Evaluation of the Effect of Fungatol and Gamma-T-ol on the Emergence and Adult Parasitoid Survival of Mummies of Cotton Aphids Parasitized by Aphidius colemani
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphids and Parasitoids
2.2. Chemicals
2.3. Effect of the Tested Formulations on A. colemani Emergence under Laboratory Conditions
2.4. Effect of Tested Formulations on Parasitoid Emergence under Glasshouse Conditions
2.5. Residual Toxicity of the Tested Formulations to Adults of A. colemani under Laboratory Conditions
2.6. Statistical Analysis
3. Results
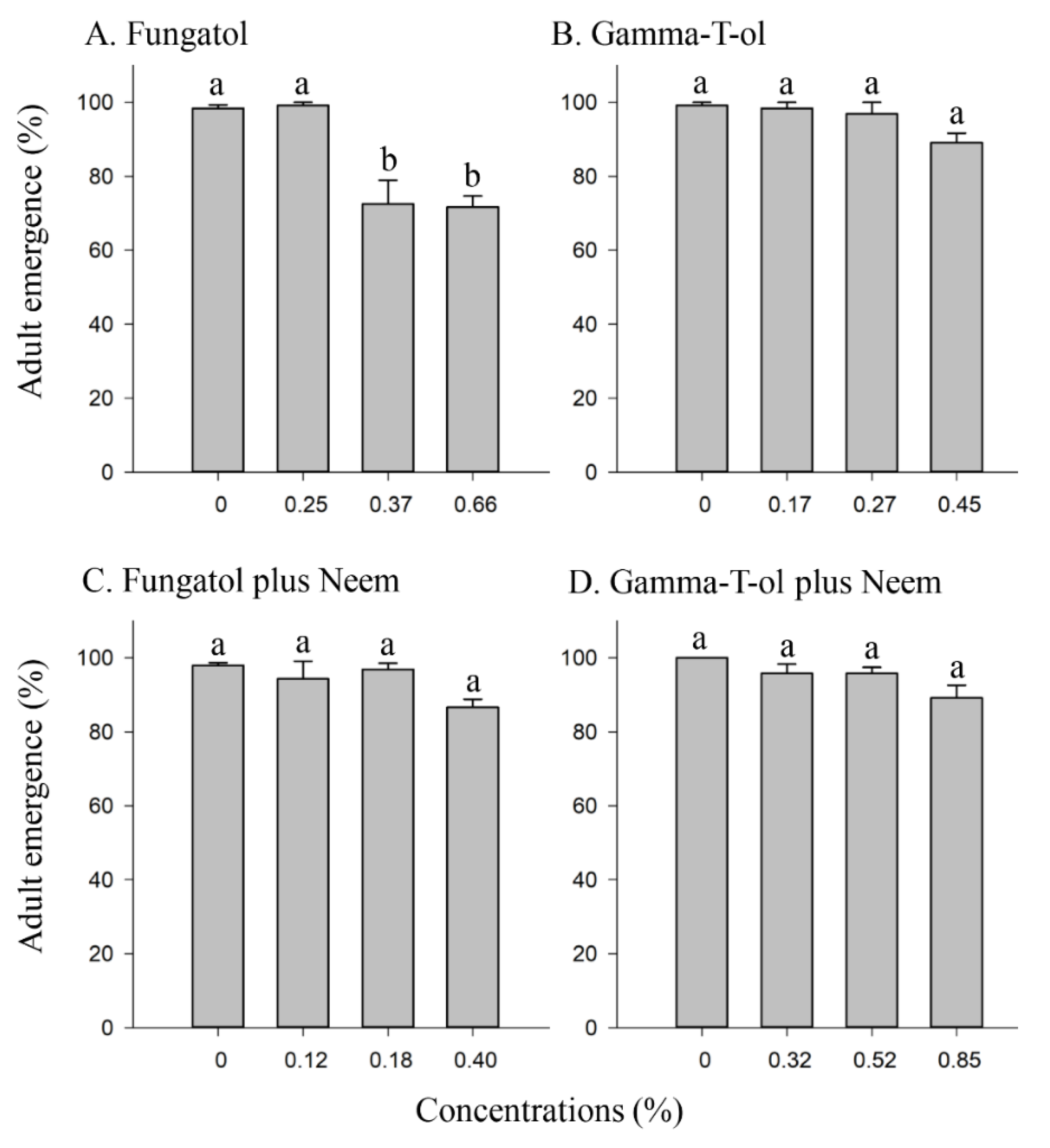
3.1. Effect of the Tested Formulations on the Emergence of A. colemani from Mummies under Laboratory Conditions
3.2. Effect of the Tested Formulations on the Emergence of A. colemani from Treated Mummies under Glasshouse Conditions
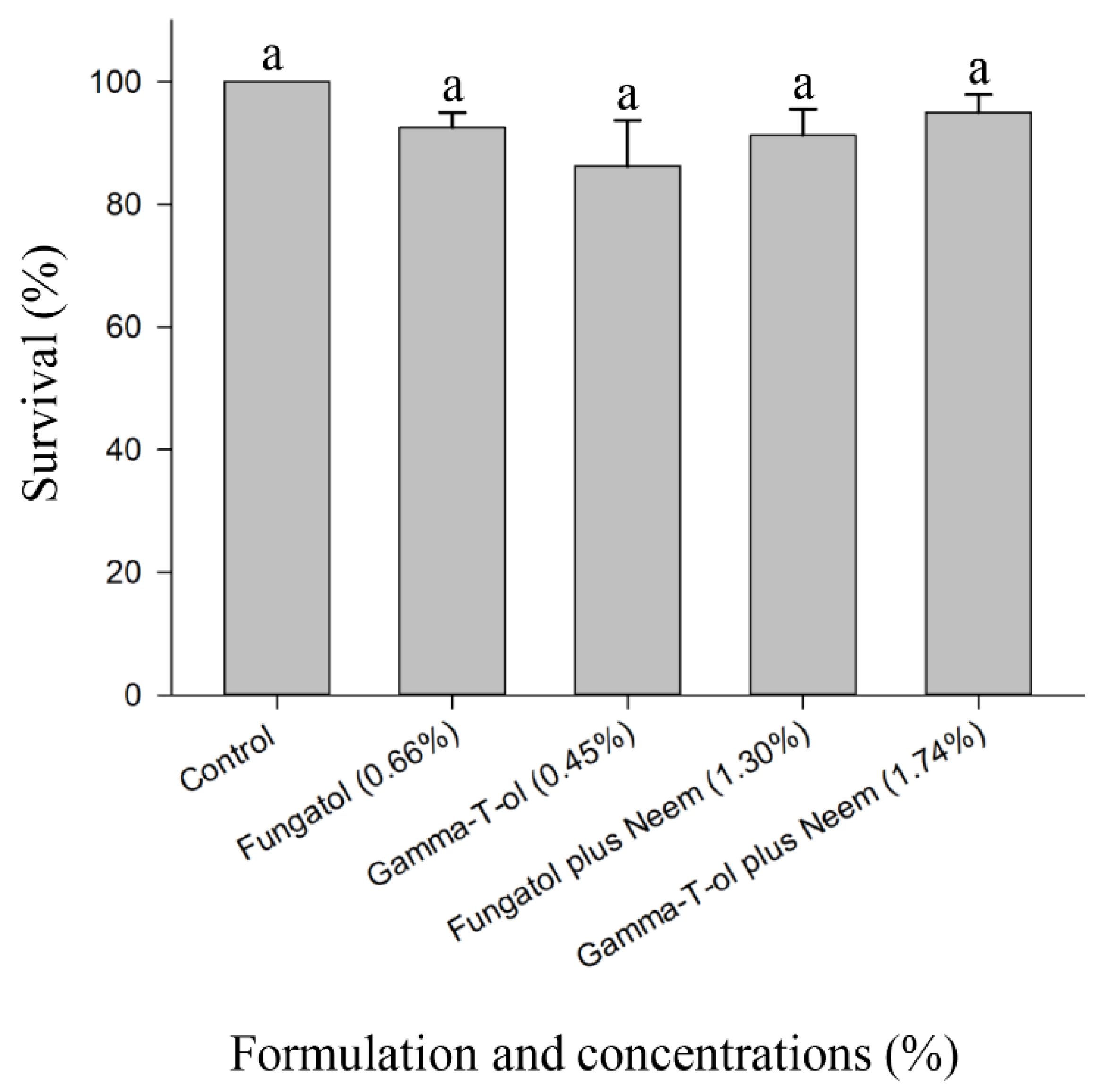
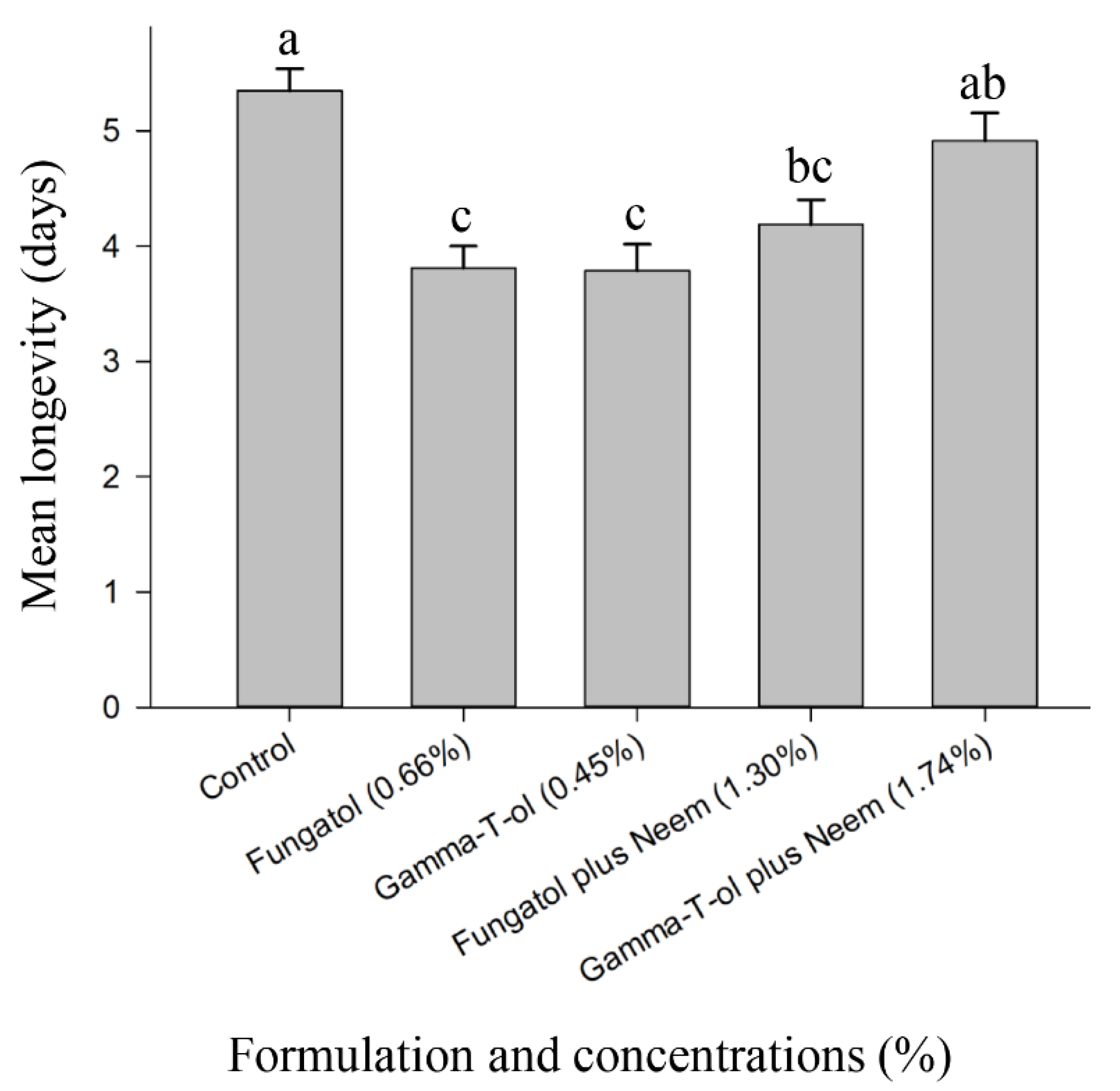
3.3. Residual Toxicity of the Tested Formulations to Adults of A. colemani under Laboratory Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, M.H.; Lauer, A.; Purtauf, T.; Thies, C.; Schaefer, M.; Tscharntke, T. Relative importance of predators and parasitoids for cereal aphid control. Proc. R. Soc. B Biol. Sci. 2003, 270, 1905–1909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer, M.J.; Elliott, N.C. Biological control of cereal aphids in North America and mediating effects of host plant and habitat manipulation. Annu. Rev. Entomol. 2004, 49, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Wu, K.; Hopper, K.R.; Li, G. Population dynamics of Aphis glycines (Homoptera: Aphididae) and impact of natural enemies in northern China. Environ. Entomol. 2007, 36, 840–848. [Google Scholar] [CrossRef]
- Carver, M.; Starý, P. A preliminary review of the Aphididae (Hymenoptera: Ichneumonoidea) of Australia and New Zealand. Aust. J. Entomol. 1974, 13, 235–240. [Google Scholar] [CrossRef]
- Carver, M.; Franzmann, B. Lysiphlebus Förster (Hymenoptera: Braconidae: Aphidiinae) in Australia. Aust. J. Entomol. 2001, 40, 198–201. [Google Scholar] [CrossRef]
- Bostanian, N.J.; Akalach, M.; Chiasson, H. Effects of a Chenopodium-based botanical insecticide/acaricide on Orius insidiosus (Hemiptera: Anthocoridae) and Aphidius colemani (Hymenoptera: Braconidae). Pest Manag. Sci. 2005, 61, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Grasswitz, T.R. Effect of adult experience on the host-location behavior of the aphid parasitoid Aphidius colemani Viereck (Hymenoptera: Aphidiidae). Biol. Control 1998, 12, 177–181. [Google Scholar] [CrossRef]
- Jones, D.B.; Giles, K.L.; Berberet, R.C.; Royer, T.A.; Elliott, N.C.; Payton, M.E. Functional responses of an introduced parasitoid and an indigenous parasitoid on greenbug at four temperatures. Environ. Entomol. 2003, 32, 425–432. [Google Scholar] [CrossRef]
- Iramu, E.T. A Critical Evaluation of the Effects of Plant Essential Oil Formulations against Two Generalised Insect Pests of Abelmoschus manihot (L.) Medik (Family: Malvaceae). Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2012. [Google Scholar]
- Preston, S.R. Aibika/Bele Abelmoschus manihot (L.) Medik. In Promoting the Conservation and Use of Underutilized and Neglected Crops; Institute of Plant Genetics and Crop Plant Research: Gatersleben, Germany; International Plant Genetic Resources Institute: Rome, Italy, 1998; p. 97. [Google Scholar]
- Sutherland, J. The effect of jassid attack on sixteen varieties of aibika. Sci. New Guinea 1984, 11, 96–104. [Google Scholar]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2006, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Fytrou, N.; Staurakaki, M.; Vontas, J.; Tsagkarakou, A. Activity of flonicamid on the sweet potato whitely Bemisia tabaci (Homoptera: Aleyrodidae) and its natural enemies. Pest Manag. Sci. 2014, 70, 1460–1467. [Google Scholar] [CrossRef]
- Stapel, J.O.; Cortesero, A.M.; Lewis, W.J. Disruptive sublethal effects of insecticides on biological control: Altered foraging ability and life span of a parasitoid after feeding on extrafloral nectar of cotton treated with systemic insecticides. Biol. Control 2000, 17, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Lowery, D.T.; Isman, M.B. Toxicity of neem to natural enemies of aphids. Phytoparasitica 1995, 23, 297–306. [Google Scholar] [CrossRef]
- Fernandes, F.L.; Bacci, L.; Fernandes, M.S. Impact and selectivity of insecticides to predators and parasitoids. EntomoBrasilis 2010, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhang, A. A floral fragrance, methyl benzoate, is an efficient green pesticide. Sci. Rep. 2017, 7, 42168. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Zhang, A. Commercially available natural benzyl esters and their synthetic analogs exhibit different toxicities against insect pests. Sci. Rep. 2018, 8, 7902. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Jhan, P.K.; Shim, J.-K.; Lee, K.-Y. Methyl benzoate exhibits insecticidal and repellent activities against Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). PLoS ONE 2018, 13, e0208552. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Shim, J.-K.; Hwang, H.-S.; Bunch, H.; Lee, K.-Y. Acaricidal effects of methyl benzoate against Tetranychus urticae Koch (Acari: Tetranychidae) on common crop plants. Pest Manag. Sci. 2020, 76, 2347–2354. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Alam, M.B.; Chi, H.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Effects of sublethal doses of methyl benzoate on the life history traits and acetylcholinesterase (AChE) activity of Aphis gossypii. Agronomy 2020, 10, 1313. [Google Scholar] [CrossRef]
- Bedini, S.; Guarino, S.; Echeverria, M.C.; Flamini, G.; Ascrizzi, R.; Loni, A.; Conti, B. Allium sativum, Rosmarinus officinalis, and Salvia officinalis essential oils: A spiced shield against blowflies. Insects 2020, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Ikbal, C.; Pavela, R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Ghabbari, M.; Guarino, S.; Caleca, V.; Saiano, F.; Sinacori, M.; Baser, N.; Mediouni-Ben Jemâa, J.; Lo Verde, G. Behavior-modifying and insecticidal effects of plant extracts on adults of Ceratitis capitata (Wiedemann) (Diptera Tephritidae). J. Pest Sci. 2018, 91, 907–917. [Google Scholar] [CrossRef]
- Isman, M.B. A renaissance for botanical insecticides? Pest Manag. Sci. 2015, 71, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Weathersbee, A.A.; Mayer, R.T. Effect of neem seed extract on the brown citrus aphid (Homoptera: Aphididae) and its parasitoid Lysiphlebus testaceipes (Hymenoptera: Aphidiidae). Environ. Entomol. 2002, 31, 172–176. [Google Scholar] [CrossRef] [Green Version]
- Mostafiz, M.M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Insecticidal efficacy of three benzoate derivatives against Aphis gossypii and its predator Chrysoperla carnea. Ecotoxicol. Environ. Saf. 2019, 184, 109653. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Lethal and sublethal effects of methyl benzoate on the predatory bug Nesidiocoris tenuis. Insects 2020, 11, 377. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from different species of the lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef] [Green Version]
- Stanley, T.D.; Ross, E.M. Flora of Southeastern Queensland; Queensland Department of Primary Industries: Brisbane, Australia, 1983; ISBN 0724217606.
- Weiss, E. Essential Oil Crops; CAB International: Wallingford, Oxfordshire, UK; New York, NY, USA, 1997; ISBN 9780851991375. [Google Scholar]
- Liao, M.; Yang, Q.Q.; Xiao, J.J.; Huang, Y.; Zhou, L.J.; Hua, R.M.; Cao, H.Q. Toxicity of Melaleuca alternifolia essential oil to the mitochondrion and NAD+/NADH dehydrogenase in Tribolium confusum. PeerJ 2018, 6, e5693. [Google Scholar] [CrossRef] [Green Version]
- Bustos-Segura, C.; Külheim, C.; Foley, W. Effects of terpene chemotypes of Melaleuca alternifolia on two specialist leaf beetles and susceptibility to myrtle rust. J. Chem. Ecol. 2015, 41, 937–947. [Google Scholar] [CrossRef]
- Liao, M.; Xiao, J.J.; Zhou, L.J.; Liu, Y.; Wu, X.W.; Hua, R.M.; Wang, G.R.; Cao, H.Q. Insecticidal activity of Melaleuca alternifolia essential oil and RNA-Seq analysis of Sitophilus zeamais transcriptome in response to oil fumigation. PLoS ONE 2016, 11, e0167748. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Xiao, J.J.; Zhou, L.J.; Yao, X.; Tang, F.; Hua, R.M.; Wu, X.W.; Cao, H.Q. Chemical composition, insecticidal and biochemical effects of Melaleuca alternifolia essential oil on the Helicoverpa armigera. J. Appl. Entomol. 2017, 141, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Kurekci, C.; Padmanabha, J.; Bishop-Hurley, S.L.; Hassan, E.; Al Jassim, R.A.M.; McSweeney, C.S. Antimicrobial activity of essential oils and five terpenoid compounds against Campylobacter jejuni in pure and mixed culture experiments. Int. J. Food Microbiol. 2013, 166, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.N.M.; Aquino, S.G.; Rossa, C.; Spolidorio, D.M.P. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages. Inflamm. Res. 2014, 63, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Kasap, I.; Kök, Ş.; Hassan, E. Effect of Fungatol and Gamma-T-ol from Melaleuca alternifolia (Maiden & Betche) Cheel on Aphis gossypii Glover (Hemiptera: Aphididae) and Tetranychus urticae Koch (Acari: Tetranychidae). Turk. Entomoloji Derg. 2016, 40, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Hassan, E.; Erdogan, P. Insecticidal efficacy Of conventional and botanical insecticides against potato tuber moth (Phthorimaea operculella (Zeller) Lepidoptera: Gelechiidae). Agron. Agric. Sci. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Kasap, İ.; Kök, Ş.; Erdoğmuş, A.; Koyun, A. Repellent effect of Fungatol and Gamma-T-ol extracts derived from Melaleuca alternifolia (Myrtaceae) against Tetranychus urticae Koch (Acari: Tetranychidae) under laboratory conditions. ÇOMÜ Zir. Fak. Derg. 2016, 4, 93–98. [Google Scholar]
- Erdogan, P. Oviposition deterrent activities of some plant extracts against tomato leaf miner, Tuta Absoluta Meyrick (Lepidoptera: Gelehiidae). J. Bacteriol. Mycol. Open Access 2019, 6, 139–142. [Google Scholar] [CrossRef] [Green Version]
- Bayindir, A.; Ozger, S.; Karaca, I.; Birgucu, A.K.; Hassan, E. Effects of some plant extracts on Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) under laboratory conditions. Adv. Food Sci. 2015, 37, 132–137. [Google Scholar]
- Borgemeister, C.; Poehling, H.M.; Dinter, A.; Höller, C. Effects of insecticides on life history parameters of the aphid parasitoid Aphidius rhopalosiphi [Hym.: Aphidiidae]. Entomophaga 1993, 38, 245–255. [Google Scholar] [CrossRef]
- Lowery, D.T. Effects of Extracts from Neem, Azadirachta indica (A. Juss), on Aphids (Homoptera: Aphididae) with Respect to their Control. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 1992. [Google Scholar]
- Hassan, S.A.; Hafes, B.; Degrande, P.E.; Herai, K. The side-effects of pesticides on the egg parasitoid Trichogramma cacoeciae Marchal (Hym., Trichogrammatidae), acute dose-response and persistence tests. J. Appl. Entomol. 1998, 122, 569–573. [Google Scholar] [CrossRef]
- Grützmacher, A.D.; Zimmermann, O.; Yousef, A.; Hassan, S.A. The side-effects of pesticides used in integrated production of peaches in Brazil on the egg parasitoid Trichogramma cacoeciae Marchal (Hym., Trichogrammatidae). J. Appl. Entomol. 2004, 128, 377–383. [Google Scholar] [CrossRef]
- SAS Institute Inc. Base SAS 9.4 Procedures Guide, Statistical Procedures, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Van De Veirk, M. Laboratory test method to evaluate the effect of 31 pesticides on the predatory bug, Orius laevigatus (Het.: Anthocoridae). Entomophaga 1996, 41, 235–243. [Google Scholar] [CrossRef]
- Hassan, S.A.; Bigler, F.; Bogenschütz, H.; Boller, E.; Brun, J.; Calis, J.N.M.; Coremans-Pelseneer, J.; Duso, C.; Grove, A.; Heimbach, U.; et al. Results of the sixth joint pesticide testing programme of the IOBC/WPRS-working group «pesticides and beneficial organisms». Entomophaga 1994, 39, 107–119. [Google Scholar] [CrossRef]
- Longley, M.; Jepson, P. Effects of life stage, substrate, and crop position on the exposure and susceptibility of Aphidius rhopalosiphi DeStefani-Perez (Hymenoptera: Braconidae) to deltamethrin. Environ. Toxicol. Chem. 1997, 16, 1034–1041. [Google Scholar] [CrossRef]
- Longley, M. A review of pesticide effects upon immature aphid parasitoids within mummified hosts. Int. J. Pest Manag. 1999, 45, 139–145. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, K.; Huang, Q.; Lei, C. Evaluation toxicity of monoterpenes to subterranean termite, Reticulitermes chinensis Snyder. Ind. Crops Prod. 2014, 53, 163–166. [Google Scholar] [CrossRef]
- Palacios, S.M.; Bertoni, A.; Rossi, Y.; Santander, R.; Urzúa, A. Efficacy of essential oils from edible plants as insecticides against the house fly, Musca domestica L. Molecules 2009, 14, 1938–1947. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Zhang, Y.; Wang, L.; Xie, Y. Fumigant toxicity of monoterpenes against fruitfly, Drosophila melanogaster. Ind. Crops Prod. 2016, 81, 147–151. [Google Scholar] [CrossRef]
- Gaire, S.; Scharf, M.E.; Gondhalekar, A.D. Toxicity and neurophysiological impacts of plant essential oil components on bed bugs (Cimicidae: Hemiptera). Sci. Rep. 2019, 9, 3961. [Google Scholar] [CrossRef] [Green Version]
- Perumalsamy, H.; Kim, N.J.; Ahn, Y.J. Larvicidal activity of compounds isolated from asarum heterotropoides against Culex pipiens pallens, Aedes aegypti, and Ochlerotatus togoi (Diptera: Culicidae). J. Med. Entomol. 2009, 46, 1420–1423. [Google Scholar] [CrossRef]
- Sarma, R.; Adhikari, K.; Mahanta, S.; Khanikor, B. Combinations of plant essential oil based terpene compounds as larvicidal and adulticidal agent against Aedes aegypti (Diptera: Culicidae). Sci. Rep. 2019, 9, 9471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeke, S.J.; Sinzogan, A.A.C.; De Almeida, R.P.; De Boer, P.W.M.; Jeong, G.; Kossou, D.K.; Van Loon, J.J.A. Side-effects of cowpea treatment with botanical insecticides on two parasitoids of Callosobruchus maculatus. Entomol. Exp. Appl. 2003, 108, 43–51. [Google Scholar] [CrossRef]
- Gonçalves-Gervásio, R.D.C.; Vendramim, J.D. Efeito de extratos de Meliáceas sobre o parasitóide de ovos Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Neotrop. Entomol. 2004, 33, 607–612. [Google Scholar] [CrossRef] [Green Version]
- B Broglio-Micheletti, S.M.F.; Santos, A.J.N.D.; Pereira-Barros, J.L. Ação de alguns produtos fitossanitários para adultos de Trichogramma galloi Zucchi, 1988 (Hymenoptera: Trichogrammatidae). Ciência e Agrotecnologia 2006, 30, 1051–1055. [Google Scholar] [CrossRef] [Green Version]
- Cóndor Golec, A. Effect of neem (Azadirachta indica A. Juss) insecticides on parasitoids. Rev. Peru. Biol. 2007, 14, 69–74. [Google Scholar]
- Akol, A.M.; Sithanantham, S.; Njagi, P.G.N.; Varela, A.; Mueke, J.M. Relative safety of sprays of two neem insecticides to Diadegma mollipla (Holmgren), a parasitoid of the diamondback moth: Effects on adult longevity and foraging behaviour. Crop Prot. 2002, 21, 853–859. [Google Scholar] [CrossRef]
- Simmonds, M.S.J.; Manlove, J.D.; Blaney, W.M.; Khambay, B.P.S. Effects of selected botanical insecticides on the behaviour and mortality of the glasshouse whitefly Trialeurodes vaporariorum and the parasitoid Encarsia formosa. Entomol. Exp. Appl. 2002, 102, 39–47. [Google Scholar] [CrossRef]
- Defagó, M.T.; Dumón, A.; Avalos, D.S.; Palacios, S.M.; Valladares, G. Effects of Melia azedarach extract on Cotesia ayerza, parasitoid of the alfalfa defoliator Colias lesbia. Biol. Control 2011, 57, 75–78. [Google Scholar] [CrossRef]
- Stenger, L.D.; Abati, R.; Pawlak, I.G.; Varpechoski, G.O.; De Souza Vismara, E.; Barbosa, L.R.; Wagner Júnior, A.; Lozano, E.R.; Potrich, M. Toxicity of essential oil of Eugenia uniflora (L.) to Thaumastocoris peregrinus (Hemiptera: Thaumastocoridae) and selectivity to the parasitoid Cleruchoides noackae (Lin & Hubert) (Hymenoptera: Mymaridae). Crop Prot. 2021, 147, 105693. [Google Scholar] [CrossRef]
- Mitchell, P.L.; Gupta, R.; Singh, A.K.; Kumar, P. Behavioral and developmental effects of neem extracts on Clavigralla scutellaris (Hemiptera: Heteroptera: Coreidae) and its egg parasitoid, Gryon fulviventre (Hymenoptera: Scelionidae). J. Econ. Entomol. 2004, 97, 916–923. [Google Scholar] [CrossRef]
- Gogi, M.D.; Syed, A.H.; Atta, B.; Sufyan, M.; Arif, M.J.; Arshad, M.; Nawaz, A.; Khan, M.A.; Mukhtar, A.; Liburd, O.E. Efficacy of biorational insecticides against Bemisia tabaci (Genn.) and their selectivity for its parasitoid Encarsia formosa Gahan on Bt cotton. Sci. Rep. 2021, 11, 2101. [Google Scholar] [CrossRef] [PubMed]

| Name | Molecular Formula | Concentrations (%) | |
|---|---|---|---|
| Active constituents of Alpha Tops | Terpinen-4-ol | C10H18O | 60.5 |
| Cineole/Eucalyptol | C10H18O | 22.5 | |
| Alpha-terpineol | C10H18O | 15.0 | |
| Active constituents of Gamma Tops | Gamma-terpinene | C10H16 | 45.5 |
| Terpinolene | C10H16 | 25.0 | |
| Alpha-terpinene | C10H16 | 20.0 |
| Formulations/Concentrations (%, v/v) | |||
|---|---|---|---|
| Fungatol | Gamma-T-ol | Fungatol Plus Neem | Gamma-T-ol Plus Neem |
| 0.25 * | 0.17 | 0.08 | 0.32 |
| 0.31 | 0.20 | 0.12 | 0.41 |
| 0.37 | 0.27 | 0.18 | 0.52 |
| 0.49 | 0.34 | 0.40 | 0.85 |
| 0.66 | 0.45 | 1.30 | 1.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, E.; Mostafiz, M.M.; Iramu, E.T.; George, D.; Lee, K.-Y. Evaluation of the Effect of Fungatol and Gamma-T-ol on the Emergence and Adult Parasitoid Survival of Mummies of Cotton Aphids Parasitized by Aphidius colemani. Insects 2022, 13, 38. https://doi.org/10.3390/insects13010038
Hassan E, Mostafiz MM, Iramu ET, George D, Lee K-Y. Evaluation of the Effect of Fungatol and Gamma-T-ol on the Emergence and Adult Parasitoid Survival of Mummies of Cotton Aphids Parasitized by Aphidius colemani. Insects. 2022; 13(1):38. https://doi.org/10.3390/insects13010038
Chicago/Turabian StyleHassan, Errol, Md Munir Mostafiz, Ellen Talairamo Iramu, Doug George, and Kyeong-Yeoll Lee. 2022. "Evaluation of the Effect of Fungatol and Gamma-T-ol on the Emergence and Adult Parasitoid Survival of Mummies of Cotton Aphids Parasitized by Aphidius colemani" Insects 13, no. 1: 38. https://doi.org/10.3390/insects13010038







