Biology of Anthonomus testaceosquamosus Linell, 1897 (Coleoptera: Curculionidae): A New Pest of Tropical Hibiscus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Weevil Colony
2.1.1. On Hibiscus Buds
2.1.2. On Artificial Diet
2.2. Instar Determination and Immature Development on Hibiscus Buds and Artificial Diet
2.3. Reproduction and Longevity on Hibiscus Buds and Artificial Diet
2.4. Life Table and Population Parameters
2.5. Adult Survival on Pollen and without Food Source
2.6. Data and Statistical Analyses
2.6.1. Instar Determination
2.6.2. Development on Hibiscus or Artificial Diet
2.6.3. Reproduction and Longevity on Hibiscus or Artificial Diet
2.6.4. Life Table and Population Parameters
2.6.5. Survival on Pollen and without Food Source
3. Results
3.1. Instar Determination and Development on Hibiscus Buds and Artificial Diet
3.2. Reproduction and Longevity on Hibiscus Buds and Artificial Diet
3.3. Adult Survival on Pollen and without Food Source
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burke, H.R.; Gates, D.B. Bionomics of Several North American Species of Anthonomus (Coleoptera: Curculionidae). Southwest. Nat. 1974, 19, 313. [Google Scholar] [CrossRef]
- Clark, W.E.; Burke, H.R.; Jones, R.W.; Anderson, R.S. The North American Species of the Anthonomus squamosus Species-Group (Coleoptera: Curculionidae: Curculioninae: Anthonomini). Coleopt. Bull. 2019, 73, 773. [Google Scholar] [CrossRef]
- Bográn, C.E.; Helnz, K.M.; Ludwlg, S. The Bud Weevil Anthonomus testaceosquamosus, a Pest of Tropical Hibiscus. In Proceedings of the SNA Research Conference Entomology, Atlanta, GA, USA, December 2003; Volume 48, pp. 147–149. [Google Scholar]
- Skelley, P.E.; Osborne, L.S. Pest Alert Anthonomus testaceosquamosus Linell, the Hibiscus Bud Weevil, New in Florida; Florida Department of Agriculture and Consumer Services: Gainesville, FL, USA, 2018.
- United States Department of Agriculture. Market Value of Agricultural Products Sold Including Food Marketing Practices and Value-Added Products: 2017 and 2012 Census of Agriculture 2017; United States Department of Agriculture: Washington, DC, USA, 2017; pp. 275–302.
- Greenberg, S.M.; Sappington, T.W.; Spurgeon, D.W.; Sétamou, M. Boll Weevil (Coleoptera: Curculionidae) Feeding and Reproduction as Functions of Cotton Square Availability. Environ. Entomol. 2003, 32, 698–704. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Sappington, T.W.; Adamczyk, J.J.; Liu, T.-X.; Setamou, M. Effects of Photoperiod on Boll Weevil (Coleoptera: Curculionidae) Development, Survival, and Reproduction. Environ. Entomol. 2008, 37, 1396–1402. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Jones, G.D.; Adamczyk, J.J., Jr.; Eischen, F.; Armstrong, J.S.; Coleman, R.J.; Sétamou, M.; Liu, T.-X. Reproductive potential of field-collected overwintering boll weevils (Coleoptera: Curculionidae) fed on pollen in the laboratory. Insect Sci. 2009, 16, 321–327. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Setamou, M.; Sappington, T.W.; Liu, T.-X.; Coleman, R.J.; Armstrong, J.S. Temperature-dependent development and reproduction of the boll weevil (Coleoptera: Curculionidae). Insect Sci. 2005, 12, 449–459. [Google Scholar] [CrossRef]
- Toapanta, M.A.; Schuster, D.J.; Stansly, P.A. Development and Life History of Anthonomus eugenii (Coleoptera: Curculionidae) at Constant Temperatures. Environ. Entomol. 2005, 34, 999–1008. [Google Scholar] [CrossRef]
- Revynthi, A.M.; Hernandez, Y.V.; Rodriguez, J.; Kendra, P.E.; Carrillo, D.; Mannion, C.M. The Hibiscus Bud Weevil (Anthonomus testaceosquamosus Linell, Coleoptera: Curculionidae); EDIS 2021, 9/2021; Funiversity of Florida: Gainesville, FL, USA, 2021; pp. 1–7. [Google Scholar]
- Stewart, F.D. Mass rearing the pink bollworm, Pectinophora gosypiella. In Advances and Challenges in Insect Rearing; King, E.G., Leppla, N.C., Eds.; Agricultural Research Service, USDA: Washington, DC, USA, 1984; pp. 176–187. [Google Scholar]
- Edwards, R.H.; Miller, E.; Becker, R.; Mossman, A.P.; Irving, D.W. Twin Screw Extrusion Processing of Diet for Mass Rearing the Pink Bollworm; Transactions of the ASAE; American Society of Agricultural Engineers: Washington, DC, USA, 1996; Volume 39, pp. 1789–1797. [Google Scholar]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell Science Ltd.: Hoboken, NJ, USA, 2000; ISBN 0632054778. [Google Scholar]
- Maia, A.D.H.N.; Luiz, A.J.B.; Campanhola, C. Statistical inference on associated fertility life table parameters using jackknife technique: Computational aspects. J. Econ. Entomol. 2000, 93, 511–518. [Google Scholar] [CrossRef]
- Ameijeiras-Alonso, J.; Crujeiras, R.M.; Rodríguez-Casal, A. Mode testing, critical bandwidth and excess mass. Test 2019, 28, 900–919. [Google Scholar] [CrossRef]
- Dyar, H.G. The Number of Molts of Lepidopterous Larvae. Psyche 1890, 5, 23871. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.H.M. Estimated Marginal Means, Aka Least-Squares Means. Available online: https://github.com/rvlenth/emmeans (accessed on 3 December 2021).
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- R Development Core Team. A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2021. [Google Scholar]
- Elmore, J.; Davis, A.; Campbell, R. The Pepper Weevil. U.S. Dep. Agric. Tech. Bull. 1934, 447, 25. [Google Scholar]
- Cohen, A.C. Ecology of Insect Rearing Systems: A Mini-Review of Insect Rearing Papers from 1906–2017. Adv. Entomol. 2018, 6, 86–115. [Google Scholar] [CrossRef]
- Toba, H.H.; Kishaba, A.N.; Pangaldan, R.; Riggs, S. Laboratory rearing of pepper weevils on artificial diets. J. Econ. Entomol. 1969, 62, 257–258. [Google Scholar] [CrossRef]
- Seal, D.R.; Martin, C.G. Laboratory Rearing of Pepper Weevils (Coleoptera: Curculionidae) Using Artificial Leaf Balls and a Boll Weevil Diet. J. Entomol. Sci. 2017, 52, 395–410. [Google Scholar] [CrossRef][Green Version]
- Fernández, D.C.; VanLaerhoven, S.L.; McCreary, C.; Labbé, R.M. An overview of the pepper weevil (Coleoptera: Curculionidae) as a pest of greenhouse peppers. J. Integr. Pest Manag. 2020, 11, 26. [Google Scholar] [CrossRef]
- Tonina, L.; Zanettin, G.; Miorelli, P.; Puppato, S.; Cuthbertson, A.G.S.; Grassi, A. Anthonomus rubi on strawberry fruit: Its biology, ecology, damage, and control from an ipm perspective. Insects 2021, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, M.A.; Fitzgerald, J.D.; Pinch, C.; Tooley, J.; Xu, X.M. Development times and fecundity of three important arthropod pests of strawberry in the United Kingdom. Ann. Appl. Biol. 2003, 143, 325–331. [Google Scholar] [CrossRef]
- Polis, G.A. The Evolution and Dynamics of Intraspecific Predation. Ann. Rev. Eeal Syst 1981, 12, 225–251. [Google Scholar] [CrossRef]
- Fox, L.R. Cannibalism in Natural Populations. Annu. Rev. Ecol. Syst. 1975, 6, 87–106. [Google Scholar] [CrossRef]
- Richardson, M.L.; Mitchell, R.F.; Reagel, P.F.; Hanks, L.M. Causes and consequences of cannibalism in non carnivorous insects. Annu. Rev. Entomol. 2010, 55, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Bolívar-Silva, D.A.; Guedes, N.M.P.; Guedes, R.N.C. Larval cannibalism and fitness in the stored grain weevils Sitophilus granarius and Sitophilus zeamais. J. Pest Sci. 2018, 91, 707–716. [Google Scholar] [CrossRef]
- de Medeiros, B.A.S.; de Cássia Bená, D.; Vanin, S.A. Curculio Curculis lupus: Biology, behavior and morphology of immatures of the cannibal weevil Anchylorhynchus eriospathae G. G. Bondar, 1943. Peer J. 2014, 2014, e502. [Google Scholar] [CrossRef][Green Version]
- van den Bosch, F.; de Roos, A.M.; Gabriel, W. Cannibalism as a life boat mechanism. J. Math. Biol. 1988, 26, 619–633. [Google Scholar] [CrossRef]
- Mayer, M.S.; Brazzel, J.R. The Mating Behavior of the Boll Weevil, Anthonomus grandis. J. Econ. Entomol. 1963, 56, 605–609. [Google Scholar] [CrossRef]
- Cardé, R.T.; Minks, A.K. Control of Moth Pests by Mating Disruption: Successes and Constraints. Annu. Rev. Entomol. 1995, 40, 559–585. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Haubruge, E.; Verheggen, F.J. Pheromone-based management strategies to control the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). A review. Agron. Soc. Environ. 2013, 17, 475–482. [Google Scholar]
- Tumlinson, J.H.; Hardee, D.D.; Gueldner, R.C.; Thompson, A.C.; Hedin, P.A.; Minyard, J.P. Sex pheromones produced by male boll weevil: Isolation, identification, and synthesis. Science 1969, 166, 1010–1012. [Google Scholar] [CrossRef]
- Eller, F.J.; Bartelt, R.J.; Shasha, B.S.; Schuster, D.J.; Riley, D.G.; Stansly, P.A.; Mueller, T.F.; Shuler, K.D.; Johnson, B.; Davis, J.H.; et al. Aggregation pheromone for the pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae): Identification and field activity. J. Chem. Ecol. 1994, 20, 1537–1555. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, P.J.; Hall, D.R.; Cross, J.V. Components of male aggregation pheromone of strawberry blossom weevil, Anthonomus rubi Herbst. (Coleoptera: Curculionidae). J. Chem. Ecol. 2001, 27, 1203–1218. [Google Scholar] [CrossRef]
- Hardee, D.D.; McKibben, G.H.; Rummel, D.R.; Huddleston, P.M.; Coppedge, J.R. Response of Boll Weevils to Component Ratios and Doses of the Pheromone, Grandlure. Environ. E 1974, 3, 135–138. [Google Scholar] [CrossRef]
- Hardee, D.D.; McKibben, G.H.; Gueldner, R.C.; Mitchell, E.B.; Tumlinson, J.H.; Cross, W.H. Boll Weevils in Nature Respond to Grandlure, a Synthetic Pheromone123. J. Econ. Entomol. 1972, 65, 97–100. [Google Scholar] [CrossRef]
- Szendrei, Z.; Averill, A.; Alborn, H.; Rodriguez-Saona, C. Identification and Field Evaluation of Attractants for the Cranberry Weevil, Anthonomus musculus Say. J. Chem. Ecol. 2011, 37, 387–397. [Google Scholar] [CrossRef]
- Cross, J.V.; Hesketh, H.; Jay, C.N.; Hall, D.R.; Innocenzi, P.J.; Farman, D.I.; Burgess, C.M. Exploiting the aggregation pheromone of strawberry blossom weevil Anthonomus rubi Herbst (Coleoptera: Curculionidae): Part 1. Development of lure and trap. Crop Prot. 2006, 25, 144–154. [Google Scholar] [CrossRef]
- Silva, D.; Salamanca, J.; Kyryczenko-Roth, V.; Alborn, H.T.; Rodriguez-Saona, C. Comparison of Trap Types, Placement, and Colors for Monitoring Anthonomus musculus (Coleoptera: Curculionidae) Adults in Highbush Blueberries. J. Insect Sci. 2018, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A. Sexual conflict over mating and fertilization: An overview. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.G. Sexual Selection and Sexual Conflict. In Sexual Selection and Reproductive Competition in Insects; Blum, M., Blum, N., Eds.; Academic Press: Cambridge, MA, USA, 1979; pp. 123–166. [Google Scholar]
- Ward, P.I.; Hemmi, J.; Roosli, T. Sexual Conflict in the Dung Fly Sepsis cynipsea. Funct. Ecol. 1992, 6, 649. [Google Scholar] [CrossRef]
- Lessells, C.M. Sexual conflict in animals. In Levels of Selection in Evolution; Keller, L., Ed.; Princeton University Press: Princeton, NJ, USA, 1999; pp. 75–99. [Google Scholar]
- Parker, G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970, 45, 525–567. [Google Scholar] [CrossRef]
- Parker, G.A. The reproductive behaviour and the nature of sexual selection in Scatophaga stercoraria L. (Diptera: Scatophagidae). IV. Epigamic recognition and competition between males for the possession of females. Behaviour 1970, 37, 113–179. [Google Scholar] [CrossRef]
- Stockley, P. Sexual conflict resulting from adaptations to sperm competition. Trends Ecol. Evol. 1997, 12, 154–159. [Google Scholar] [CrossRef]
- Davies, N.B. Cooperation and conflict among dunnocks, Prunella modularis, in a variable mating system. Anim. Behav. 1985, 33, 628–648. [Google Scholar] [CrossRef]
- Rowe, L.; Arnqvist, G.; Sih, A.; Krupa, J. Sexual conflict and the evolutionary ecology of mating patterns: Water striders as a model system. Trends Ecol. Evol. 1994, 9, 289–293. [Google Scholar] [CrossRef]
- Roff, D.A. The Evolution of Life Histories; Routledge, Chapman & Hall, Inc.: New York, NY, USA, 1992; ISBN 0412023911. [Google Scholar]
- Moreau, J.; Benrey, B.; Thiery, D. Assessing larval food quality for phytophagous insects: Are the facts as simple as they appear? Funct. Ecol. 2006, 20, 592–600. [Google Scholar] [CrossRef]
- Ryne, C.; Nilsson, P.A.; Siva-Jothy, M.T. Dietary glycerol and adult access to water: Effects on fecundity and longevity in the almond moth. J. Insect Physiol. 2004, 50, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Erdem, M.; Buyukguzel, E.; Buyukguzel, K. Effect of dietary sodium tetraborate on adult longevity and fecundity of Drosophila melanogaster (Diptera: Drosphilidae). J. Entomol. Sci. 2016, 51, 305–313. [Google Scholar] [CrossRef]
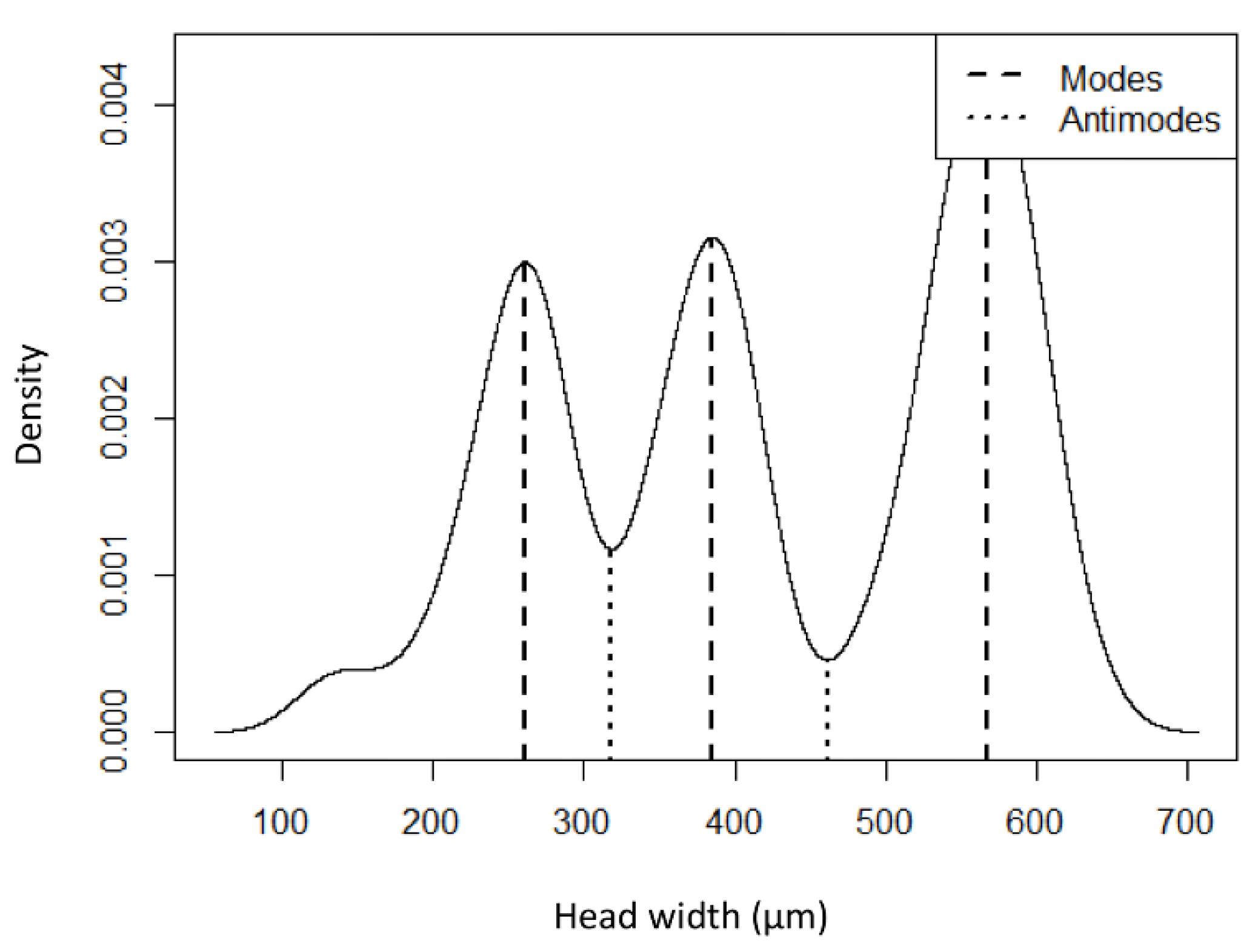
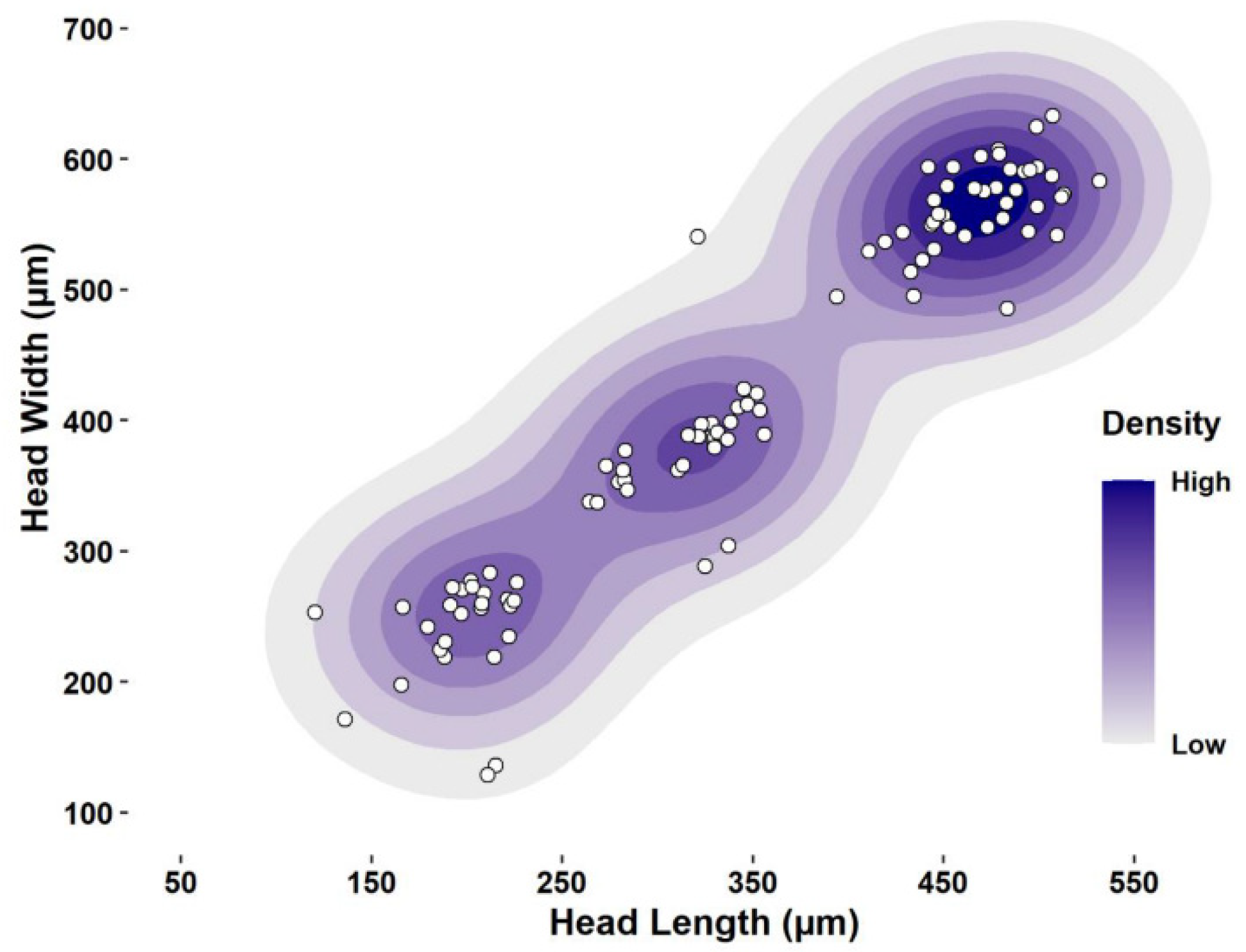
| Instar | n | Width ± SE | Range | Dyar’s Constant |
|---|---|---|---|---|
| First | 29 | 248.14 ± 8.57a | 129–318.37 | - |
| Second | 26 | 383.31 ± 4.65b | 318.38–461.38 | 1.48 |
| Third | 43 | 563.23 ± 5.07c | 461.39–633 | 1.48 |
| Food Source | Temperature (°C) | Egg (n) | First Instar (n) | Second Instar (n) | Third Instar (n) | Pupa (n) | Egg to Adult (n) |
|---|---|---|---|---|---|---|---|
| Hibiscus buds | 10 | 78.2 ± 0.55a (20) | - | - | - | - | - |
| 15 | 13 ± 1.33b (20) | 4.9 ± 0.86a (10) | 12.75 ± 2.46a (4) | 87 ± 14.01a (3) | - | - | |
| 27 | 3.35 ± 0.31d (20) | 2.6 ± 0.24a (20) | 3.73 ± 0.48a (19) | 2.05 ± 0.19b (18) | 4.1 ± 0.27 (18) | 15.78 ± 0.83 (18) | |
| 34 | 5.5 ± 0.29c (20) | 2.53 ± 0.29a (19) | 8.92 ± 1.3b (13) | 25.5 ± 8.86ac (6) | - | - | |
| Artificial diet | 27 | 2.22 ± 0.05e (129) | 1.94 ± 0.05b (128) | 3.9 ± 0.08a (128) | 4.25 ± 0.23b (128) | 4.21 ± 0.07 (128) | 16.47 ± 0.3 (128) |
| Development, Feeding and Oviposition | Fecundity * (n) | Fertility ** (n) | Pre-Oviposition Period *** (n) | Oviposition Period *** (n) | Post-Oviposition Period *** (n) |
|---|---|---|---|---|---|
| Hibiscus buds | 5.85 ± 0.48a (20) | 55.2 ± 2.32 (10) | 4.05 ± 0.4c (20) | 40.35 ± 3.53 (20) | 4.45 ± 1b (20) |
| Artificial diet | 0.2 ± 0.04b (21) | NA | 6.33 ± 0.3b (21) | 32.29 ± 3.48 (21) | 19.85 ± 3.94a (21) |
| Artificial diet + Hibiscus buds | 0.73 ± 0.57b (20) | 62 ± 0.03 (25) | 11.35 ± 1.21a (20) | 38.45 ± 3.15 (20) | 19.85 ± 3.2a (20) |
| Development, Feeding and Oviposition | n | Net Reproductive Rate (Ro) * | Intrinsic Rate of Increase (rm) ** | Generation Time (T) *** | Doubling Time (Dt) *** | Finite Rate of Increase (λ) *** |
|---|---|---|---|---|---|---|
| Hibiscus buds | 20 | 136.73a 135.54–137.91 | 0.4547a 0.4522–0.4573 | 10.82a 10.76–10.88 | 1.52a 1.51–1.53 | 1.5758a 1.5717–1.5798 |
| Artificial diet | 21 | 7.65c 7.48–7.83 | 0.0578c 0.0572–0.0584 | 35.2c 35.03–35.3 | 11.99c 11.86–12.13 | 1.0599c 1.0588–1.0601 |
| Artificial diet and Hibiscus buds | 20 | 20.85b 20.46–21.23 | 0.0841b 0.0834–0.0847 | 36.09b 35.96–36.09 | 8.24b 8.18–8.30 | 1.0877b 1.0871–1.0885 |
| Trial | Gender | n | Longevity ± SE | Max Longevity | Min Longevity |
|---|---|---|---|---|---|
| (A) Status | |||||
| Paired | Female | 20 | 47.3 ± 4.5a | 89 | 13 |
| Male | 20 | 111.1 ± 8.4b | 169 | 58 | |
| Solitary | Female | 10 | 109.2 ± 12.8b | 162 | 14 |
| Male | 10 | 86 ± 9.9b | 134 | 42 | |
| (B) Food/oviposition source | |||||
| Hibiscus buds | Female | 20 | 69.65 ± 6.1a | 127 | 35 |
| Male | 20 | 61.85 ± 5.9b | 115 | 11 | |
| Artificial diet | Female | 21 | 75.62 ± 4.5a | 107 | 30 |
| Male | 21 | 60.33 ± 5.0b | 107 | 25 | |
| (C) Access to water | |||||
| With | Female | 4 | 25.5 ± 4.51a | 34 | 14 |
| Male | 6 | 30.1 ± 4.5b | 50 | 16 | |
| Without | Female | 6 | 13.7 ± 1.5a | 18 | 9 |
| Male | 4 | 19.5 ± 2.9b | 28 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revynthi, A.M.; Velazquez Hernandez, Y.; Canon, M.A.; Greene, A.D.; Vargas, G.; Kendra, P.E.; Mannion, C.M. Biology of Anthonomus testaceosquamosus Linell, 1897 (Coleoptera: Curculionidae): A New Pest of Tropical Hibiscus. Insects 2022, 13, 13. https://doi.org/10.3390/insects13010013
Revynthi AM, Velazquez Hernandez Y, Canon MA, Greene AD, Vargas G, Kendra PE, Mannion CM. Biology of Anthonomus testaceosquamosus Linell, 1897 (Coleoptera: Curculionidae): A New Pest of Tropical Hibiscus. Insects. 2022; 13(1):13. https://doi.org/10.3390/insects13010013
Chicago/Turabian StyleRevynthi, Alexandra M., Yisell Velazquez Hernandez, Maria A. Canon, A. Daniel Greene, German Vargas, Paul E. Kendra, and Catharine M. Mannion. 2022. "Biology of Anthonomus testaceosquamosus Linell, 1897 (Coleoptera: Curculionidae): A New Pest of Tropical Hibiscus" Insects 13, no. 1: 13. https://doi.org/10.3390/insects13010013
APA StyleRevynthi, A. M., Velazquez Hernandez, Y., Canon, M. A., Greene, A. D., Vargas, G., Kendra, P. E., & Mannion, C. M. (2022). Biology of Anthonomus testaceosquamosus Linell, 1897 (Coleoptera: Curculionidae): A New Pest of Tropical Hibiscus. Insects, 13(1), 13. https://doi.org/10.3390/insects13010013







