The Diversity of Bacteria Associated with the Invasive Gall Wasp Dryocosmus kuriphilus, Its Galls and a Specialist Parasitoid on Chestnuts
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
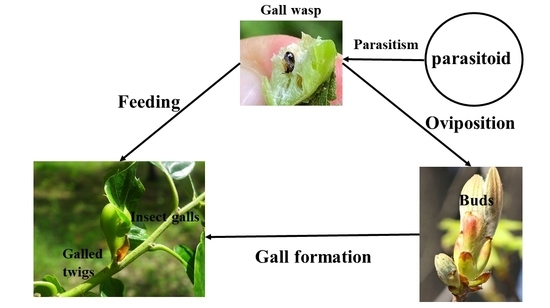
2.1. Collection and Pretreatment of Specimens
2.2. DNA Extraction, PCR Amplification and High-Throughput Sequencing
2.3. Bioinformatics and Statistical Analysis
3. Results
3.1. Bacterial Diversity and Community Composition of D. kuriphilus, T. sinensis, D. kuriphilus Galls and Galled Twigs
3.2. Unique, Common and Predominant Bacterial Species Associated with D. kuriphilus, T. sinensis, D. kuriphilus Galls and Galled Twigs
4. Discussion
4.1. Possibility of Horizontal Transmission of Bacteria among D. kuriphilus, T. sinensis, D. kuriphilus Galls and Galled Twigs
4.2. Differences in Bacterial Community Structures among D. kuriphilus, T. sinensis, D. kuriphilus Galls and Galled Twigs
4.3. Predominant Bacteria Associated with D. kuriphilus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dodueva, I.E.; Lebedeva, M.A.; Kuznetsova, K.A.; Gancheva, M.S.; Paponova, S.S.; Lutova, L.L. Plant tumors: A hundred years of study. Planta 2020, 251, 82. [Google Scholar] [CrossRef]
- Harris, M.O.; Pitzschke, A. Plants make galls to accommodate foreigners: Some are friends, most are foes. New Phytol. 2020, 225, 1852–1872. [Google Scholar] [CrossRef]
- Egan, S.P.; Hood, G.R.; Martinson, E.O.; Ott, J.R. Cynipid gall wasps. Curr. Biol. 2018, 28, R1370–R1374. [Google Scholar] [CrossRef] [Green Version]
- Avtzis, D.N.; Melika, G.; Matošević, D.; Coyle, D.R. The Asian chestnut gall wasp Dryocosmus kuriphilus: A global invader and a successful case of classical biological control. J. Pest. Sci. 2019, 92, 107–115. [Google Scholar] [CrossRef]
- Gehring, E.; Bellosi, B.; Reynaud, N.; Conedera, M. Chestnut tree damage evolution due to Dryocosmus kuriphilus attacks. J. Pest. Sci. 2020, 93, 103–115. [Google Scholar] [CrossRef]
- Bernardo, U.; Iodice, L.; Sasso, R.; Tutore, V.A.; Cascone, P.; Guerrieri, E. Biology and monitoring of Dryocosmus kuriphilus on Castanea sativa in southern Italy. Agric. For. Entomol. 2013, 15, 65–76. [Google Scholar] [CrossRef]
- Cooper, W.R.; Rieske, L.K. Community associates of an exotic gallmaker, Dryocosmus kuriphilus (Hymenoptera: Cynipidae), in eastern north America. Ann. Entomol. Soc. Am. 2007, 100, 236–244. [Google Scholar] [CrossRef]
- Reale, L.; Tedeschini, E.; Rondoni, G.; Ricci, C.; Bin, F.; Frenguelli, G.; Ferranti, F. Histological investigation on gall development induced by a worldwide invasive pest, Dryocosmus kuriphilus, on Castanea sativa. Plant Biosyst. 2016, 150, 35–42. [Google Scholar] [CrossRef]
- Matosevic, D.; Lackovic, N.; Melika, G.; Kos, K.; Franic, I.; Kriston, E.; Bozso, M.; Seljak, G.; Rot, M. Biological control of invasive Dryocosmus kuriphilus with introduced parasitoid Torymus sinensis in Croatia, Slovenia and Hungary. Period. Biol. 2016, 117, 471–477. [Google Scholar] [CrossRef]
- Aebi, A.; Schönrogge, K.; Melika, G.; Alma, A.; Bosio, G.; Quacchia, A.; Picciau, L.; Abe, Y.; Moriya, S.; Yara, K. Parasitoid recruitment to the globally invasive chestnut gall wasp Dryocosmus kuriphilus. In Galling Arthropods and Their Associates; Ozaki, K., Ed.; Springer: Tokyo, Japan, 2006; pp. 103–121. [Google Scholar]
- Graziosi, I.; Rieske, L.K. Response of Torymus sinensis, a parasitoid of the gallforming Dryocosmus kuriphilus, to olfactory and visual cues. Biol. Control 2013, 67, 137–142. [Google Scholar] [CrossRef]
- Quacchia, A.; Moriya, S.; Bosio, G.; Radócz, L.; Botu, M.; Bolvanský, M. Effectiveness of Torymus sinensis in the biological control of Dryocosmus kuriphilus in Italy. Acta Hortic. 2014, 199–204. [Google Scholar] [CrossRef]
- Yara, K. Interaction between Torymus sinensis (Hymenoptera: Torymidae) and T. beneficus, introduced and indigenous parasitoids of the chestnut gall wasp Dryocosmus kuriphilus (Hymenoptera: Cynipidae). Jarq-Jpn. Agr. Res. Q. 2014, 48, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Hayward, A.; Stone, G.N. Oak gall wasp communities: Evolution and ecology. Basic Appl. Ecol. 2005, 6, 435–443. [Google Scholar] [CrossRef]
- Hansen, A.K.; Moran, N.A. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol. Ecol. 2014, 23, 1473–1496. [Google Scholar] [CrossRef]
- Guo, C.; Peng, X.; Zheng, X.; Wang, X.; Wang, R.; Huang, Z.; Yang, Z. Comparison of bacterial diversity and abundance between sexes of Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae) from China. PeerJ 2020, 8, e8411. [Google Scholar]
- Liu, Y.; Xu, L.; Zhang, Z.; Huang, Z.; Fang, D.; Zheng, X.; Yang, Z.; Lu, M. Isolation, identification, and analysis of potential functions of culturable bacteria associated with an invasive gall wasp. Leptocybe invasa. Microb. Ecol. 2021, 1–16. [Google Scholar] [CrossRef]
- Wu, H.; Chen, X.; Hang, C.; Qin, L.; Chen, M.S. Variation and diversification of the microbiome of Schlechtendalia chinensis on two alternate host plants. PLoS ONE 2018, 13, e0200049. [Google Scholar] [CrossRef] [Green Version]
- Yates, A.D.; Michel, A. Mechanisms of aphid adaptation to host plant resistance. Curr. Opin. Insect Sci. 2018, 26, 41–49. [Google Scholar] [CrossRef]
- Correa, C.C.; Ballard, J.W.O. Wolbachia associations with insects: Winning or losing against a master manipulator. Front. Ecol. Evol. 2016, 3, 153. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.H.; He, Y.Y.; Fan, Y.S.; Ma, M.Y.; Peng, D.L. Negative evidence of parthenogenesis induction by Wolbachia in a gallwasp species, Dryocosmus kuriphilus. Entomol. Exp. Appl. 2007, 124, 279–284. [Google Scholar] [CrossRef]
- Iskender, N.A.; Algur, O.F.; Aksu, Y.; Saral, A. Isolation, identification and characterization of biotechnologically important bacteria from microflora of Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae). Biotechnol. Biotechnol. Equip. 2017, 31, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Bansal, R.; Hulbert, S.H.; Reese, J.C.; Whitworth, R.J.; Stuart, J.J.; Chen, M.S. Pyrosequencing reveals the predominance of pseudomonadaceae in gut microbiome of a gall midge. Pathogens 2014, 3, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Ojha, A.; Sinha, D.K.; Padmakumari, A.P.; Bentur, J.S.; Nair, S. Bacterial community structure in the Asian rice gall midge reveals a varied microbiome rich in Proteobacteria. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Medina, R.F.; Nachappa, P.; Tamborindeguy, C. Differences in bacterial diversity of host-associated populations of Phylloxera notabilis Pergande (Hemiptera: Phylloxeridae) in pecan and water hickory. J. Evol. Biol. 2011, 24, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Michell, C.T.; Nyman, T. Microbiomes of willow-galling sawflies: Effects of host plant, gall type, and phylogeny on community structure and function. Genome 2021, 64, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Clerck-Floate, R.D.; Tooker, J.F.; Price, P.W.; Miller, D.G.; Connor, E.F. Are bacterial symbionts associated with gall induction in insects? Arthropod-Plant Inte. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Hou, H.Q.; Zhao, G.Z.; Su, C.Y.; Zhu, D.H. Wolbachia prevalence patterns: Horizontal transmission, recombination, and multiple infections in chestnut gall wasp-parasitoid communities. Entomol. Exp. Appl. 2020, 168, 752–765. [Google Scholar] [CrossRef]
- Yang, X.-M.; Hui, Y.; Zhao, L.-Q.; Zhu, D.-H.; Zeng, Y.; Yang, X.-H. Comparison of auxin and cytokinins concentrations, and the structure of bacterial community between host twigs and Lithosaphonecrus arcoverticus galls. Insects 2021, 12, 982. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. Flash: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Marc, L.; Bjoern, U. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package ‘vegan’. Community Ecol. Package 2013, 2, 1–295. [Google Scholar]
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial endophyte colonization and distribution within plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [Green Version]
- Johnston-Monje, D.; Raizada, M.N. Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and ecology. PLoS ONE 2011, 6, e20396. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Kikuchi, Y. Impact of the insect gut microbiota on ecology, evolution, and industry. Curr. Opin. Insect Sci. 2020, 41, 33–39. [Google Scholar] [CrossRef]
- Janson, E.M.; Stireman, J.O., III; Singer, M.S.; Abbot, P. Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution 2008, 62, 997–1012. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.H.; Zhu, D.H.; Liu, Z.; Zhao, L.; Su, C.Y. High levels of multiple infections, recombination and horizontal transmission of Wolbachia in the Andricus mukaigawae (Hymenoptera; Cynipidae) communities. PLoS ONE 2013, 8, e78970. [Google Scholar] [CrossRef] [Green Version]
- Chiel, E.; Zchori-Fein, E.; Inbar, M.; Gottlieb, Y.; Adachi-Hagimori, T.; Kelly, S.E.; Asplen, M.K.; Hunter, M.S. Almost there: Transmission routes of bacterial symbionts between trophic levels. PLoS ONE 2009, 4, e4767. [Google Scholar] [CrossRef]
- Tanaka, Y.; Okada, K.; Asami, T.; Suzuki, Y. Phytohormones in Japanese mugwort gall induction by a gall-inducing gall midge. Biosci. Biotech. Bioch 2013, 77, 1942–1948. [Google Scholar] [CrossRef] [Green Version]
- Takei, M.; Yoshida, S.; Kawai, T.; Hasegawa, M.; Suzuki, Y. Adaptive significance of gall formation for a gall-inducing aphids on Japanese elm trees. J. Insect Physiol. 2015, 72, 43–51. [Google Scholar] [CrossRef]
- Mapes, C.C.; Davies, P.J. Indole-3-acetic acid and ball gall development on Solidago altissima. New Phytol. 2001, 151, 195–202. [Google Scholar] [CrossRef]
- Straka, J.R.; Hayward, A.R.; Emery, R.N. Gall-inducing Pachypsylla celtidis (Psyllidae) infiltrate hackberry trees with high concentrations of phytohormones. J. Plant Interact. 2010, 5, 197–203. [Google Scholar] [CrossRef]
- Kai, S.; Kumashiro, S.; Adachi, S.; Suzuki, Y.; Shiomi, Y.; Matsunaga, K.; Gyoutoku, N.; Asami, T.; Tokuda, M. Life history of Stenopsylla nigricornis (Hemiptera: Psylloidea: Triozidae) and phytohormones involved in its gall induction. Arthropod-Plant Inte. 2017, 11, 99–108. [Google Scholar] [CrossRef]
- Tokuda, M.; Jikumaru, Y.; Matsukura, K.; Takebayashi, Y.; Kumashiro, S.; Matsumura, M.; Kamiya, Y. Phytohormones related to host plant manipulation by a gall-inducing leafhopper. PLoS ONE 2013, 8, e62350. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Tanaka, H.; Hasegawa, M.; Tokuda, M.; Asami, T.; Suzuki, Y. Phytohormones and willow gall induction by a gall-inducing sawfly. New Phytol. 2012, 196, 586–595. [Google Scholar] [CrossRef]
- Li, X.Q.; Liu, Y.Z.; Guo, W.F.; Solanki, M.K.; Yang, Z.D.; Xiang, Y.; Ma, Z.C.; Wen, Y.G. The gall wasp Leptocybe invasa (Hymenoptera: Eulophidae) stimulates different chemical and phytohormone responses in two Eucalyptus varieties that vary in susceptibility to galling. Tree Physiol. 2017, 37, 1208–1217. [Google Scholar] [CrossRef]
- Wood, B.W.; Payne, J.A. Growth regulators in chestnut shoot galls infected with oriental ghestnut gall wasp, Dryocosmus kuriphilus (Hymenoptera: Cynipidae). Environ. Entomol. 1988, 17, 915–920. [Google Scholar] [CrossRef]
- Donati, A.J.; Lee, H.-I.; Leveau, J.H.J.; Chang, W.-S. Effects of indole-3-acetic acid on the transcriptional activities and stress tolerance of Bradyrhizobium japonicum. PLoS ONE 2013, 8, e76559. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [Green Version]
- Berens, M.L.; Berry, H.M.; Mine, A.; Argueso, C.T.; Tsuda, K. Evolution of hormone signaling networks in plant defense. Annu. Rev. Phytopathol. 2017, 55, 401–425. [Google Scholar] [CrossRef]
- Ikai, N.; Hijii, N. Manipulation of tannins in oaks by galling cynipids. J. Forest Res. 2007, 12, 316–319. [Google Scholar] [CrossRef]
- Koncz, N.K.; Szabó, L.J.; Máthé, C.; Jámbrik, K.; M-Hamvas, M. Histological study of quercus galls of Neuroterus quercusbaccarum (Linnaeus, 1758) (Hymenoptera: Cynipidae). Acta Bio. Szeg. 2011, 55, 247–253. [Google Scholar]
- Allison, S.D.; Schultz, J.C. Biochemical responses of chestnut oak to a galling cynipid. J. Chem. Ecol. 2005, 31, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-M.; Yang, X.-H. Comparison of the contents or activities of nutritional and defensive substances between the larval galls and host plants of Dryocosmus kuriphilus. Life Sci. Res. 2019, 23, 214–218. [Google Scholar]
- Yang, X.-H.; Li, X.-M.; Zhu, D.-H. Alteration of free amino acid concentrations in insect galls induced by Andricus mukaigawae (Hymenoptera; Cynipidae). Ecol. Entomol. 2020, 45, 945–954. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Cui, K.; Lu, Q.; Wang, C.; Wu, H.X.; Yang, Z.X.; Ding, W.F.; Shao, S.X.; Wang, H.Y.; et al. Molecular mechanisms of tannin accumulation in Rhus galls and genes involved in plant-insect interactions. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Ferracini, C.; Ferrari, E.; Pontini, M.; Nova, L.K.H.; Saladini, M.A.; Alma, A. Post-release evaluation of non-target effects of Torymus sinensis, the biological control agent of Dryocosmus kuriphilus in Italy. BioControl 2017, 62, 445–456. [Google Scholar] [CrossRef]
- Gibbs, M.; Schonrogge, K.; Alma, A.; Melika, G.; Quacchia, A.; Stone, G.N.; Aebi, A. Torymus sinensis: A viable management option for the biological control of Dryocosmus kuriphilus in Europe? BioControl 2011, 56, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Amit, L.; Ben-Shlomo, R.; Chiel, E. Are microbial symbionts involved in the speciation of the gall-inducing aphid Slavum wertheimae? Arthropod-Plant Int. 2017, 11, 475–484. [Google Scholar] [CrossRef]
- Morrow, J.L.; Hall, A.; Riegler, M. Symbionts in waiting: The dynamics of incipient endosymbiont complementation and replacement in minimal bacterial communities of psyllids. Microbiome 2017, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Van Baarlen, P.; Van Belkum, A.; Summerbell, R.C.; Crous, P.W.; Thomma, B.P.H.J. Molecular mechanisms of pathogenicity: How do pathogenic microorganisms develop cross-kingdom host jumps? FEMS Microbiol. Rev. 2007, 31, 239–277. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Sañudo, I.; Mazzon, L.; Simonato, M.; Avtzis, D.; Pujade-Villar, J.; Faccoli, M. Tracking the origin and dispersal of the Asian chestnut gall wasp Dryocosmus kuriphilus Yasumatsu (Hymenoptera, Cynipidae) in Europe with molecular markers. Bull. Entomol. Res. 2019, 109, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.N.; Schönrogge, K.; Atkinson, R.J.; Bellido, D.; Pujade-Villar, J. The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annu. Rev. Entomol. 2002, 47, 633–668. [Google Scholar] [CrossRef] [Green Version]


| Phylum | Class | Order | Family | Genus | Species | OTU | |
|---|---|---|---|---|---|---|---|
| T. sinensis | 10 | 15 | 54 | 80 | 125 | 182 | 221 |
| D. kuriphilus | 8 | 12 | 40 | 64 | 98 | 138 | 162 |
| D. kuriphilus galls | 13 | 19 | 60 | 96 | 170 | 253 | 350 |
| Galled twigs | 14 | 20 | 61 | 100 | 176 | 266 | 366 |
| Total | 14 | 20 | 63 | 103 | 181 | 273 | 373 |
| Genus of Predominant Bacteria | Reported in T. sinensis | Reported in Galling Insects | Reported in D. kuriphilus galls | Reported in C. mollissima |
|---|---|---|---|---|
| TorS group | ||||
| Massilia | No | Leptocybe invasa (Liu et al., 2021) | No | No |
| Ralstonia | No | No | No | |
| Pseudomonas | No | D. kuriphilus (Iskender et al., 2017), L. invasa (Liu et al., 2021), Gall midges (Bansal et al., 2014; Ojha et al., 2017), Gall aphids (Medina et al., 2011; Wu et al., 2018), Galling sawflies (Michell and Nyman, 2021), Thrips (Hammer et al., 2021) | No | No |
| Dryk group | ||||
| Serratia | No | Gall wasps (Liu et al., 2021), Gall aphids (Amit et al., 2017; Medina et al., 2011; Wu et al., 2018), psyllids (Morrow et al., 2017) | No | Chen et al., 2019 |
| Pseudomonas | No | D. kuriphilus (Iskender et al., 2017), L. invasa (Liu et al., 2021), Gall midges (Bansal et al., 2014; Ojha et al., 2017), Gall aphids (Medina et al., 2011; Wu et al., 2018), Galling sawflies (Michell and Nyman, 2021), Thrips (Hammer et al., 2021) | No | Ni, 1998; |
| InsG group | No | |||
| Pantoea | No | L. invasa (Liu et al., 2021), Gall midges (Bansal et al., 2014; Ojha et al., 2017), Thrips (Hammer et al., 2021) | No | Zhang et al., 2019 |
| Rhodococcus | No | Gall aphids (Wu et al., 2018) | No | No |
| CasM group | ||||
| Sphingomonas | No | L. invasa (Guo et al., 2020), Galling sawflies (Michell and Nyman, 2021), Gall aphids (Wu et al., 2018) | No | Chen et al., 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Hui, Y.; Zhu, D.; Zeng, Y.; Zhao, L.; Yang, X.; Wang, Y. The Diversity of Bacteria Associated with the Invasive Gall Wasp Dryocosmus kuriphilus, Its Galls and a Specialist Parasitoid on Chestnuts. Insects 2022, 13, 86. https://doi.org/10.3390/insects13010086
Yang X, Hui Y, Zhu D, Zeng Y, Zhao L, Yang X, Wang Y. The Diversity of Bacteria Associated with the Invasive Gall Wasp Dryocosmus kuriphilus, Its Galls and a Specialist Parasitoid on Chestnuts. Insects. 2022; 13(1):86. https://doi.org/10.3390/insects13010086
Chicago/Turabian StyleYang, Xiaohui, Yu Hui, Daohong Zhu, Yang Zeng, Lvquan Zhao, Xuemei Yang, and Yumei Wang. 2022. "The Diversity of Bacteria Associated with the Invasive Gall Wasp Dryocosmus kuriphilus, Its Galls and a Specialist Parasitoid on Chestnuts" Insects 13, no. 1: 86. https://doi.org/10.3390/insects13010086
APA StyleYang, X., Hui, Y., Zhu, D., Zeng, Y., Zhao, L., Yang, X., & Wang, Y. (2022). The Diversity of Bacteria Associated with the Invasive Gall Wasp Dryocosmus kuriphilus, Its Galls and a Specialist Parasitoid on Chestnuts. Insects, 13(1), 86. https://doi.org/10.3390/insects13010086