil (Sitophilus granarius L.)" Insects 12, no. 9: 806. https://doi.org/10.3390/insects12090806
APA StyleNietupski, M., Ludwiczak, E., Cabaj, R., Purwin, C., & Kordan, B. (2021). Fatty Acids Present in Wheat Kernels Influence the Development of the Grain Weevil (Sitophilus granarius L.). Insects, 12(9), 806. https://doi.org/10.3390/insects12090806






