Identification and Expression Profile of Chemosensory Receptor Genes in Aromia bungii (Faldermann) Antennal Transcriptome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Tissue Collections
2.2. RNA Isolation and cDNA Library Construction
2.3. Transcriptome Sequencing and Data Assembly
2.4. Phylogenetic Analysis
2.5. Quantitative Real-Time PCR Analysis of Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Transcriptome Sequence and Homologous Assembly
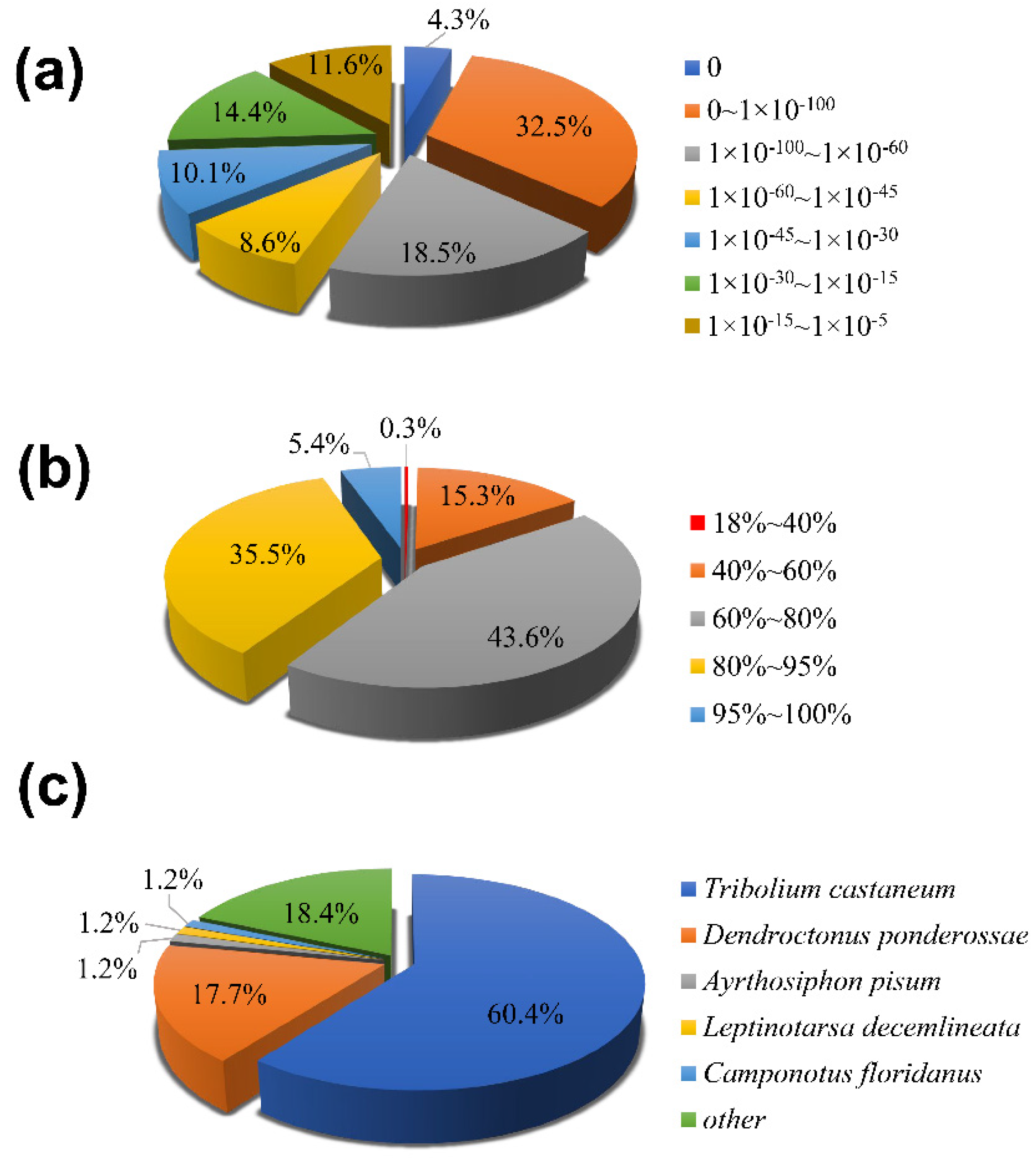
3.2. Functional Annotation and Expression Level
3.3. Identification of Putative Odorant Receptors
3.4. Tissue- and Sex-Specific Expression Analysis of Putative Odorant Receptors
3.5. Identification of Putative Gustatory Receptors
3.6. Tissue- and Sex-Specific Expression Analysis of Putative Gustatory Receptors
3.7. Identification and Expression Analysis of Putative Ionotropic Receptors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montagné, N.; de Fouchier, A.; Newcomb, R.D.; Jacquin-Joly, E. Advances in the identification and characterization of olfactory receptors in insects. Prog. Mol. Biol. Transl. Sci. 2015, 130, 55–80. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Benton, R. Molecular mechanisms of olfactory detection in insects: Beyond receptors. Open Biol. 2020, 10, 200252. [Google Scholar] [CrossRef]
- Wicher, D.; Miazzi, F. Functional properties of insect olfactory receptors: Ionotropic receptors and odorant receptors. Cell Tissue Res. 2021, 383, 7–19. [Google Scholar] [CrossRef]
- Vogt, R.G.; Riddiford, L.M. Pheromone binding and inactivation by moth antennae. Nature 1981, 293, 161–163. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Younus, F.; Chertemps, T.; Pearce, S.L.; Pandey, G.; Bozzolan, F.; Coppin, C.W.; Russell, R.J.; Maïbèche-Coisne, M.; Oakeshott, J.G. Identification of candidate odorant degrading gene/enzyme systems in the antennal transcriptome of Drosophila melanogaster. Insect Biochem. Mol. Biol. 2014, 53, 30–43. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ma, H.; Xie, H.; Xuan, N.; Guo, X.; Fan, Z.; Rajashekar, B.; Arnaud, P.; Offmann, B.; Picimbon, J.F. Biotype Characterization, Developmental Profiling, Insecticide Response and Binding Property of Bemisia tabaci Chemosensory Proteins: Role of CSP in Insect Defense. PLoS ONE 2016, 11, e0154706. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.D.; Dahanukar, A. Recent advances in the genetic basis of taste detection in Drosophila. CMLS 2020, 77, 1087–1101. [Google Scholar] [CrossRef]
- Zhang, H.J.; Xu, W.; Chen, Q.M.; Sun, L.N.; Anderson, A.; Xia, Q.Y.; Papanicolaou, A. A phylogenomics approach to characterizing sensory neuron membrane proteins (SNMPs) in Lepidoptera. Insect Biochem. Mol. Biol. 2020, 118, 103313. [Google Scholar] [CrossRef]
- Ache, B.W.; Young, J.M. Olfaction: Diverse species, conserved principles. Neuron 2005, 48, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Gracia, A.; Vieira, F.G.; Rozas, J. Molecular evolution of the major chemosensory gene families in insects. Heredity 2009, 103, 208–216. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Ann. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Pennisi, E. Fruit fly odor receptors found. Science 1999, 283, 1239. [Google Scholar] [CrossRef]
- Gao, Q.; Chess, A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics 1999, 60, 31–39. [Google Scholar] [CrossRef]
- Clyne, P.J.; Warr, C.G.; Carlson, J.R. Candidate taste receptors in Drosophila. Science 2000, 287, 1830–1834. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Zufall, F.; Domingos, A.I. The structure of Orco and its impact on our understanding of olfaction. J. Gen. Physiol. 2018, 150, 1602–1605. [Google Scholar] [CrossRef] [Green Version]
- Martin, F.; Alcorta, E. Regulation of olfactory transduction in the orco channel. Front. Cell. Neurosci. 2011, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Brand, P.; Robertson, H.M.; Lin, W.; Pothula, R.; Klingeman, W.E.; Jurat-Fuentes, J.L.; Johnson, B.R. The origin of the odorant receptor gene family in insects. eLife 2018, 7, e38340. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, P.; Qin, Q.; Li, M.; Meng, R.; Zhang, T. Characterizing the Role of Orco Gene in Detecting Aggregation Pheromone and Food Resources in Protaetia brevitarsis Leiws (Coleoptera: Scarabaeidae). Front. Physiol. 2021, 12, 649590. [Google Scholar] [CrossRef]
- Yang, Y.; Krieger, J.; Zhang, L.; Breer, H. The olfactory co-receptor Orco from the migratory locust (Locusta migratoria) and the desert locust (Schistocerca gregaria): Identification and expression pattern. Int. J. Biol. Sci. 2012, 8, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Yang, Y.T.; Wu, Q.J.; Wang, S.L.; Xie, W.; Zhang, Y.J. Genome-wide analysis of odorant-binding proteins and chemosensory proteins in the sweet potato whitefly, Bemisia tabaci. Insect Sci. 2019, 26, 620–634. [Google Scholar] [CrossRef] [Green Version]
- Bohbot, J.; Pitts, R.J.; Kwon, H.W.; Rützler, M.; Robertson, H.M.; Zwiebel, L.J. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol. Biol. 2007, 16, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Robertson, H.M.; Wanner, K.W. The chemoreceptor superfamily in the honey bee, Apis mellifera: Expansion of the odorant, but not gustatory, receptor family. Genome Res. 2006, 16, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Scieuzo, C.; Nardiello, M.; Farina, D.; Scala, A.; Cammack, J.A.; Tomberlin, J.K.; Vogel, H.; Salvia, R.; Persaud, K.; Falabella, P. Hermetia illucens (L.) (Diptera: Stratiomyidae) Odorant Binding Proteins and Their Interactions with Selected Volatile Organic Compounds: An In Silico Approach. Insects 2021, 12, 814. [Google Scholar] [CrossRef]
- Carey, A.F.; Carlson, J.R. Insect olfaction from model systems to disease control. Proc. Natl. Acad. Sci. USA 2011, 108, 12987–12995. [Google Scholar] [CrossRef] [Green Version]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Carey, A.F.; Carlson, J.R.; Zwiebel, L.J. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2010, 107, 4418–4423. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.Y.; Li, W.; Liu, H.Y.; Xiang, F.; Kang, Y.K.; Yin, X.; Huang, A.P.; Wang, Y.J. Systemic identification and analyses of genes potentially involved in chemosensory in the devastating tea pest Basilepta melanopus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 31, 100586. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Gibbs, R.A.; Weinstock, G.M.; Brown, S.J.; Denell, R.; Beeman, R.W.; Gibbs, R.; Beeman, R.W.; Brown, S.J.; Bucher, G.; et al. The genome of the model beetle and pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, G.; Scala, A.; Garzone, V.; Salvia, R.; Yalcin, C.; Vernile, P.; Aresta, A.M.; Facini, O.; Baraldi, R.; Bufo, S.A.; et al. Chemical Ecology of Capnodis tenebrionis (L.) (Coleoptera: Buprestidae): Behavioral and Biochemical Strategies for Intraspecific and Host Interactions. Front. Physiol. 2019, 10, 604. [Google Scholar] [CrossRef]
- Yi, J.K.; Yang, S.; Wang, S.; Wang, J.; Zhang, X.X.; Liu, Y.; Xi, J.H. Identification of candidate chemosensory receptors in the antennal transcriptome of the large black chafer Holotrichia parallela Motschulsky (Coleoptera: Scarabaeidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 28, 63–71. [Google Scholar] [CrossRef]
- Cui, X.; Liu, D.; Sun, K.; He, Y.; Shi, X. Expression Profiles and Functional Characterization of Two Odorant-Binding Proteins From the Apple Buprestid Beetle Agrilus mali (Coleoptera: Buprestidae). J. Econ. Entomol. 2018, 111, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, S.; Zhang, J.; Wang, X.; Wang, S.; Liu, M.; Xi, J. Identification and expression analysis of candidate chemosensory receptors based on the antennal transcriptome of Lissorhoptrus oryzophilus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 30, 133–142. [Google Scholar] [CrossRef]
- Andersson, M.N.; Grosse-Wilde, E.; Keeling, C.I.; Bengtsson, J.M.; Yuen, M.M.; Li, M.; Hillbur, Y.; Bohlmann, J.; Hansson, B.S.; Schlyter, F. Antennal transcriptome analysis of the chemosensory gene families in the tree killing bark beetles, Ips typographus and Dendroctonus ponderosae (Coleoptera: Curculionidae: Scolytinae). BMC Genom. 2013, 14, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.C.; Zhang, Y.N.; Kang, K.; Dong, S.L.; Zhang, L.W. Antennal Transcriptome Analysis of Odorant Reception Genes in the Red Turpentine Beetle (RTB), Dendroctonus valens. PLoS ONE 2015, 10, e0125159. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ju, Q.; Jie, W.; Li, F.; Jiang, X.; Hu, J.; Qu, M. Chemosensory gene families in adult antennae of Anomala corpulenta Motschulsky (Coleoptera: Scarabaeidae: Rutelinae). PLoS ONE 2015, 10, e0121504. [Google Scholar] [CrossRef]
- Annemarie, H.; Hanghui, K.; Irina, B.; Michael, V.T.; Till, T.; Armin, G.T.; Elisabeth, W.; Wittko, F.; Ulrich, M.; Stefan, D. Deceptive Ceropegia dolichophylla fools its kleptoparasitic fly pollinators with exceptional floral scent. Front. Ecol. Environ. 2015, 3, 66. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Rao, X.J.; Li, M.Y.; Feng, M.F.; He, M.Z.; Li, S.G. Identification of candidate chemosensory genes in the antennal transcriptome of Tenebrio molitor (Coleoptera: Tenebrionidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2015, 13, 44–51. [Google Scholar] [CrossRef]
- Gonzalez, F.; Johny, J.; Walker, W.B.; Guan, Q.; Mfarrej, S.; Jakše, J.; Montagné, N.; Jacquin-Joly, E.; Alqarni, A.S.; Al-Saleh, M.A.; et al. Antennal transcriptome sequencing and identification of candidate chemoreceptor proteins from an invasive pest, the American palm weevil, Rhynchophorus palmarum. Sci. Rep. 2021, 11, 8334. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Hughes, D.T.; Luetje, C.W.; Millar, J.G.; Soriano-Agatón, F.; Hanks, L.M.; Robertson, H.M. Sequencing and characterizing odorant receptors of the cerambycid beetle Megacyllene caryae. Insect Biochem. Mol. Biol. 2012, 42, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, Y.N.; Qian, J.L.; Kang, K.; Zhang, X.Q.; Deng, J.D.; Tang, Y.P.; Chen, C.; Hansen, L.; Xu, T.; et al. Identification and Expression Patterns of Anoplophora chinensis (Forster) Chemosensory Receptor Genes from the Antennal Transcriptome. Front. Physiol. 2018, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.L.; Mang, D.Z.; Lv, G.C.; Ye, J.; Li, Z.Q.; Chu, B.; Sun, L.; Liu, Y.J.; Zhang, L.W. Identification and Expression Profile of Olfactory Receptor Genes Based on Apriona germari (Hope) Antennal Transcriptome. Front. Physiol. 2020, 11, 807. [Google Scholar] [CrossRef]
- Hu, P.; Wang, J.; Cui, M.; Tao, J.; Luo, Y. Antennal transcriptome analysis of the Asian longhorned beetle Anoplophora glabripennis. Sci. Rep. 2016, 6, 26652. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, D.Z.; Min, S.F.; Mi, F.; Zhou, S.S.; Wang, M.Q. Analysis of chemosensory gene families in the beetle Monochamus alternatus and its parasitoid Dastarcus helophoroides. Comp. Biochem. Physiol. Part D Genom. Proteom. 2014, 11, 1–8. [Google Scholar] [CrossRef]
- Xu, T.; Yasui, H.; Teale, S.A.; Fujiwara-Tsujii, N.; Wickham, J.D.; Fukaya, M.; Hansen, L.; Kiriyama, S.; Hao, D.; Nakano, A.; et al. Identification of a male-produced sex-aggregation pheromone for a highly invasive cerambycid beetle, Aromia bungii. Sci. Rep. 2017, 7, 7330. [Google Scholar] [CrossRef]
- Pea, E.D.L.; Schrader, G.; Vos, S. Pest survey card on Aromia bungii. EFSA Support. Publ. 2019, 16, EN-1731. [Google Scholar] [CrossRef] [Green Version]
- Yasui, H.; Fujiwara-Tsujii, N.; Yasuda, T.; Fukaya, M.; Kiriyama, S.; Nakano, A.; Watanabe, T.; Mori, K. Electroantennographic responses and field attraction of an emerging invader, the red-necked longicorn beetle Aromia bungii (Coleoptera: Cerambycidae), to the chiral and racemic forms of its male-produced aggregation-sex pheromone. Appl. Entomol. Zool. 2018, 54, 109–114. [Google Scholar] [CrossRef]
- Wang, W.C.; Cao, D.D.; Men, J. (R)-(+)-citronellal identified as a female-produced sex pheromone of Aromia bungii Faldermann (Coleoptera: Cerambycidae). Egypt. J. Biol. Pest Control 2018, 28, 77. [Google Scholar] [CrossRef]
- Antony, B.; Johny, J.; Aldosari, S.A. Silencing the Odorant Binding Protein RferOBP1768 Reduces the Strong Preference of Palm Weevil for the Major Aggregation Pheromone Compound Ferrugineol. Front. Physiol. 2018, 9, 252. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Mamidala, P.; Wijeratne, A.J.; Wijeratne, S.; Poland, T.; Qazi, S.S.; Doucet, D.; Cusson, M.; Beliveau, C.; Mittapalli, O. Identification of odor-processing genes in the emerald ash borer, Agrilus planipennis. PLoS ONE 2013, 8, e56555. [Google Scholar] [CrossRef] [Green Version]
- Engsontia, P.; Sanderson, A.P.; Cobb, M.; Walden, K.K.; Robertson, H.M.; Brown, S. The red flour beetle’s large nose: An expanded odorant receptor gene family in Tribolium castaneum. Insect Biochem. Mol. Biol. 2008, 38, 387–397. [Google Scholar] [CrossRef]
- Wu, Z.; Bin, S.; He, H.; Wang, Z.; Li, M.; Lin, J. Differential Expression Analysis of Chemoreception Genes in the Striped Flea Beetle Phyllotreta striolata Using a Transcriptomic Approach. PLoS ONE 2016, 11, e0153067. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Simon, P. Q-Gene: Processing quantitative real-time RT-PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef] [Green Version]
- Ebbs, M.L.; Amrein, H. Taste and pheromone perception in the fruit fly Drosophila melanogaster. Pflug. Arch. 2007, 454, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Plewa, R.; Jaworski, T.; Hilszczański, J.; Horák, J. Investigating the biodiversity of the forest strata: The importance of vertical stratification to the activity and development of saproxylic beetles in managed temperate deciduous forests. For. Ecol. Manag. 2017, 402, 186–193. [Google Scholar] [CrossRef]
- Sweeney, J.D.; Silk, P.J.; Gutowski, J.M.; Wu, J.; Lemay, M.A.; Mayo, P.D.; Magee, D.I. Effect of chirality, release rate, and host volatiles on response of Tetropium fuscum (F.), Tetropium cinnamopterum Kirby, and Tetropium castaneum (L.) to the aggregation pheromone, Fuscumol. J. Chem. Ecol. 2010, 36, 1309–1321. [Google Scholar] [CrossRef]
- Ando, T.; Inomata, S.; Yamamoto, M. Lepidopteran sex pheromones. Top. Curr. Chem. 2004, 239, 51–96. [Google Scholar] [CrossRef]
- Abuin, L.; Bargeton, B.; Ulbrich, M.H.; Isacoff, E.Y.; Kellenberger, S.; Benton, R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron 2011, 69, 44–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Wei, H.; Shu, C.; Zhang, S.; Cao, Y.; Luo, C.; Yin, J. Identification and comparison of candidate odorant receptor genes in the olfactory and non-olfactory organs of Holotrichia oblita Faldermann by transcriptome analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 24, 1–11. [Google Scholar] [CrossRef]
- Mitchell, R.F.; Schneider, T.M.; Schwartz, A.M.; Andersson, M.N.; McKenna, D.D. The diversity and evolution of odorant receptors in beetles (Coleoptera). Insect Mol. Biol. 2020, 29, 77–91. [Google Scholar] [CrossRef]
- Zhao, T.; Ganji, S.; Schiebe, C.; Bohman, B.; Weinstein, P.; Krokene, P.; Borg-Karlson, A.K.; Unelius, C.R. Convergent evolution of semiochemicals across Kingdoms: Bark beetles and their fungal symbionts. ISME J. 2019, 13, 1535–1545. [Google Scholar] [CrossRef]
- Hou, X.Q.; Yuvaraj, J.K.; Roberts, R.E.; Zhang, D.D.; Unelius, C.R.; Löfstedt, C.; Andersson, M.N. Functional Evolution of a Bark Beetle Odorant Receptor Clade Detecting Monoterpenoids of Different Ecological Origins. Mol. Biol. Evol. 2021, 38, 4934–4947. [Google Scholar] [CrossRef]
- Abdel-Latief, M. A family of chemoreceptors in Tribolium castaneum (Tenebrionidae: Coleoptera). PLoS ONE 2007, 2, e1319. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, Y.; Shen, C.; Xia, D. Identification and Expression Profiling of Odorant Receptor Protein Genes in Sitophilus zeamais (Coleoptera: Curculionoidea) Using RT-qPCR. Neotrop. Entomol. 2019, 48, 538–551. [Google Scholar] [CrossRef]
- Xu, W.; Anderson, A. Carbon dioxide receptor genes in cotton bollworm Helicoverpa armigera. Naturwissenschaften 2015, 102, 11. [Google Scholar] [CrossRef]
- Bin, S.Y.; Qu, M.Q.; Li, K.M.; Peng, Z.Q.; Wu, Z.Z.; Lin, J.T. Antennal and abdominal transcriptomes reveal chemosensory gene families in the coconut hispine beetle, Brontispa longissima. Sci. Rep. 2017, 7, 2809. [Google Scholar] [CrossRef] [Green Version]
- Kozma, M.T.; Schmidt, M.; Ngo-Vu, H.; Sparks, S.D.; Senatore, A.; Derby, C.D. Chemoreceptor proteins in the Caribbean spiny lobster, Panulirus argus: Expression of Ionotropic Receptors, Gustatory Receptors, and TRP channels in two chemosensory organs and brain. PLoS ONE 2018, 13, e0203935. [Google Scholar] [CrossRef]
- Croset, V.; Rytz, R.; Cummins, S.F.; Budd, A.; Brawand, D.; Kaessmann, H.; Gibson, T.J.; Benton, R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010, 6, e1001064. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Statistics Project | Number |
|---|---|
| Total Raw Reads | 45,642,924 |
| Total Clean Reads | 43,302,906 |
| Clean bases | 6.5G |
| Q20 percentage | 97.74% |
| Q30 percentage | 94.10% |
| GC percentage | 43.80% |
| Transcripts | 79,280 |
| Mean length of transcripts | 783 |
| N50 of transcripts | 1435 |
| Unigenes | 42,197 |
| Mean length of Unigenes | 1215 |
| N50 of Unigenes | 1744 |
| Gene Name | ORF Length (bp) | Complete ORF | Transmembrane Helix | FPKM | Best Blastx Match | ||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Acc.number | Species | E-Value | Identity (%) | |||||
| AbunOR1 | 330 | Yes | 1 | 1.64 | olfactory receptor OR1 | AJO62220.1 | Tenebrio molitor | 4 × 10−22 | 43% |
| AbunOR2 | 1182 | Yes | 4 | 12.81 | odorant receptor Or1-like | XP_018566530.1 | Anoplophora glabripennis | 3 × 10−121 | 48% |
| AbunOR3 | 330 | Yes | 0 | 1.64 | odorant receptor Or2-like | XP_018567067.1 | Anoplophora glabripennis | 1 × 10−8 | 72% |
| AbunOR4 | 402 | Yes | 2 | 1.17 | odorant receptor 4-like | XP_023027241.1 | Leptinotarsa decemlineata | 6 × 10−37 | 49% |
| AbunOR5 | 1308 | Yes | 7 | 1.71 | odorant receptor | AUF73018.1 | Anoplophora chinensis | 9 × 10−162 | 55% |
| AbunOR6 | 1179 | Yes | 6 | 0.69 | odorant receptor Or2-like | XP_018579015.1 | Anoplophora glabripennis | 1 × 10−81 | 39% |
| AbunOR7 | 594 | Yes | 3 | 3.89 | odorant receptor Or2-like | XP_018576526.1 | Anoplophora glabripennis | 1 × 10−95 | 69% |
| AbunOR8 | 600 | Yes | 2 | 2.4 | odorant receptor Or1-like | XP_023310030.1 | Anoplophora glabripennis | 2 × 10−90 | 78% |
| AbunOR9 | 1137 | Yes | 7 | 0.33 | odorant receptor 22b-like | XP_023311541.1 | Anoplophora glabripennis | 6 × 10−45 | 33% |
| AbunOR10 | 276 | Yes | 0 | 0.76 | odorant receptor 63a-like | XP_023016125.1 | Leptinotarsa decemlineata | 4 × 10−17 | 46% |
| AbunOR11 | 150 | Yes | 1 | 1.81 | odorant receptor 22c-like | XP_018579026.1 | Anoplophora glabripennis | 3 × 10−10 | 53% |
| AbunOR12 | 996 | Yes | 4 | 3.14 | odorant receptor 63a-like | XP_023016125.1 | Leptinotarsa decemlineata | 8 × 10−50 | 35% |
| AbunOR13 | 630 | Yes | 0 | 3.28 | odorant receptor | AUF73043.1 | Anoplophora chinensis | 2 × 10−44 | 37% |
| AbunOR14 | 90 | Yes | 0 | 52.82 | odorant receptor 49b-like | XP_018577261.1 | Anoplophora glabripennis | 4 × 10−13 | 74% |
| AbunOR15 | 897 | NO | 4 | 3.27 | odorant receptor 49b-like | XP_022917715.1 | Onthophagus taurus | 8 × 10−28 | 29% |
| AbunOR16 | 507 | NO | 2 | 1.58 | odorant receptor 1 | APC94305.1 | Pyrrhalta aenescens | 3 × 10−33 | 39% |
| AbunOR17 | 1086 | Yes | 7 | 3.44 | odorant receptor 94a-like | XP_018560823.1 | Anoplophora glabripennis | 5 × 10−32 | 30% |
| AbunOR18 | 486 | NO | 0 | 1.04 | odorant receptor 4-like | XP_018577142.1 | Anoplophora glabripennis | 2 × 10−34 | 45% |
| AbunOR19 | 411 | Yes | 3 | 2.27 | odorant receptor | AUF73037.1 | Anoplophora chinensis | 4 × 10−38 | 48% |
| AbunOR20 | 321 | Yes | 1 | 3.51 | odorant receptor OR32 | ALR72575.1 | Colaphellus bowringi | 6 × 10−17 | 35% |
| AbunOR21 | 654 | Yes | 3 | 1.9 | odorant receptor 4-like | XP_023027241.1 | Leptinotarsa decemlineata | 3 × 10−37 | 33% |
| AbunOR22 | 366 | Yes | 1 | 3.25 | odorant receptor Or2-like | XP_018579015.1 | Anoplophora glabripennis | 6 × 10−48 | 50% |
| AbunOR23 | 576 | Yes | 2 | 3.7 | odorant receptor 94a-like | XP_018560823.1 | Anoplophora glabripennis | 4 × 10−47 | 36% |
| AbunOR24 | 297 | Yes | 0 | 2.21 | odorant receptor 63a-like | XP_023016125.1 | Leptinotarsa decemlineata | 6 × 10−15 | 38% |
| AbunOR25 | 1440 | Yes | 7 | 49.99 | odorant receptor coreceptor | XP_018568191.1 | Anoplophora glabripennis | 0 | 92% |
| AbunOR26 | 936 | Yes | 4 | 6.25 | odorant receptor 18 | APC94230.1 | Pyrrhalta maculicollis | 9 × 10−90 | 41% |
| AbunOR27 | 1158 | Yes | 6 | 0.66 | odorant receptor Or2-like | XP_018579015.2 | Anoplophora glabripennis | 5 × 10−95 | 41% |
| AbunOR28 | 894 | Yes | 4 | 2.79 | odorant receptor | AUF73043.1 | Anoplophora chinensis | 2 × 10−64 | 41% |
| AbunOR29 | 735 | Yes | 5 | 2.76 | odorant receptor OR36 | ALR72579.1 | Colaphellus bowringi | 4 × 10−113 | 61% |
| AbunOR30 | 318 | Yes | 2 | 1.58 | odorant receptor Or2-like | XP_018576526.1 | Anoplophora glabripennis | 1 × 10−31 | 55% |
| AbunOR31 | 1134 | Yes | 7 | 2.69 | odorant receptor 4-like | XP_018577142.1 | Anoplophora glabripennis | 2 × 10−88 | 41% |
| AbunOR32 | 1143 | Yes | 6 | 1.34 | odorant receptor 19, partial | AVN97831.1 | Anoplophora chinensis | 3 × 10−99 | 43% |
| AbunOR33 | 762 | Yes | 1 | 3.09 | odorant receptor 18 | APC94230.1 | Pyrrhalta maculicollis | 6 × 10−105 | 54% |
| AbunOR34 | 381 | Yes | 1 | 1.83 | odorant receptor 43a-like | XP_018573343.1 | Anoplophora glabripennis | 7 × 10−32 | 46% |
| AbunOR35 | 1170 | Yes | 6 | 3.22 | odorant receptor | AUF73043.1 | Anoplophora chinensis | 2 × 10−45 | 28% |
| AbunOR36 | 171 | Yes | 2 | 2.63 | odorant receptor 85b-like | XP_018564120.1 | Anoplophora glabripennis | 7 × 10−13 | 57% |
| AbunOR37 | 726 | Yes | 4 | 5.48 | odorant receptor OR24 | ALR72568.1 | Colaphellus bowringi | 3 × 10−92 | 47% |
| AbunOR38 | 1104 | Yes | 7 | 3.11 | odorant receptor 49b-like | XP_018570955.1 | Anoplophora glabripennis | 1 × 10−171 | 65% |
| AbunOR39 | 627 | Yes | 2 | 0.96 | odorant receptor 286 | EFA01418.1 | Tribolium castaneum | 6 × 10−17 | 26% |
| AbunOR40 | 387 | Yes | 0 | 0.69 | odorant receptor OR20 | ALR72565.1 | Colaphellus bowringi | 4 × 10−26 | 39% |
| AbunOR41 | 840 | Yes | 4 | 1.06 | odorant receptor OR9 | ALR72554.1 | Colaphellus bowringi | 3 × 10−87 | 43% |
| AbunOR42 | 372 | NO | 2 | 1.61 | odorant receptor 19, partial | AVN97831.1 | Anoplophora chinensis | 4 × 10−9 | 35% |
| AbunOR43 | 333 | Yes | 2 | 1.87 | odorant receptor 26 | QNH68050.1 | Apriona germari | 4 × 10−78 | 69% |
| AbunOR44 | 537 | NO | 2 | 1.13 | odorant receptor Or2-like | XP_023311850.1 | Anoplophora glabripennis | 4 × 10−68 | 56% |
| AbunOR45 | 543 | Yes | 2 | 2.00 | odorant receptor 3, partial | AVN97815.1 | Anoplophora chinensis | 5 × 10−34 | 35% |
| Gene Name | ORF Length (bp) | Complete ORF | Transmembrane Helix | FPKM Value | Best Blastx Match | ||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Acc.number | Species | E-Value | Identity (%) | |||||
| AbunGR1 | 243 | NO | 0 | 2.59 | putative gustatory receptor GR9 | ALR72586.1 | Colaphellus bowringi | 1 × 10−17 | 44% |
| AbunGR2 | 198 | NO | 0 | 8.34 | gustatory receptor Gr83 | NP_001138948.1 | Tribolium castaneum | 1 × 10−18 | 66% |
| AbunGR3 | 297 | NO | 0 | 1.77 | putative gustatory receptor 2a | XP_015840061.1 | Tribolium castaneum | 1 × 10−9 | 38% |
| AbunGR4 | 597 | NO | 3 | 3.22 | gustatory receptor 68a-like | XP_018567270.1 | Anoplophora glabripennis | 6 × 10−37 | 39% |
| AbunGR5 | 522 | NO | 4 | 1.53 | gustatory receptor 1 | EFA07594.2 | Tribolium castaneum | 2 × 10−115 | 81% |
| AbunGR6 | 510 | NO | 2 | 4.1 | gustatory receptor 3 | AKC58580.2 | Anomala corpulenta | 9 × 10−103 | 83% |
| Gene Name | ORF Length (bp) | Complete ORF | Transmembrane Helix | FPKM Value | Best Blastx Match | ||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Acc.number | Species | E-Value | Identity (%) | |||||
| AbunIR1 | 174 | NO | 0 | 2.59 | ionotropic receptor 1 | QNH68025.1 | Apriona germari | 1 × 10−13 | 52% |
| AbunIR2 | 1257 | Yes | 3 | 6.03 | ionotropic receptor 3 | ANQ46495.1 | Phyllotreta striolata | 1 × 10−110 | 44% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Ye, J.; Qian, J.; Purba, E.R.; Zhang, Q.; Zhang, L.; Mang, D. Identification and Expression Profile of Chemosensory Receptor Genes in Aromia bungii (Faldermann) Antennal Transcriptome. Insects 2022, 13, 96. https://doi.org/10.3390/insects13010096
Wu Z, Ye J, Qian J, Purba ER, Zhang Q, Zhang L, Mang D. Identification and Expression Profile of Chemosensory Receptor Genes in Aromia bungii (Faldermann) Antennal Transcriptome. Insects. 2022; 13(1):96. https://doi.org/10.3390/insects13010096
Chicago/Turabian StyleWu, Zhenchen, Jia Ye, Jiali Qian, Endang Rinawati Purba, Qinghe Zhang, Longwa Zhang, and Dingze Mang. 2022. "Identification and Expression Profile of Chemosensory Receptor Genes in Aromia bungii (Faldermann) Antennal Transcriptome" Insects 13, no. 1: 96. https://doi.org/10.3390/insects13010096
APA StyleWu, Z., Ye, J., Qian, J., Purba, E. R., Zhang, Q., Zhang, L., & Mang, D. (2022). Identification and Expression Profile of Chemosensory Receptor Genes in Aromia bungii (Faldermann) Antennal Transcriptome. Insects, 13(1), 96. https://doi.org/10.3390/insects13010096






