Climate Change Increases the Expansion Risk of Helicoverpa zea in China According to Potential Geographical Distribution Estimation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
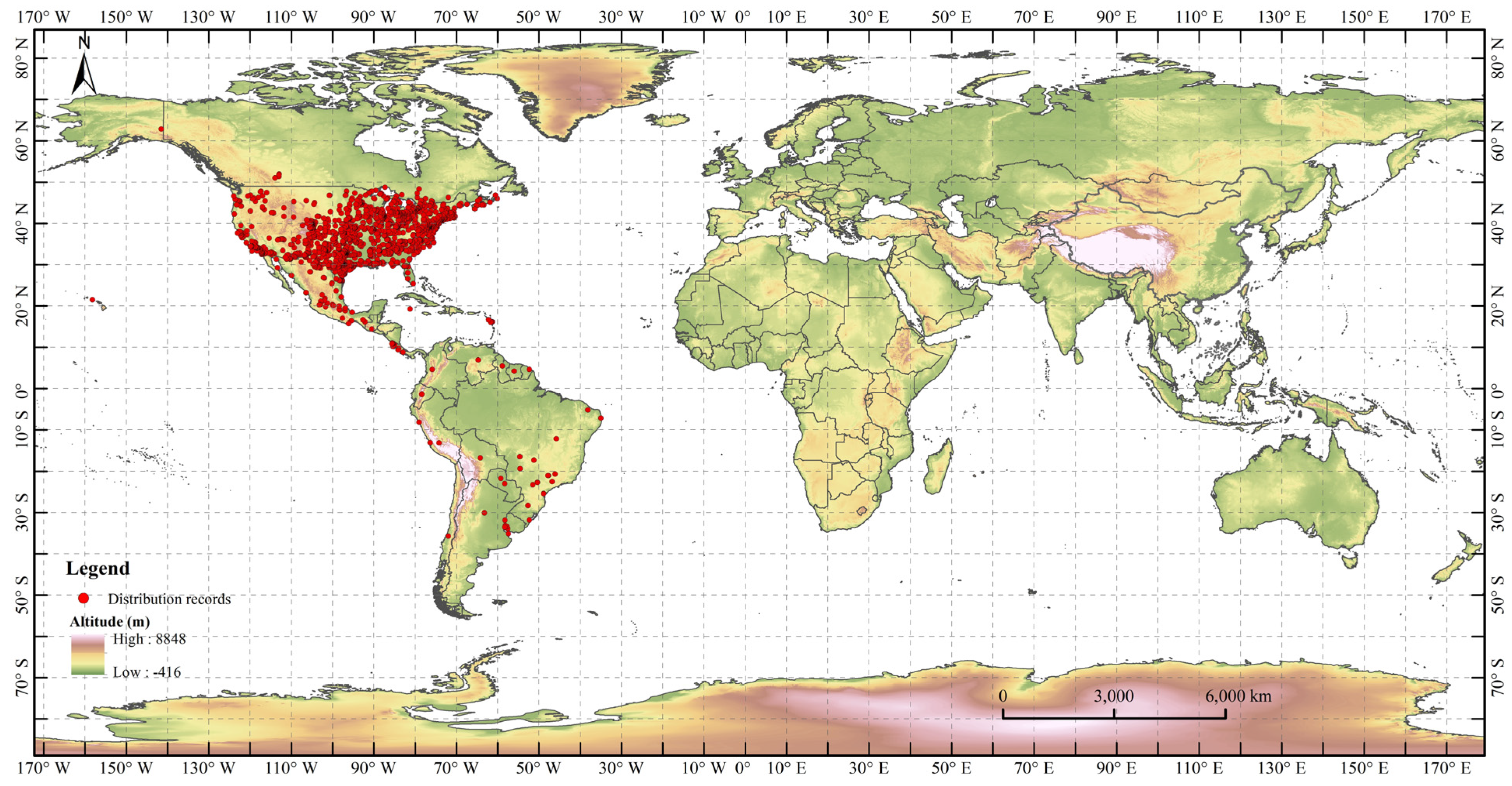
2.1. Distribution Records of H. zea
2.2. Environmental Variables, Map and Model
2.3. MaxEnt Model Calibration
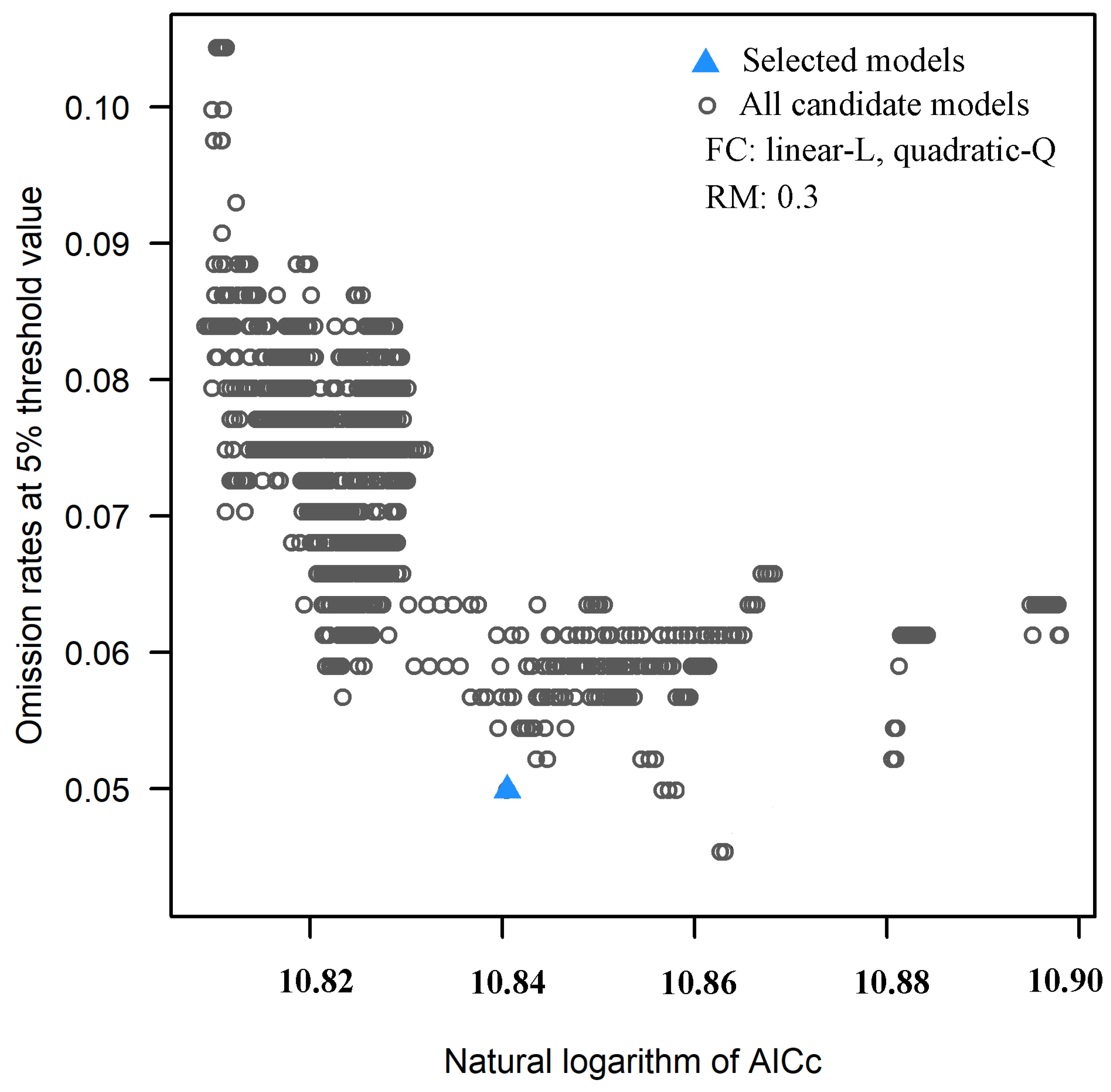
2.4. Model Settings and Evaluation
3. Results
3.1. FC and RM of the Optimal Model
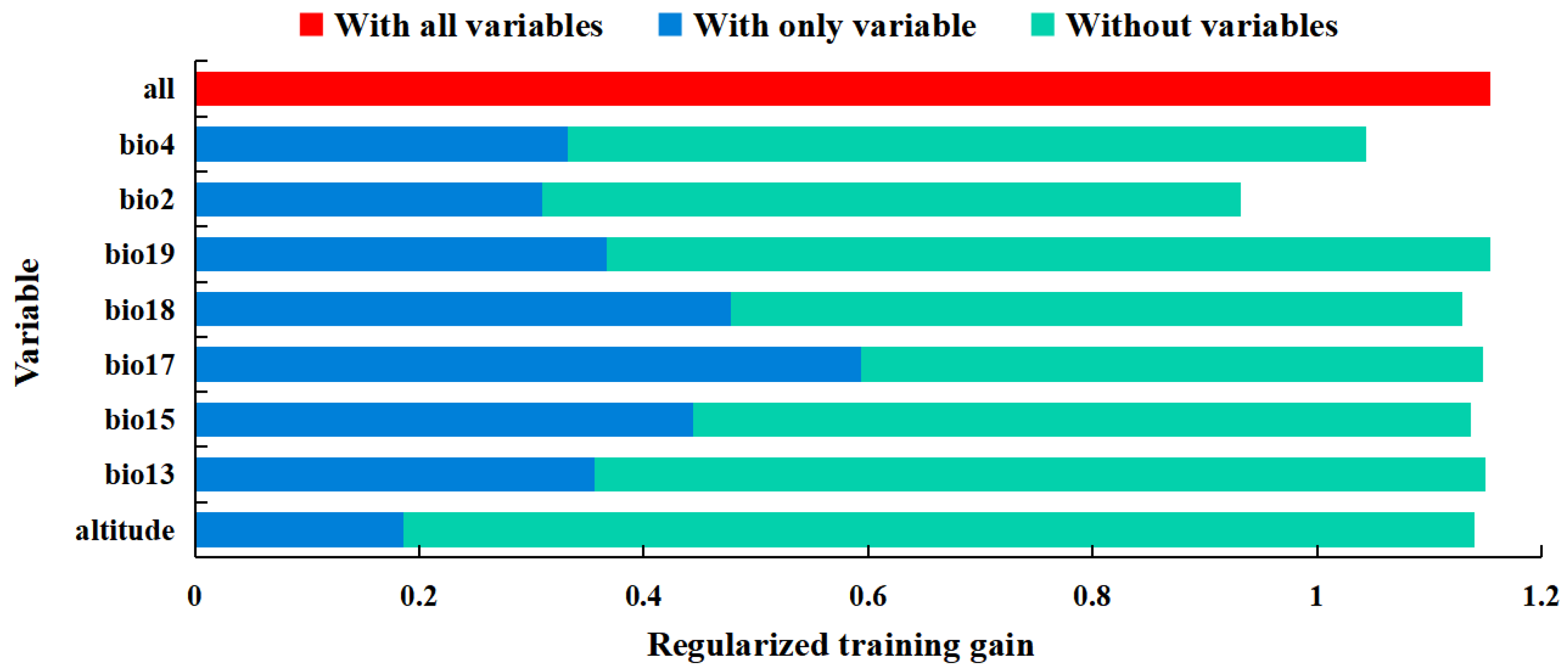
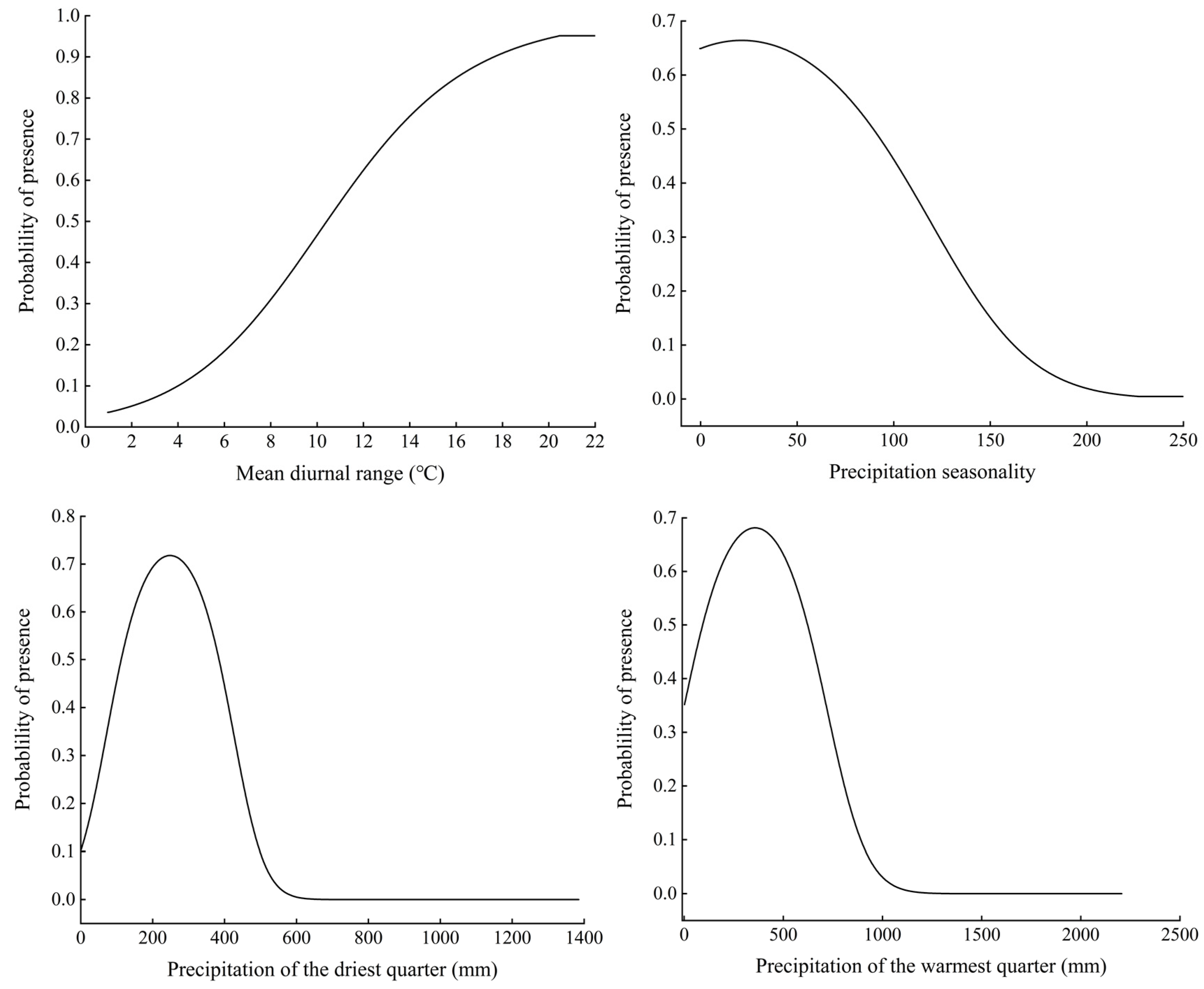
3.2. Significant Environmental Variables
3.3. Potential Suitable Habitats of H. zea under the Current Climate
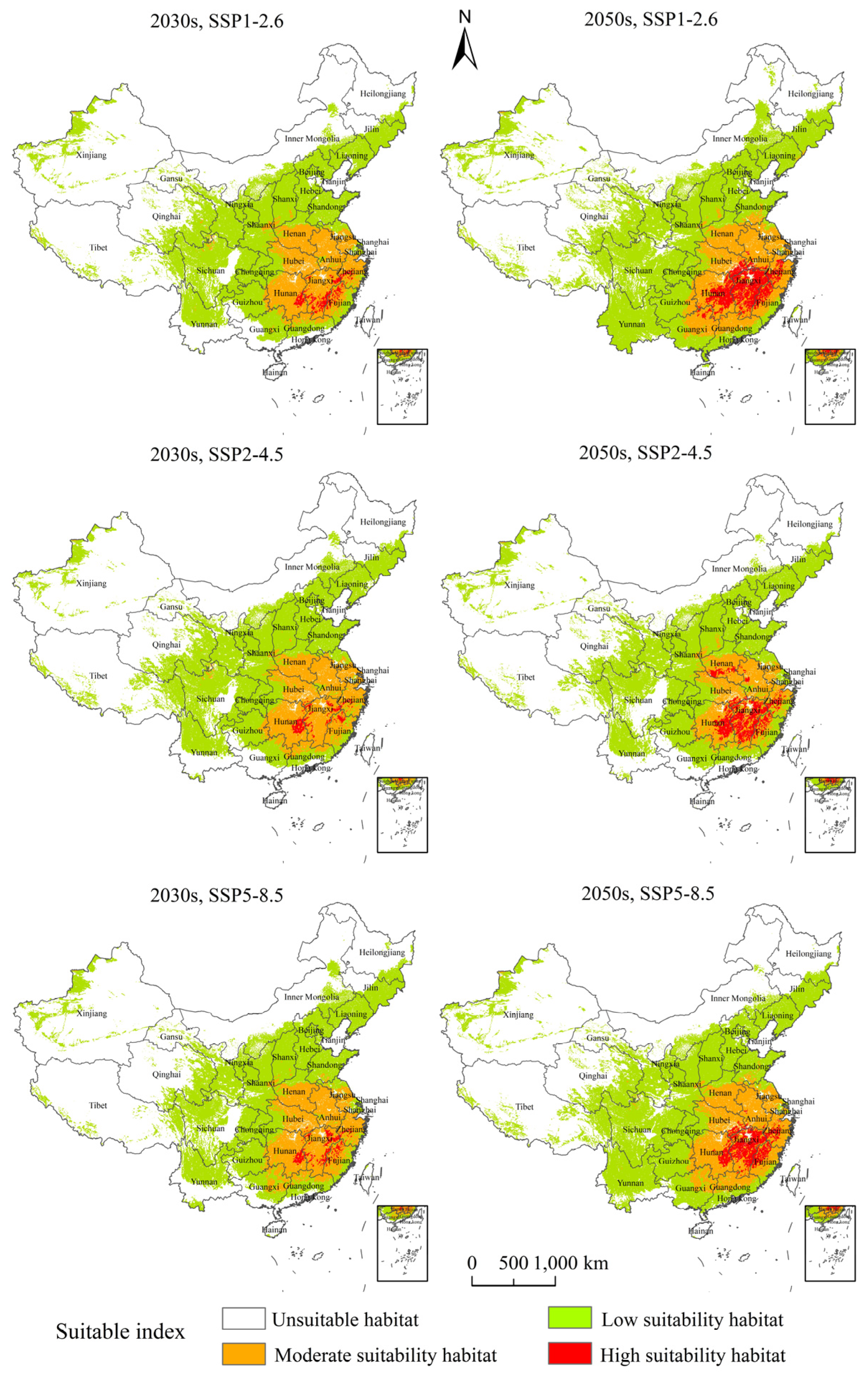
3.4. Potential Suitable Habitats, Changes, and Centroid Distributional Shifts of H. zea under Projected Climate Change
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Regan, E.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global Threats from Invasive Alien Species in the Twenty-First Century and Nation-al Response Capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar]
- Chapman, D.; Purse, B.V.; Roy, H.E.; Bullock, J.M. Global Trade Networks Determine the Distribution of Invasive Non-native Species. Glob. Ecol. Biogeogr. 2017, 26, 907–917. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Vaz, A.S.; Silva, J.; Van Loo, M.; Alonso, A.; Aponte, C.; Bayón, A.; Bellingham, P.J.; Chiuffo, M.C.; DiManno, N.; et al. Global Effects of Non-native Tree Species on Multiple Ecosystem Services. Biol. Rev. 2019, 94, 1477–1501. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti-Ambrogi, A. Global Change and Marine Communities: Alien Species and Climate Change. Mar. Pollut. Bull. 2007, 55, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Pintó-Marijuan, M.; Munné-Bosch, S. Ecophysiology of Invasive Plants: Osmotic Adjustment and Antioxidants. Trends Plant. Sci. 2013, 18, 660–666. [Google Scholar] [CrossRef]
- Ryding, S.; Klaassen, M.; Tattersall, G.J.; Gardner, J.L.; Symonds, M.R. Shape-Shifting: Changing Animal Morphologies as a Response to Climatic Warming. Trends Ecol. Evol. 2021, 36, 1036–1048. [Google Scholar] [CrossRef]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; van Kleunen, M.; Kühn, I.; et al. Projecting the Continental Accumulation of Alien Species through to 2050. Glob. Chang. Biol. 2020, 27, 970–982. [Google Scholar] [CrossRef]
- Kent, A.; Drezner, T.D.; Bello, R. Climate Warming and the Arrival of Potentially Invasive Species into Boreal Forest and Tundra in the Hudson Bay Lowlands, Canada. Polar Biol. 2018, 41, 2007–2022. [Google Scholar] [CrossRef]
- Paul, R.; Subudhi, D.K.; Sahoo, C.K.; Banerjee, K. Invasion of Lantana camara L. and Its Response to Climate Change in the Mountains of Eastern Ghats. Biologia 2021, 76, 1391–1408. [Google Scholar] [CrossRef]
- Cornelissen, B.; Neumann, P.; Schweiger, O. Global Warming Promotes Biological Invasion of a Honey Bee Pest. Glob. Chang. Biol. 2019, 25, 3642–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skendžić, S.; Zovko, M.; Živković, I.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cleland, E. The Phenology of Plant Invasions: A Community Ecology Perspective. Front. Ecol. Environ. 2010, 9, 287–294. [Google Scholar] [CrossRef]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate Change and Forest Diseases. Plant. Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Schippers, P.; Abarca, E.L.; Verboom, J.; Wamelink, G.W.W.; Vos, C.C.; de Boer, W.F.; Harvey, J.A.; Essens, T.; Grashof-Bokdam, C.J.; WallisDeVries, M.F.; et al. Biodiversity Conservation in Climate Change Driven Transient Communities. Biodivers. Conserv. 2021, 30, 2885–2906. [Google Scholar] [CrossRef]
- Clements, D.R.; Jones, V.L. Rapid Evolution of Invasive Weeds under Climate Change: Present Evidence and Future Research Needs. Front. Agron. 2021, 3, 664034. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) IPCC, 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Hulme, P.E. Climate Change and Biological Invasions: Evidence, Expectations, and Response Options. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Porfirio Luciana, L.; Harris, R.M.B.; Lefroy, E.C.; Hugh, S.; Gould, S.F.; Lee, G.; Bindoff, N.L.; Mackey, B. Improving the Use of Species Distribution Models in Conservation Planning and Management under Climate Change. PLoS ONE 2014, 9, e113749. [Google Scholar] [CrossRef] [Green Version]
- Jarvie, S.; Svenning, J.-C. Using Species Distribution Modelling to Determine Opportunities for Trophic Rewilding under Future Scenarios of Climate Change. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condro, A.; Prasetyo, L.; Rushayati, S.; Santikayasa, I.; Iskandar, E. Predicting Hotspots and Prioritizing Protected Areas for Endangered Primate Species in Indonesia under Changing Climate. Biology 2021, 10, 154. [Google Scholar] [CrossRef]
- Cerasoli, F.; Besnard, A.; Marchand, M.; D’Alessandro, P.; Iannella, M.; Biondi, M. Determinants of habitat suitability models transferability across geographically disjunct populations: Insights from Vipera ursinii Ursinii. Ecol. Evol. 2021, 11, 3991–4011. [Google Scholar] [CrossRef]
- Barbosa, F.G.; Schneck, F. Characteristics of the Top-Cited Papers in Species Distribution Predictive Models. Ecol. Model. 2015, 313, 77–83. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of Maxent for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Hsin-Ting, Y.; Cheah, H.; Chiu, M.; Liao, J.; Chiun-Cheng, K. Assessment of Potential Invasion for Six Phytophagous Quarantine Pests in Taiwan. Sci. Rep. 2021, 11, 10666. [Google Scholar]
- Simon, V.; David, J.C. Can Habitat Suitability Estimated from Maxent Predict Colonizations and Extinctions? Divers. Distrib. 2021, 27, 873–886. [Google Scholar]
- Tang, X.; Yuan, Y.; Li, X.; Zhang, J. Maximum Entropy Modeling to Predict the Impact of Climate Change on Pine Wilt Disease in China. Front. Plant. Sci. 2021, 12, 652500. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; He, J.; Ning, C.; Xu, B.; Wang, G.; Lai, J.; Jiang, J.; Ye, L.; Liang, H. Maxent Modeling for Predicting the Potential Distribution of Global Talaromycosis. bioRxiv 2021. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field Validation of an Invasive Species Maxent Model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tang, J.; Ren, G.; Zhao, K.; Wang, X. Global Potential Distribution Prediction of Xanthium italicum based on Maxent model. Sci. Rep. 2021, 11, 16545. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Tay, W.T.; McGaughran, A.; Gordon, K.; Walsh, T.K. Population Structure and Gene Flow in the Global Pest, Helicoverpa armigera. Mol. Ecol. 2016, 25, 5296–5311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J. Effects of Climate Change on Different Geographical Populations of the Cotton Bollworm Helicoverpa armigera (Lepidoptera, Noctuidae). Ecol. Evol. 2020, 10, 1324–1338. [Google Scholar] [CrossRef] [Green Version]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.; Miret, J.A.J.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; et al. Pest Categorisation of Helicoverpa zea. EFSA J. 2020, 18, e06177. [Google Scholar] [CrossRef]
- EPPO A1 List of Pests Recommended for Regulation as Quarantine Pests, Version 2021-09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list (accessed on 9 December 2021).
- Cordeiro, E.M.G.; Pantoja-Gomez, L.M.; de Paiva, J.B.; Nascimento, A.R.B.; Omoto, C.; Michel, A.P.; Correa, A.S. Hybridization and Introgression between Helicoverpa armigera and H. Zea: An Adaptational Bridge. BMC Evol. Biol. 2020, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A Toolbox for Comparative Studies of Environmental Niche Models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent Modeling for Predicting the Potential Geographical Distribution of Two Peony Species under Climate Change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Radosavljevic, A.; Anderson, R.P. Making Better Maxent Models of Species Distributions: Complexity, Overfitting and Evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A Practical Guide to Maxent for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. Kuenm: An R Package for Detailed Development of Ecological Niche Models Using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Upadhye, S.; Worster, A. Understanding Receiver Operating Characteristic (ROC) Curves. Can. J. Emerg. Med. 2006, 8, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Swets, J. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Strubbe, D.; Matthysen, E. Predicting the Potential Distribution of Invasive Ring-Necked Parakeets Psittacula Krameri in Northern Belgium Using an Ecological Niche Modelling Approach. Biol. Invasions 2009, 11, 497–513. [Google Scholar] [CrossRef]
- Lippitt, C.D.; Rogan, J.; Toledano, J.; Sangermano, F.; Eastman, J.R.; Mastro, V.; Sawyer, A. Incorporating Anthropogenic Variables into a Species Distribution Model to Map Gypsy Moth Risk. Ecol. Model. 2008, 210, 339–350. [Google Scholar] [CrossRef]
- Régnière, J.; Bentz, B. Modeling Cold Tolerance in the Mountain Pine Beetle, Dendroctonus Ponderosae. J. Insect Physiol. 2007, 53, 559–572. [Google Scholar] [CrossRef]
- Peterson, A.; Williams, R.; Chen, G. Modeled Global Invasive Potential of Asian Gypsy Moths, Lymantria Dispar. Entomol. Exp. Appl. 2007, 125, 39–44. [Google Scholar] [CrossRef]
- Kumar, S.; Graham, J.; West, A.M.; Evangelista, P.H. Using District-Level Occurrences in Maxent for Predicting the Invasion Potential of an Exotic Insect Pest in India. Comput. Electron. Agric. 2014, 103, 55–62. [Google Scholar] [CrossRef]
- Jones, C.M.; Parry, H.; Tay, W.T.; Reynolds, D.R.; Chapman, J.W. Movement Ecology of Pest Helicoverpa: Implications for Ongoing Spread. Annu. Rev. Entomol. 2019, 64, 277–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Guo, Y. The Evolution of Cotton Pest Management Practices in China. Annu. Rev. Entomol. 2005, 50, 31–52. [Google Scholar] [CrossRef] [PubMed]
- FAO: Climate Change. Available online: https://www.fao.org/climate-change/zh/ (accessed on 9 December 2021).
- Yan, Y.; Xian, X.; Jiang, M.; Wan, F. Biological Invasion and Its Research in China: An Overview. In Biological Invasions and Its Management in China; Wan, F., Jiang, M., Zhan, A., Eds.; Springer: Dordrecht, The Netherlands, 2017; Volume 1. [Google Scholar]
- Wan, F.-H.; Yang, N.-W. Invasion and Management of Agricultural Alien Insects in China. Annu. Rev. Entomol. 2016, 61, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, J.K.; López, J.D. Long-Distance Migration inHelicoverpa zea1: What We Know and Need to Know. Southwest. Entomol. 2010, 35, 355–360. [Google Scholar] [CrossRef]
- Ding, J.; Mack, R.N.; Lu, P.; Ren, M.; Huang, H. China’s Booming Economy Is Sparking and Accelerating Biological Invasions. BioScience 2008, 58, 317–324. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Roques, A.; Battisti, A. Forest Insects and Climate Change. Curr. For. Rep. 2018, 4, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Menzel, F.; Feldmeyer, B. How Does Climate Change Affect Social Insects? Curr. Opin. Insect Sci. 2021, 46, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and Recent Climate Change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Harvey, J.A.; Biere, A.; Gols, R. Rain Downpours Affect Survival and Development of Insect Herbivores: The Specter of Climate Change? Ecology 2019, 100, e02819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, G.D. Bollworm: Development in Relation to Temperature and Larval Food. Environ. Entomol. 1976, 5, 520–522. [Google Scholar] [CrossRef]
- Duan, R.; Huang, G.; Li, Y.; Zheng, R.; Wang, G.; Xin, B.; Tian, C.; Ren, J. Ensemble Temperature and Precipitation Projection for Multi-Factorial Interactive Effects of GCMs and SSPs: Application to China. Front. Environ. Sci. 2021, 9, 742326. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model. Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Wang, Y.; Feng, C.; Wan, P.M.; Chang, K.T. Potential Distributional Changes of Invasive Crop Pest Species Associated with Global Climate Change. Appl. Geogr. 2019, 82, 83–92. [Google Scholar] [CrossRef]
- Walther, G.-R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien Species in a Warmer World: Risks and Opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.P.; Bertelsmeier, C.; Clusella-Trullas, S.; Garnas, J.; Robertson, M.P.; Terblanche, J.S. Predicted Decrease in Global Climate Suitability Masks Regional Complexity of Invasive Fruit Fly Species Response to Climate Change. Biol. Invasions 2016, 18, 1105–1119. [Google Scholar] [CrossRef] [Green Version]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-S.; Zhang, W.; Peng, Y.; Zhao, F.; Chang, X.-Q.; Xing, K.; Zhu, L.; Ma, G.; Yang, H.-P.; Rudolf, V.H.W. Climate Warming promotes Pesticide Resistance through Expanding Overwintering Range of a Global Pest. Nat. Commun. 2021, 12, 5351. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vaamonde, C.; Agassiz, D.; Augustin, S.; de Prins, J.; de Prins, W.; Gomboc, S.; Ivinskis, P.; Karsholt, O.; Koutroumpas, A.; Koutroumpa, F.; et al. Lepidoptera. Chapter 11. BioRisk 2010, 4, 603–668. [Google Scholar] [CrossRef] [Green Version]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward Shifts in Geographical Ranges of butterfly Species Associated with regional Warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Mironidis, G.K.; Savopoulou-Soultani, M. Effects of Heat Shock on Survival and Reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) Adults. J. Therm. Biol. 2010, 35, 59–69. [Google Scholar] [CrossRef] [PubMed]
- de Sassi, C.; Lewis, O.T.; Tylianakis, J.M. Plant-Mediated and Nonadditive Effects of Two Global Change Drivers on an Insect Herbivore Community. Ecology 2012, 93, 1892–1901. [Google Scholar] [CrossRef] [PubMed]




| Variable | Description | In the Model (Yes/No) | Unit |
|---|---|---|---|
| Bio1 | Annual mean temperature | No | °C |
| Bio2 | Mean diurnal range | Yes | °C |
| Bio3 | Isothermality | No | - |
| Bio4 | Temperature seasonality | Yes | °C |
| Bio5 | Max temperature of the warmest month | No | °C |
| Bio6 | Min temperature of the coldest month | No | °C |
| Bio7 | Temperature annual range | No | °C |
| Bio8 | Mean temperature of the wettest quarter | No | °C |
| Bio9 | Mean temperature of the driest quarter | No | °C |
| Bio10 | Mean temperature of the warmest quarter | No | °C |
| Bio11 | Mean temperature of the coldest quarter | No | °C |
| Bio12 | Annual precipitation | No | mm |
| Bio13 | Precipitation of the wettest month | Yes | mm |
| Bio14 | Precipitation of the driest month | No | mm |
| Bio15 | Precipitation seasonality | Yes | - |
| Bio16 | Precipitation of the wettest quarter | No | mm |
| Bio17 | Precipitation of the driest quarter | Yes | mm |
| Bio18 | Precipitation of the warmest quarter | Yes | mm |
| Bio19 | Precipitation of the coldest quarter | Yes | mm |
| Altitude | Altitude | Yes | m |
| Pathways | Description |
|---|---|
| SSP1-2.6 | A world of sustainability-focused growth and equality, radiative forcing stabilizes at 2.6 W/m2 in 2100 |
| SSP2-4.5 | A “middle of the road” world where trends broadly follow their historical patterns, radiative forcing stabilizes at 4.5 W/m2 in 2100 |
| SSP5-8.5 | A world of rapid and unconstrained growth in economic output and energy use, radiative forcing stabilizes at 8.5 W/m2 in 2100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Xian, X.; Zhao, Z.; Zhang, G.; Liu, W.; Wan, F. Climate Change Increases the Expansion Risk of Helicoverpa zea in China According to Potential Geographical Distribution Estimation. Insects 2022, 13, 79. https://doi.org/10.3390/insects13010079
Zhao H, Xian X, Zhao Z, Zhang G, Liu W, Wan F. Climate Change Increases the Expansion Risk of Helicoverpa zea in China According to Potential Geographical Distribution Estimation. Insects. 2022; 13(1):79. https://doi.org/10.3390/insects13010079
Chicago/Turabian StyleZhao, Haoxiang, Xiaoqing Xian, Zihua Zhao, Guifen Zhang, Wanxue Liu, and Fanghao Wan. 2022. "Climate Change Increases the Expansion Risk of Helicoverpa zea in China According to Potential Geographical Distribution Estimation" Insects 13, no. 1: 79. https://doi.org/10.3390/insects13010079
APA StyleZhao, H., Xian, X., Zhao, Z., Zhang, G., Liu, W., & Wan, F. (2022). Climate Change Increases the Expansion Risk of Helicoverpa zea in China According to Potential Geographical Distribution Estimation. Insects, 13(1), 79. https://doi.org/10.3390/insects13010079







