Is Forensic Entomology Lost in Space?
Abstract
Simple Summary
Abstract
1. Introduction
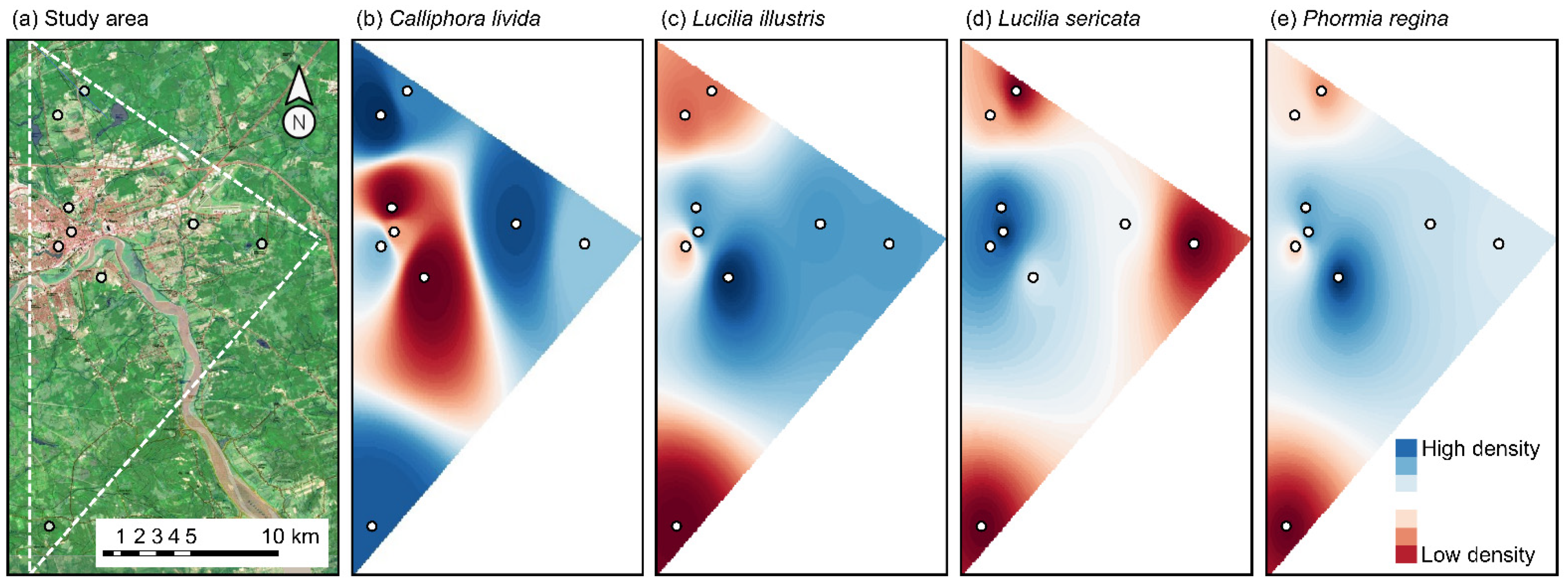
2. Materials and Methods
2.1. Source of Data
2.2. Statistical Analyses
3. Results
4. Discussion
4.1. Spatial Dynamics of Calliphoridae Species
4.2. Contribution of Spatial Statistics to Forensic Science
4.3. Recommendations for Forensic Entomology
- The thumb rule of using a minimum distance of 50 m between carcasses (e.g., [3,8,47,48]) to achieve some independence amongst experimental units needs to be re-evaluated using approaches documenting distance-dependent autocorrelation while accounting for time dependence associated with repeated sampling (e.g., spatio-temporal semivariograms). Although the 50 m distance limits larval movement between carcasses [15], it does not preclude synchronization of carcass dynamics through the movement of Diptera and Coleoptera adults from one carcass to another. The documentation in the current study of strong autocorrelation patterns in L. illustris and L. sericata under distances of a few kilometers indicates that the potential for this to occur is very real. The intention here is not to make forensic experiments any harder but rather to understand the consequences of the methodology used on the dynamics of decomposition and the organisms involved.
- Researchers should consider georeferencing experimental units at all scales (i.e., both the position of cadavers/carcasses in the field and the position of study sites for larger-scale projects). Multi-site studies cannot afford to ignore spatial and scale effects as these may be more influential than the variables under study.
- If researchers are not comfortable with spatial statistics, they can also choose other ways to incorporate spatial effects into their statistical models. For example, additive models lend themselves very well to the inclusion of geographic coordinates and autoregressive structures using, for example, tensor product smoothing. It is also possible to check the autocorrelation of residuals in most statistical procedures. Spatial analysis can be complex, but it is not necessary to know every facet of it to account for spatial interdependence.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrd, J.H.; Castner, J.L. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: Boca Raton, FL, USA, 2001; p. 387. [Google Scholar]
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and diptera colonization. Forensic Sci. Int. 2001, 120, 18–27. [Google Scholar] [CrossRef]
- Moreau, G.; Michaud, J.-P.; Schoenly, K.G. Experimental design, inferential statistics, and computer modeling. In Forensic Entomology: International Dimensions and Frontiers; Tomberlin, J.K., Benbow, M.E., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon on Thames, UK, 2015; pp. 205–230. [Google Scholar]
- Moreau, G. The Pitfalls in the Path of Probabilistic Inference in Forensic Entomology: A Review. Insects 2021, 12, 240. [Google Scholar] [CrossRef]
- Cruickshank, I.; Wall, R. Aggregation and habitat use by Lucilia blowflies (Diptera: Calliphoridae) in pasture. Bull. Entomol. Res. 2002, 92, 153–158. [Google Scholar] [CrossRef]
- Charabidze, D.; Gosselin, M.; Hedouin, V. Use of necrophagous insects as evidence of cadaver relocation: Myth or reality? PeerJ 2017, 5, e3506. [Google Scholar] [CrossRef]
- Zabala, J.; Díaz, B.; Saloña-Bordas, M.I. Seasonal blowfly distribution and abundance in fragmented landscapes. Is it useful in forensic inference about where a corpse has been decaying? PLoS ONE 2014, 9, e99668. [Google Scholar] [CrossRef]
- Perez, A.E.; Haskell, N.H.; Wells, J.D. Commonly used intercarcass distances appear to be sufficient to ensure independence of carrion insect succession pattern. Ann. Entomol. Soc. Am. 2015, 109, 72–80. [Google Scholar] [CrossRef]
- Nestel, D.; Carvalho, J.; Nemny-Lavy, E. The Spatial Dimension in the Ecology of Insect Pests and its Relevance to Pest Management. In Insect Pest Management; Horowitz, A.R., Ishaaya, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 45–63. [Google Scholar]
- Griffith, D. Towards a theory of spatial statistics. Geogr. Anal. 2010, 12, 325–339. [Google Scholar] [CrossRef]
- Goguen, J.; Moreau, G. Exogenous and endogenous factors acting on the spatial distribution of a chrysomelid in extensively managed blueberry fields. Agric. For. Entomol. 2014, 17, 181–187. [Google Scholar] [CrossRef]
- Schoenly, K.G.; Michaud, J.-P.; Moreau, G. Design and Analysis of Field Studies in Carrion Ecology. In Carrion Ecology, Evolution and Their Applications; Benbow, M.E., Tomberlin, J.K., Tarone, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 129–148. [Google Scholar]
- Kautz, M.; Schopf, R.; Ohser, J. The “sun-effect”: Microclimatic alterations predispose forest edges to bark beetle infestations. Eur. J. For. Res. 2013, 132, 453–465. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Lewis, A.J.; Benbow, M.E. When entomological evidence crawls away: Phormia regina en masse larval dispersal. J. Med. Entomol. 2011, 48, 1112–1119. [Google Scholar] [CrossRef]
- Fortin, M.J. Spatial Statistics in Landscape Ecology. In Landscape Ecological Analysis; Klopatek, J., Gardner, R., Eds.; Springer: New York, NY, USA, 1999; pp. 253–279. [Google Scholar]
- Amarasekare, P.; Nisbet, R.M. Spatial heterogeneity, source-sink dynamics, and the local coexistence of competing species. Am. Nat. 2001, 158, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Michaud, J.-P.; Majka, C.G.; Privé, J.-P.; Moreau, G. Natural and anthropogenic changes in the insect fauna associated with carcasses in the North American Maritime lowlands. Forensic Sci. Int. 2010, 202, 64–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lichstein, J.W.; Simons, T.R.; Shriner, S.A.; Franzreb, K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002, 72, 445–463. [Google Scholar] [CrossRef]
- Tobler, W.R. A Computer Movie Simulating Urban Growth in the Detroit Region. Econ. Geogr. 1970, 46, 234. [Google Scholar] [CrossRef]
- Cressie, N. Statistics for Spatial Data; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Boudreau, D.R.; Hammami, N.; Moreau, G. Environmental and evolutionary factors favouring the coexistence of sarcosaprophagous Calliphoridae species competing for animal necromass. Ecol. Entomol. 2021, 46, 1293–1300. [Google Scholar] [CrossRef]
- LeBlanc, K.; Boudreau, D.; Moreau, G. Small bait traps may not accurately reflect the composition of necrophagous Diptera associated to remains. Insects 2021, 12, 261. [Google Scholar] [CrossRef]
- Vilte, R.; Gleiser, R.M.; Horenstein, M.B. Necrophagous fly assembly: Evaluation of species bait preference in field experiments. J. Med. Entomol. 2019, 57, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Weidner, L.M.; Gemmellaro, M.D.; Tomberlin, J.K.; Hamilton, G.C. Evaluation of bait traps as a means to predict initial blow fly (Diptera: Calliphoridae) communities associated with decomposing swine remains in New Jersey, USA. Forensic Sci. Int. 2017, 278, 95–100. [Google Scholar] [CrossRef]
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Ann. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef]
- Marshall, S.A.; Whitworth, T.; Roscoe, L. Blow flies (Diptera; Calliphoridae) of eastern Canada with a key to Calliphoridae subfamilies and genera of eastern North America, and a key to the eastern Canadian species of Calliphorinae, Luciliinae and Chrysomyiinae. Can. J. Arthropod. Identif. 2011, 11. [Google Scholar] [CrossRef]
- Tantawi, T.I.; Whitworth, T. First record of L. bufonivora Moniez, 1876 (Diptera: Calliphoridae) from North America and key to North American species of the L. bufonivora species group. Zootaxa 2014, 3881, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Whitworth, T.; Marshall, S.A. Blow flies of North America: Keys to the subfamilies and genera of Calliphoridae, and to the species of the subfamilies Calliphorinae, Luciliinae and Chrysomyinae. Can. J. Arthropod Identif. 2019, 39, 1–191. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 17 December 2021).
- De’Ath, G. Multivariate regression trees: A new technique for modeling species–environment relationships. Ecology 2002, 83, 1105–1117. [Google Scholar] [CrossRef]
- Rock, D.; Greenberg, D.M.; Hallmayer, J. cyclical changes of homicide rates. J. Interpers. Violence 2003, 18, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Matheron, G. Principles of geostatistics. Econ. Geol. 1963, 58, 1246–1266. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Introna, F. The forensic entomologist in the context of the forensic pathologist’s role. Forensic Sci. Int. 2001, 120, 132–139. [Google Scholar] [CrossRef]
- Matuszewski, S. Estimating the pre-appearance interval from temperature in Necrodes littoralis L. (Coleoptera: Silphidae). Forensic Sci. Int. 2011, 212, 180–188. [Google Scholar] [CrossRef]
- Simpson, G.; Strongman, D.B. Carrion insects on pig carcasses at a rural and an urban site in Nova Scotia. Can. Soc. Forensic Sci. J. 2002, 35, 123–143. [Google Scholar] [CrossRef]
- Brundage, A.; Bros, S.; Honda, J.Y. Seasonal and habitat abundance and distribution of some forensically important blow flies (Diptera: Calliphoridae) in Central California. Forensic Sci. Int. 2011, 212, 115–120. [Google Scholar] [CrossRef]
- Johnson, M.D. Seasonal and microseral variations in the insect populations on carrion. Am. Midl. Nat. 1975, 93, 79. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Watt, A.D.; Leather, S.R. Aggregation, habitat quality and coexistence: A case study on carrion fly communities in slug cadavers. J. Anim. Ecol. 2002, 71, 131–140. [Google Scholar] [CrossRef]
- Kouki, J.; Hanski, I. Population aggregation facilitates coexistence of many competing carrion fly species. Oikos 1995, 72, 223. [Google Scholar] [CrossRef]
- Michaud, J.-P.; Moreau, G. Predicting the visitation of carcasses by carrion-related insects under different rates of degree-day accumulation. Forensic Sci. Int. 2009, 185, 78–83. [Google Scholar] [CrossRef]
- Baz, A.; Cifrian, B.; Díaz-Äranda, L.M.; Martín-Vega, D. The distribution of adult blow-flies (Diptera: Calliphoridae) along an altitudinal gradient in Central Spain. Ann. Société Entomol. Fr. 2007, 43, 289–296. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Community diversity: Relative roles of local and regional processes. Science 1987, 235, 167–171. [Google Scholar] [CrossRef]
- Lutz, L.; Amendt, J.; Moreau, G. Carcass concealment alters assemblages and reproduction of forensically important beetles. Forensic Sci. Int. 2018, 291, 124–132. [Google Scholar] [CrossRef]
- Levin, S. The Problem of pattern and scale in ecology: The Robert H. MacArthur award lecture. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Rollinson, C.R.; Finley, A.O.; Alexander, M.R.; Banerjee, S.; Hamil, K.-A.D.; E Koenig, L.; Locke, D.H.; DeMarche, M.L.; Tingley, M.W.; Wheeler, K.; et al. Working across space and time: Nonstationarity in ecological research and application. Front. Ecol. Environ. 2021, 19, 66–72. [Google Scholar] [CrossRef]
- Anderson, G.S.; Van Laerhoven, S.L. Initial studies on insect succession on carrion in south western British Columbia. J. Forensic Sci. 1996, 41, 617–625. [Google Scholar] [CrossRef]
- Bourel, B.; Luck, M.-B.; Hedouin, V.; Cailliez, J.-C.; Derout, D.; Gosset, D. Necrophilous insect succession on rabbit carrion in sand dune habitats in Northern France. J. Med. Entomol. 1999, 36, 420–425. [Google Scholar] [CrossRef]
- Griffith, D.A. What is spatial autocorrelation? Reflections on the past 25 years of spatial statistics. L’Espace Géographique 1992, 21, 265–280. [Google Scholar] [CrossRef]
- Manhein, M.H.; Listi, G.A.; Leitner, M. The application of geographic information systems and spatial analysis to assess dumped and subsequently scattered human remains. J. Forensic Sci. 2006, 51, 469–474. [Google Scholar] [CrossRef]
- Abraham, J.; Champod, C.; Lennard, C.; Roux, C. Spatial analysis of corresponding fingerprint features from match and close non-match populations. Forensic Sci. Int. 2013, 230, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Tuller, H.; Hofmeister, U.; Tuller, H.; Hofmeister, U. Spatial analysis of mass grave mapping data to assist in the reassociation of disarticulated and commingled human remains. In Commingled Human Remains: Methods in Recovery Analysis and Identification; Adams., B.J., Byrd, J.E., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 7–32. [Google Scholar]
- Groff, E.R.; Lockwood, B. Criminogenic facilities and crime across street segments in Philadelphia. J. Res. Crime Delinq. 2014, 51, 277–314. [Google Scholar] [CrossRef]
- Sabetta, L.; Zaccarelli, N.; Mancinelli, G.; Mandrone, S.; Salvatori, R.; Costantini, M.L.; Zurlini, G.; Rossi, L. Mapping litter decomposition by remote-detected indicators. Ann. Geophys. 2009, 49, 219–226. [Google Scholar] [CrossRef]
- Louzada, J.; Lima, A.P.; Matavelli, R.; Zambaldi, L.; Barlow, J. Community structure of dung beetles in Amazonian savannas: Role of fire disturbance, vegetation and landscape structure. Landsc. Ecol. 2010, 25, 631–641. [Google Scholar] [CrossRef]
- Mourant, A.; LeComte, N.; Moreau, G. Size matters: When resource accessibility by ecosystem engineering elicits wood-boring beetle demographic responses. Ecol. Evol. 2021, 11, 784–795. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudreau, D.R.; Moreau, G. Is Forensic Entomology Lost in Space? Insects 2022, 13, 11. https://doi.org/10.3390/insects13010011
Boudreau DR, Moreau G. Is Forensic Entomology Lost in Space? Insects. 2022; 13(1):11. https://doi.org/10.3390/insects13010011
Chicago/Turabian StyleBoudreau, Denis R., and Gaétan Moreau. 2022. "Is Forensic Entomology Lost in Space?" Insects 13, no. 1: 11. https://doi.org/10.3390/insects13010011
APA StyleBoudreau, D. R., & Moreau, G. (2022). Is Forensic Entomology Lost in Space? Insects, 13(1), 11. https://doi.org/10.3390/insects13010011





