Effect of Organic Farming and Agricultural Abandonment on Beneficial Arthropod Communities Associated with Olive Groves in Western Spain: Implications for Bactrocera oleae Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
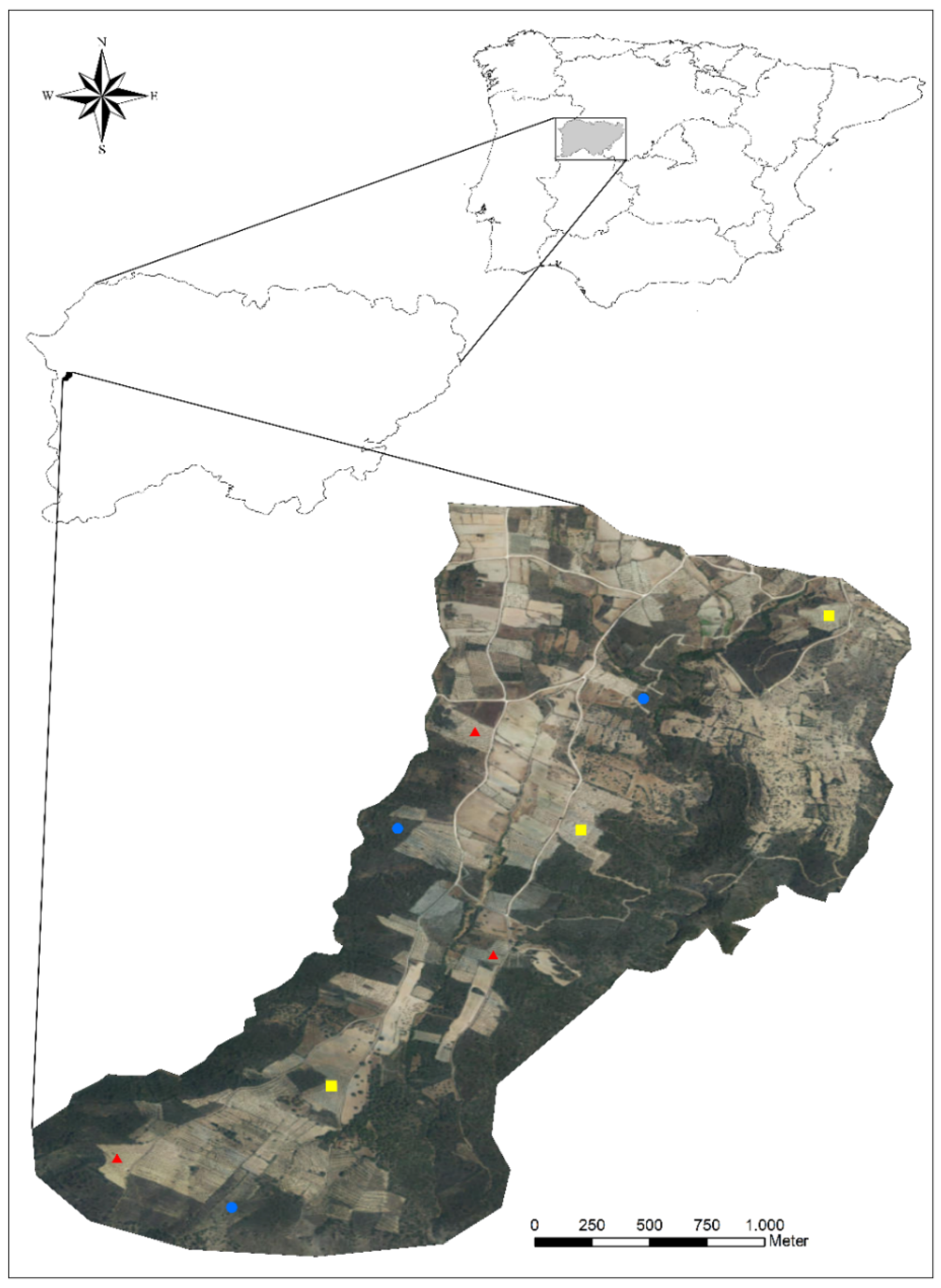
2.1. Study Area
2.2. Sampling Design
2.3. Statistical Analyses
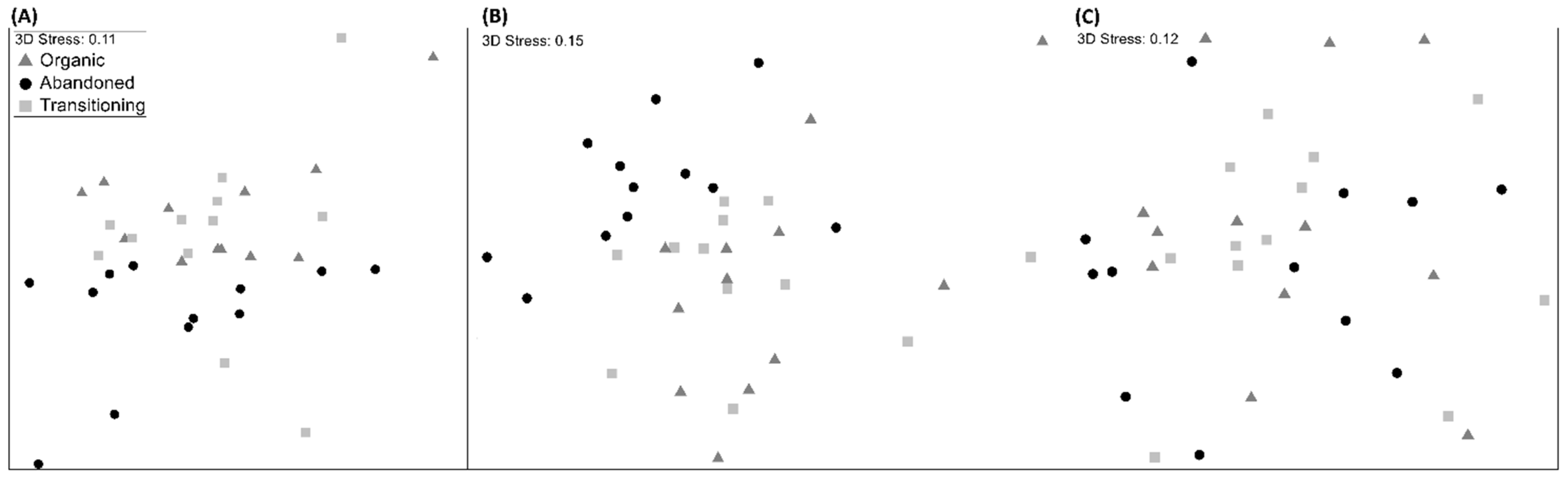
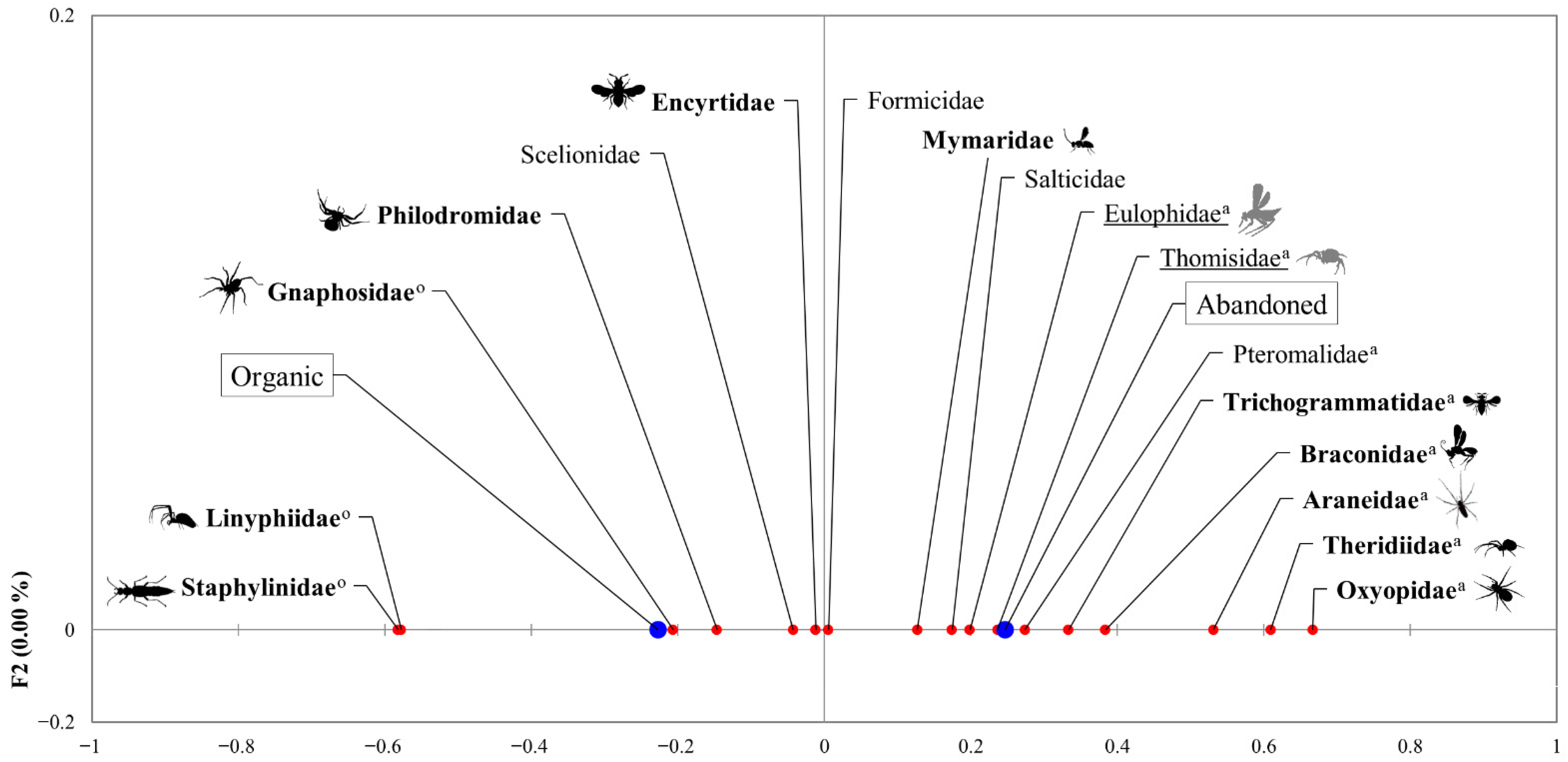
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Bongaarts, J. IPBES Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Popul. Dev. Rev. 2019, 45, 680–681. [Google Scholar]
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, 1–6. [Google Scholar] [CrossRef]
- Lichtenberg, E.M.; Kennedy, C.M.; Kremen, C.; Batáry, P.; Berendse, F.; Bommarco, R.; Bosque-Pérez, N.A.; Carvalheiro, L.G.; Snyder, W.E.; Williams, N.M.; et al. A global synthesis of the effects of diversified farming systems on arthropod diversity within fields and across agricultural landscapes. Glob. Chang. Biol. 2017, 23, 4946–4957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef] [Green Version]
- Tuck, S.L.; Winqvist, C.; Mota, F.; Ahnström, J.; Turnbull, L.A.; Bengtsson, J. Land-use intensity and the effects of organic farming on biodiversity: A hierarchical meta-analysis. J. Appl. Ecol. 2014, 51, 746–755. [Google Scholar] [CrossRef]
- Bengtsson, J.; Ahnström, J.; Weibull, A.C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Concepción, E.D.; Díaz, M.; Kleijn, D.; Báldi, A.; Batáry, P.; Clough, Y.; Gabriel, D.; Herzog, F.; Holzschuh, A.; Knop, E.; et al. Interactive effects of landscape context constrain the effectiveness of local agri-environmental management. J. Appl. Ecol. 2012, 49, 695–705. [Google Scholar] [CrossRef]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batáry, P.; Bengtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.F.; et al. Landscape moderation of biodiversity patterns and processes—Eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef] [PubMed]
- Bruggisser, O.T.; Schmidt-Entling, M.H.; Bacher, S. Effects of vineyard management on biodiversity at three trophic levels. Biol. Conserv. 2010, 143, 1521–1528. [Google Scholar] [CrossRef] [Green Version]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Cramer, V.; Hobbs, R.; Standish, R. What’s new about old fields? Land abandonment and ecosystem assembly. Trends Ecol. Evol. 2008, 23, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Benayas, J.M.R.; Martins, A.; Nicolau, J.M.; Schulz, J.J. Abandonment of agricultural land: An overview of drivers and consequences. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Baldock, D.; Beaufoy, G.; Bennett, G.; Clark, J. Nature Conservation and New Directions in the Common Agricultural Policy: The Potential Role of EC Policies in Maintaining Farming and Management Systems of High Nature Value in the Community; Institute for European Environmental Policy (IEEP): London, UK, 1993; ISBN 90-74095-03-8. [Google Scholar]
- Fischer, J.; Hartel, T.; Kuemmerle, T. Conservation policy in traditional farming landscapes. Conserv. Lett. 2012, 5, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Keenleyside, C.; Beaufoy, G.; Tucker, G.; Jones, G. High Nature Value Farming throughout EU-27 and Its Financial Support under the CAP; Institute for European Environmental Policy (IEEP): London, UK, 2014; ISBN 9789279379581. [Google Scholar]
- Queiroz, C.; Beilin, R.; Folke, C.; Lindborg, R. Farmland abandonment: Threat or opportunity for biodiversity conservation? A global review. Front. Ecol. Environ. 2014, 12, 288–296. [Google Scholar] [CrossRef]
- Plieninger, T.; Hui, C.; Gaertner, M.; Huntsinger, L. The Impact of Land Abandonment on Species Richness and Abundance in the Mediterranean Basin: A Meta-Analysis. PLoS ONE 2014, 9, e98355. [Google Scholar] [CrossRef]
- Cremene, C.; Groza, G.; Rakosy, L.; Schileyko, A.A.; Baur, A.; Erhardt, A.; Baur, B. Alterations of Steppe-Like Grasslands in Eastern Europe: A Threat to Regional Biodiversity Hotspots. Conserv. Biol. 2005, 19, 1606–1618. [Google Scholar] [CrossRef]
- Öckinger, E.; Eriksson, A.K.; Smith, H.G. Effects of grassland abandonment, restoration and management on butterflies and vascular plants. Biol. Conserv. 2006, 133, 291–300. [Google Scholar] [CrossRef]
- Fartmann, T.; Krämer, B.; Stelzner, F.; Poniatowski, D. Orthoptera as ecological indicators for succession in steppe grassland. Ecol. Indic. 2012, 20, 337–344. [Google Scholar] [CrossRef]
- Nardi, D.; Marini, L. Role of abandoned grasslands in the conservation of spider communities across heterogeneous mountain landscapes. Agric. Ecosyst. Environ. 2021, 319, 107526. [Google Scholar] [CrossRef]
- Bonet, A.; Pausas, J.G. Species richness and cover along a 60-year chronosequence in old-fields of southeastern Spain. Plant Ecol. 2004, 174, 257–270. [Google Scholar] [CrossRef]
- Randlkofer, B.; Obermaier, E.; Hilker, M.; Meiners, T. Vegetation complexity-The influence of plant species diversity and plant structures on plant chemical complexity and arthropods. Basic Appl. Ecol. 2010, 11, 383–395. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Weissteiner, C.J.; Boschetti, M.; Böttcher, K.; Carrara, P.; Bordogna, G.; Brivio, P.A. Spatial explicit assessment of rural land abandonment in the Mediterranean area. Glob. Planet. Chang. 2011, 79, 20–36. [Google Scholar] [CrossRef]
- Blondel, J.; Aronson, J.; Bodiou, J.Y.; Boeuf, G. The Mediterranean Region: Biological Diversity in Space and Time, 2nd ed.; Oxford University Press: New York, NY, USA, 2010; ISBN 978-0-19-955798-1. [Google Scholar]
- Loumou, A.; Giourga, C. Olive groves: “The life and identity of the Mediterranean”. Agric. Hum. Values 2003, 20, 87–95. [Google Scholar] [CrossRef]
- FAOSTAT, F. Food and Agriculture Data. 2019. Available online: https://www.fao.org/faostat/en/#home (accessed on 10 July 2021).
- Daane, K.M.; Johnson, M.W. Olive Fruit Fly: Managing an Ancient Pest in Modern Times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef]
- Ordano, M.; Engelhard, I.; Rempoulakis, P.; Nemny-Lavy, E.; Blum, M.; Yasin, S.; Lensky, I.M.; Papadopoulos, N.T.; Nestel, D. Olive Fruit Fly (Bactrocera oleae) Population Dynamics in the Eastern Mediterranean: Influence of Exogenous Uncertainty on a Monophagous Frugivorous Insect. PLoS ONE 2015, 10, e0127798. [Google Scholar] [CrossRef]
- Marchini, D.; Petacchi, R.; Marchi, S. Bactrocera oleae reproductive biology: New evidence on wintering wild populations in olive groves of Tuscany (Italy). Bull. Insectology 2017, 70, 121–128. [Google Scholar]
- Ortega, M.; Pascual, S.; Rescia, A.J. Spatial structure of olive groves and scrublands affects Bactrocera oleae abundance: A multi-scale analysis. Basic Appl. Ecol. 2016, 17, 696–705. [Google Scholar] [CrossRef]
- Albertini, A.; Marchi, S.; Ratti, C.; Burgio, G.; Petacchi, R.; Magagnoli, S. Bactrocera oleae pupae predation by Ocypus olens detected by molecular gut content analysis. BioControl 2018, 63, 227–239. [Google Scholar] [CrossRef]
- Potts, S.G.; Petanidou, T.; Roberts, S.; O’Toole, C.; Hulbert, A.; Willmer, P. Plant-pollinator biodiversity and pollination services in a complex Mediterranean landscape. Biol. Conserv. 2006, 129, 519–529. [Google Scholar] [CrossRef]
- Solomou, A.D.; Sfougaris, A.I.; Sfenthourakis, S. Terrestrial isopods as bioindicators for environmental monitoring in olive groves and natural ecosystems. J. Nat. Hist. 2019, 53, 1721–1735. [Google Scholar] [CrossRef]
- Sánchez-Fernández, J.; Vílchez-Vivanco, J.; Navarro, F.; Castro-RodrÍguez, J. Farming system and soil management affect butterfly diversity in sloping olive groves. Insect Conserv. Divers. 2020, 13, 456–469. [Google Scholar] [CrossRef]
- Sanz, C.; Mata, R.; Gómez, J.; Allende, F.; López, N.; Molina, P.; Galiana, L. Atlas de los paisajes de España; Ministerio de Medio Ambiente: Madrid, Spain, 2003. [Google Scholar]
- Ruano, F.; Lozano, C.; Garcia, P.; Pena, A.; Tinaut, A.; Pascual, F.; Campos, M. Use of arthropods for the evaluation of the olive-orchard management regimes. Agric. For. Entomol. 2004, 6, 111–120. [Google Scholar] [CrossRef]
- Picchi, M.S.; Bocci, G.; Petacchi, R.; Entling, M.H. Effects of local and landscape factors on spiders and olive fruit flies. Agric. Ecosyst. Environ. 2016, 222, 138–147. [Google Scholar] [CrossRef]
- Avinent, L.; Llacer, G. Adaptación de un aspirador de jardín para la captura de insectos. Boletín Sanid. Veg. Plagas 1995, 21, 329–335. [Google Scholar]
- de Oliveira, S.S.; Ortega, J.C.G.; dos Santos Ribas, L.G.; Lopes, V.G.; Bini, L.M. Higher taxa are sufficient to represent biodiversity patterns. Ecol. Indic. 2020, 111, 105994. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. User’s Guide and Application. 2009. Available online: http://purl.oclc.org/estimates (accessed on 12 September 2021).
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2018; ISBN 978-1-4419-7975-9. [Google Scholar]
- Zuur, A.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science & Business Media: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual; Plymouth Marine Laboratory: Plymouth, UK, 2006. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Addinsoft XLSTAT 2014. Data Analysis and Statistical Solution for Microsoft Excel. 2014. Available online: https://www.xlstat.com (accessed on 6 November 2021).
- Cronin, J.T.; Reeve, J.D. Host–parasitoid spatial ecology: A plea for a landscape-level synthesis. Proc. R. Soc. B Biol. Sci. 2005, 272, 2225–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [Green Version]
- Puech, C.; Baudry, J.; Joannon, A.; Poggi, S.; Aviron, S. Organic vs. conventional farming dichotomy: Does it make sense for natural enemies? Agric. Ecosyst. Environ. 2014, 194, 48–57. [Google Scholar] [CrossRef]
- Alignier, A.; Raymond, L.; Deconchat, M.; Menozzi, P.; Monteil, C.; Sarthou, J.P.; Vialatte, A.; Ouin, A. The effect of semi-natural habitats on aphids and their natural enemies across spatial and temporal scales. Biol. Control 2014, 77, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Tscharntke, T.; Rand, T.A.; Bianchi, F.J.J.A. The landscape context of trophic interactions: Insect spillover across the crop-noncrop interface. Ann. Zool. Fennici 2005, 42, 421–432. [Google Scholar]
- Cooper, T.; Arblaster, K.; Baldock, D.; Farmer, M.; Beaufoy, G.; Jones, G.; Poux, X.; McCracken, D.; Bignal, E.; Elbersen, B.; et al. Final Report on the Study of HNV Indicators for Evaluation; Institute for European Environmental Policy: London, UK, 2007. [Google Scholar]
- Horak, J.; Peltanova, A.; Podavkova, A.; Safarova, L.; Bogusch, P.; Romportl, D.; Zasadil, P. Biodiversity responses to land use in traditional fruit orchards of a rural agricultural landscape. Agric. Ecosyst. Environ. 2013, 178, 71–77. [Google Scholar] [CrossRef]
- Pulliam, H.R. Sources, Sinks, and Population Regulation. Am. Nat. 1988, 132, 652–661. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Shelton, A.M.; Nault, B.A. Evaluating Trap Crops for Diamondback Moth, Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2004, 97, 1365–1372. [Google Scholar] [CrossRef]
- Fanadzo, M.; Dalicuba, M.; Dube, E. Application of Conservation Agriculture Principles for the Management of Field Crops Pests. In Sustainable Agriculture Reviews 28; Gaba, S., Smith, B., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 125–152. ISBN 978-94-007-5448-5. [Google Scholar]
- Kajtoch, Ł. The importance of traditional orchards for breeding birds: The preliminary study on Central European example. Acta Oecologica 2017, 78, 53–60. [Google Scholar] [CrossRef]
- Barton, P.S.; Evans, M.J.; Foster, C.N.; Cunningham, S.A.; Manning, A.D. Environmental and spatial drivers of spider diversity at contrasting microhabitats. Austral Ecol. 2017, 42, 700–710. [Google Scholar] [CrossRef] [Green Version]
- Theron, K.J.; Gaigher, R.; Pryke, J.S.; Samways, M.J. High quality remnant patches in a complex agricultural landscape sustain high spider diversity. Biol. Conserv. 2020, 243, 108480. [Google Scholar] [CrossRef]
- Uetz, G.W.; Halaj, J.; Cady, A.B. Guild Structure of Spiders in Major Crops. J. Arachnol. 1999, 27, 270–280. [Google Scholar]
- Rosas-Ramos, N.; Baños-Picón, L.; Tobajas, E.; de Paz, V.; Tormos, J.; Asís, J.D. Value of ecological infrastructure diversity in the maintenance of spider assemblages: A case study of Mediterranean vineyard agroecosystems. Agric. Ecosyst. Environ. 2018, 265, 244–253. [Google Scholar] [CrossRef]
- Rosas-Ramos, N.; Baños-Picón, L.; Tormos, J.; Asís, J.D. The complementarity between ecological infrastructure types benefits natural enemies and pollinators in a Mediterranean vineyard agroecosystem. Ann. Appl. Biol. 2019, 175, 193–201. [Google Scholar] [CrossRef]
- Benhadi-Marín, J.; Pereira, J.A.; Sousa, J.P.; Santos, S.A.P. Distribution of the spider community in the olive grove agroecosystem (Portugal): Potential bioindicators. Agric. For. Entomol. 2020, 22, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Markó, V.; Keresztes, B.; Fountain, M.T.; Cross, J.V. Prey availability, pesticides and the abundance of orchard spider communities. Biol. Control 2009, 48, 115–124. [Google Scholar] [CrossRef]
- Spears, L.R.; MacMahon, J.A. An experimental study of spiders in a shrub-steppe ecosystem: The effects of prey availability and shrub architecture. J. Arachnol. 2012, 40, 218–227. [Google Scholar] [CrossRef]
- Álvarez, H.A.; Morente, M.; Oi, F.S.; Rodríguez, E.; Campos, M.; Ruano, F. Semi-natural habitat complexity affects abundance and movement of natural enemies in organic olive orchards. Agric. Ecosyst. Environ. 2019, 285, 106618. [Google Scholar] [CrossRef]
- Rusch, A.; Valantin-Morison, M.; Sarthou, J.-P.; Roger-Estrade, J. Biological Control of Insect Pests in Agroecosystems. In Advances in Agronomy; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; Volume 109, pp. 219–259. [Google Scholar]
- Meyhofer, R.; Hindayana, D. Effects of intraguild predation on aphid parasitoid survival. Entomol. Exp. Appl. 2000, 97, 115–122. [Google Scholar] [CrossRef]
- Finke, D.L.; Denno, R.F. Intraguild predation diminished in complex-structured vegetation: Implications for prey suppression. Ecology 2002, 83, 643–652. [Google Scholar] [CrossRef]
- Stinner, B.R.; House, G.J. Arthropods and Other Invertebrates in Conservation-Tillage Agriculture. Annu. Rev. Entomol. 1990, 35, 299–318. [Google Scholar] [CrossRef]
- Arnoldi, J.-F.; Bideault, A.; Loreau, M.; Haegeman, B. How ecosystems recover from pulse perturbations: A theory of short- to long-term responses. J. Theor. Biol. 2018, 436, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Tamburini, G.; De Simone, S.; Sigura, M.; Boscutti, F.; Marini, L. Conservation tillage mitigates the negative effect of landscape simplification on biological control. J. Appl. Ecol. 2016, 53, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Rowen, E.K.; Regan, K.H.; Barbercheck, M.E.; Tooker, J.F. Is tillage beneficial or detrimental for insect and slug management? A meta-analysis. Agric. Ecosyst. Environ. 2020, 294, 106849. [Google Scholar] [CrossRef]
- Mates, S.G.; Perfecto, I.; Badgley, C. Parasitoid wasp diversity in apple orchards along a pest-management gradient. Agric. Ecosyst. Environ. 2012, 156, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Kruess, A.; Tscharntke, T. Habitat Fragmentation, Species Loss, and Biological Control. Science 1994, 264, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.B.; Gratton, C. Local and landscape scale variables impact parasitoid assemblages across an urbanization gradient. Landsc. Urban Plan. 2012, 104, 26–33. [Google Scholar] [CrossRef]
- Aartsma, Y.; Bianchi, F.J.J.A.; Werf, W.; Poelman, E.H.; Dicke, M. Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol. 2017, 216, 1054–1063. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, P.; Pekár, S.; Jocqué, R.; Coddington, J.A. Global Patterns of Guild Composition and Functional Diversity of Spiders. PLoS ONE 2011, 6, e21710. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Ramos, N.; Baños-Picón, L.; Tormos, J.; Asís, J.D. Farming system shapes traits and composition of spider assemblages in Mediterranean cherry orchards. PeerJ 2020, 8, e8856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardenas, M.; Castro, J.; Campos, M. Short-term response of soil spiders to cover-crop removal in an organic olive orchard in a mediterranean setting. J. Insect Sci. 2012, 12, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Bellmann, H. Nueva Guía de Campo de los Arácnidos de Europa; Omega: Barcelona, Spain, 2011; ISBN 9788428215589. [Google Scholar]
- Balog, A.; Markó, V. Community structure of rove beetles (Coleoptera: Staphylinidae) in apple orchards under different pest management system programs in Hungary. Acta Phytopathol. Entomol. Hung. 2007, 42, 377–385. [Google Scholar] [CrossRef]
- Balog, A.; Markó, V. Species composition and community structure of the rove beetles (Coleoptera: Staphylinidae) in an experimental vineyard under different vineyard management systems. Acta Phytopathol. Entomol. Hung. 2007, 42, 367–376. [Google Scholar] [CrossRef]
- Honěk, A.; Kocian, M.; Martinková, Z. Rove beetles (Coleoptera: Staphylinidae) in an apple orchard. Plant Prot. Sci. 2012, 48, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Altieri, M.A.; Schmidt, L.L. Abundance patterns and foraging activity of ant communities in abandoned, organic and commercial apple orchards in Northern California. Agric. Ecosyst. Environ. 1984, 11, 341–352. [Google Scholar] [CrossRef]
- Gómez, C.; Casellas, D.; Oliveras, J.; Bas, J.M. Structure of ground-foraging ant assemblages in relation to land-use change in the northwestern Mediterranean region. Biodivers. Conserv. 2003, 12, 2135–2146. [Google Scholar] [CrossRef]
- Robinson, J.V. The Effect of Architectural Variation in Habitat on a Spider Community: An Experimental Field Study. Ecology 1981, 62, 73–80. [Google Scholar] [CrossRef]
- McCain, C.; Szewczyk, T.; Bracy Knight, K. Population variability complicates the accurate detection of climate change responses. Glob. Chang. Biol. 2016, 22, 2081–2093. [Google Scholar] [CrossRef]
- Rivers-Moore, N.A.; Samways, M.J. Game and cattle trampling, and impacts of human dwellings on arthropods at a game park boundary. Biodivers. Conserv. 1996, 5, 1545–1556. [Google Scholar] [CrossRef]

| Response Variable | Explanatory Variables | d.f. | Pseudo-F | p-Value |
|---|---|---|---|---|
| Natural enemies | System | 2 | 2.3117 | 0.0001 |
| Sampling month | 3 | 8.9107 | 0.0001 | |
| Spiders | System | 2 | 3.0515 | 0.0001 |
| Sampling month | 3 | 6.2039 | 0.0001 | |
| Parasitoids | System | 2 | 0.7927 | 0.7016 |
| Sampling month | 3 | 5.3291 | 0.0001 | |
| Pairwise Comparisons | Pseudo-t | p-Value | ||
| Natural enemies | Abandoned, organic | 1.7466 | 0.0001 | |
| Abandoned, traditional | 1.7311 | 0.0002 | ||
| Organic, traditional | 0.9225 | 0.6299 | ||
| Spiders | Abandoned, organic | 2.0979 | 0.0001 | |
| Abandoned, traditional | 2.0336 | 0.0001 | ||
| Organic, traditional | 0.7412 | 0.8070 | ||
| Parasitoids | Abandoned, organic | 0.6894 | 0.8338 | |
| Abandoned, traditional | 1.0252 | 0.4277 | ||
| Organic, traditional | 0.9476 | 0.5434 | ||
| Response Variable | Explanatory Variable | Value/Estimate | Std. Error | t-Value/z-Value | p-Value | * |
|---|---|---|---|---|---|---|
| Natural enemy family richness a | Intercept | 20.667 | 1.208 | 17.114 | <0.001 | *** |
| System (organic) | −0.667 | 1.080 | −0.617 | 0.544 | ns | |
| Natural enemy abundance a | Intercept | 247.081 | 33.023 | 7.482 | <0.001 | *** |
| System (organic) | 4.5057 | 31.315 | 0.144 | 0.887 | ns | |
| Natural enemy diversity (H) a | Intercept | 1.557 | 0.159 | 9.789 | <0.001 | *** |
| System (organic) | −0.155 | 0.142 | −1.087 | 0.291 | ns | |
| Spider family richness (square root) a | Intercept | 2.838 | 0.094 | 30.134 | <0.001 | *** |
| System (organic) | −0.157 | 0.072 | −2.161 | 0.044 | * | |
| Spider abundance b | Intercept | 3.200 | 0.126 | 25.352 | <0.001 | *** |
| System (organic) | −0.346 | 0.136 | −2.555 | 0.011 | * | |
| Spider diversity (H) a | Intercept | 1.913 | 0.140 | 13.675 | <0.001 | *** |
| System (organic) | 0.075 | 0.183 | 0.409 | 0.689 | ns | |
| Sampling month (June): system (organic) | −0.135 | 0.258 | −0.524 | 0.608 | ns | |
| Sampling month (August): system (organic) | 0.423 | 0.205 | 2.059 | 0.056 | · | |
| Sampling month (October): system (organic) | 0.467 | 0.247 | 1.894 | 0.077 | · | |
| Parasitoid family richness c | Intercept | 2.368 | 0.107 | 22.136 | <0.001 | *** |
| System (organic) | −0.034 | 0.099 | −0.347 | 0.732 | ns | |
| Parasitoid abundance d | Intercept | 48.917 | 8.685 | 5.632 | <0.001 | *** |
| System (organic) | −5.833 | 10.328 | −0.565 | 0.602 | ns | |
| Parasitoid diversity a | Intercept | 2.144 | 0.130 | 16.445 | <0.001 | *** |
| System (organic) | −0.473 | 0.184 | −2.568 | 0.021 | * | |
| Sampling month (June): system (organic) | 0.421 | 0.200 | 2.103 | 0.052 | · | |
| Sampling month (August): system (organic) | 0.108 | 0.277 | 0.389 | 0.702 | ns | |
| Sampling month (October): system (organic) | 0.701 | 0.303 | 2.317 | 0.034 | * |
| Response Variable | Explanatory Variable | Value/Estimate | Std. Error | t-Value/z-Value | p-Value | * |
|---|---|---|---|---|---|---|
| Araneidae abundance a | Intercept | 1.680 | 0.244 | 6.875 | <0.001 | *** |
| System (organic) | −1.069 | 0.244 | −4.376 | <0.001 | *** | |
| Gnaphosidae abundance a | Intercept | 0.784 | 0.491 | 1.597 | 0.110 | ns |
| System (organic) | 0.584 | 0.650 | 0.899 | 0.369 | ns | |
| Sampling month (June): system (organic) | −0.657 | 0.557 | −1.179 | 0.238 | ns | |
| Sampling month (August): system (organic) | 0.208 | 0.601 | 0.346 | 0.730 | ns | |
| Sampling month (October): system (organic) | 1.594 | 0.865 | 1.843 | 0.065 | · | |
| Linyphiidae abundance a | Intercept | 0.654 | 0.474 | 1.379 | 0.168 | ns |
| System (organic) | 0.758 | 0.599 | 1.266 | 0.205 | ns | |
| Sampling month (June): system (organic) | 1.391 | 0.608 | 2.287 | 0.022 | * | |
| Sampling month (August): system (organic) | −5.11E-05 | 0.625 | 0 | 0.999 | ns | |
| Sampling month (October): system (organic) | −0.074 | 0.688 | −0.108 | 0.914 | ns | |
| Oxyopidae abundance a | Intercept | 0.834 | 0.388 | 2.152 | 0.031 | * |
| System (organic) | −1.946 | 1.068 | −1.823 | 0.068 | · | |
| Sampling month (June): system (organic) | −0.251 | 1.500 | −0.168 | 0.867 | ns | |
| Sampling month (August): system (organic) | −1.571 | 0.538 | −2.92 | 0.004 | ** | |
| Sampling month (October): system (organic) | −2.683 | 0.624 | −4.301 | <0.001 | *** | |
| Philodromidae abundance (square root) b | Intercept | 0.334 | 0.378 | 0.884 | 0.388 | ns |
| System (organic) | 0.576 | 0.114 | 5.058 | <0.001 | *** | |
| Salticidae abundance b | Intercept | 2.000 | 0.645 | 3.098 | 0.006 | ** |
| System (organic) | −0.667 | 0.577 | −1.155 | 0.263 | ns | |
| Theridiidae abundance a | Intercept | 0.245 | 0.466 | 0.525 | 0.600 | ns |
| System (organic) | −1.287 | 0.310 | −4.15 | <0.001 | *** | |
| Thomisidae abundance a | Intercept | 0.835 | 0.390 | 2.141 | 0.032 | * |
| System (organic) | −0.002 | 0.550 | −0.004 | 0.997 | ns | |
| Sampling month (June): system (organic) | 0.167 | 0.673 | 0.248 | 0.804 | ns | |
| Sampling month (August): system (organic) | −1.447 | 0.770 | −1.879 | 0.060 | · | |
| Sampling month (October): system (organic) | −0.442 | 0.683 | −0.646 | 0.518 | ns | |
| Braconidae abundance (square root) b | Intercept | 2.943 | 0.529 | 5.567 | <0.001 | *** |
| System (organic) | −2.000 | 0.748 | −2.675 | 0.017 | * | |
| Sampling month (June): system (organic) | 1.576 | 0.835 | 1.889 | 0.077 | · | |
| Sampling month (August): system (organic) | 1.805 | 0.857 | 2.105 | 0.051 | · | |
| Sampling month (October): system (organic) | 2.667 | 0.933 | 2.858 | 0.011 | * | |
| Encyrtidae abundance a | Intercept | 1.194 | 0.334 | 3.576 | <0.001 | *** |
| System (organic) | −1.625 | 0.789 | −2.061 | 0.039 | * | |
| Sampling month (June): system (organic) | 2.197 | 0.837 | 2.625 | 0.007 | ** | |
| Sampling month (August): system (organic) | −0.056 | 0.876 | −0.064 | 0.949 | ns | |
| Sampling month (October): system (organic) | 3.091 | 0.848 | 3.644 | <0.001 | *** | |
| Eulophidae abundance a | Intercept | 1.814 | 0.200 | 9.058 | <0.001 | *** |
| System (organic) | −0.321 | 0.173 | −1.853 | 0.064 | · | |
| Mymaridae abundance b | Intercept | 1.843 | 0.197 | 9.337 | <0.001 | *** |
| System (organic) | −0.530 | 0.175 | −3.034 | 0.007 | ** | |
| Pteromalidae abundance a | Intercept | 0.668 | 0.530 | 1.259 | 0.208 | ns |
| System (organic) | −0.372 | 0.664 | −0.561 | 0.575 | ns | |
| Scelionidae abundance a | Intercept | 2.557 | 0.133 | 19.257 | <0.001 | *** |
| System (organic) | 0.169 | 0.120 | 1.401 | 0.161 | ns | |
| Trichogrammatidae abundance b | Intercept | 1.426 | 0.233 | 6.126 | <0.001 | *** |
| System (organic) | −0.600 | 0.228 | −2.634 | 0.016 | * | |
| Staphylinidae abundance a | Intercept | 1.936 | 0.353 | 5.479 | <0.001 | *** |
| System (organic) | 1.405 | 0.469 | 2.998 | 0.003 | ** | |
| Bactrocera oleae abundance a | Intercept | 1.662 | 0.566 | 2.938 | 0.003 | ** |
| System (traditional) | 0.346 | 0.788 | 0.439 | 0.660 | ns | |
| System (organic) | 0.859 | 0.775 | 1.108 | 0.268 | ns | |
| Sampling month (October): system (traditional) | 1.820 | 0.302 | 6.027 | <0.001 | *** | |
| Sampling month (October): system (organic) | 1.531 | 0.263 | 5.817 | <0.001 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Paz, V.; Tobajas, E.; Rosas-Ramos, N.; Tormos, J.; Asís, J.D.; Baños-Picón, L. Effect of Organic Farming and Agricultural Abandonment on Beneficial Arthropod Communities Associated with Olive Groves in Western Spain: Implications for Bactrocera oleae Management. Insects 2022, 13, 48. https://doi.org/10.3390/insects13010048
de Paz V, Tobajas E, Rosas-Ramos N, Tormos J, Asís JD, Baños-Picón L. Effect of Organic Farming and Agricultural Abandonment on Beneficial Arthropod Communities Associated with Olive Groves in Western Spain: Implications for Bactrocera oleae Management. Insects. 2022; 13(1):48. https://doi.org/10.3390/insects13010048
Chicago/Turabian Stylede Paz, Víctor, Estefanía Tobajas, Natalia Rosas-Ramos, José Tormos, Josep Daniel Asís, and Laura Baños-Picón. 2022. "Effect of Organic Farming and Agricultural Abandonment on Beneficial Arthropod Communities Associated with Olive Groves in Western Spain: Implications for Bactrocera oleae Management" Insects 13, no. 1: 48. https://doi.org/10.3390/insects13010048
APA Stylede Paz, V., Tobajas, E., Rosas-Ramos, N., Tormos, J., Asís, J. D., & Baños-Picón, L. (2022). Effect of Organic Farming and Agricultural Abandonment on Beneficial Arthropod Communities Associated with Olive Groves in Western Spain: Implications for Bactrocera oleae Management. Insects, 13(1), 48. https://doi.org/10.3390/insects13010048






