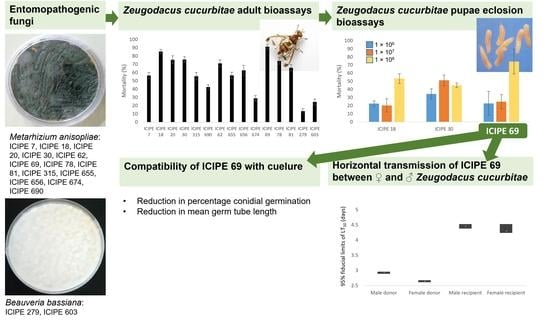
The Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana for Management of the Melon Fly Zeugodacus cucurbitae: Pathogenicity, Horizontal Transmission, and Compatability with Cuelure
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source and Rearing Conditions
2.2. Fungal Sources and Maintenance of Fungal Cultures
2.3. Virulence of Entomopathogenic Fungal Isolates to Zeugodacus cucurbitae Adults
2.4. Effect of Selected Metarhizium anisopliae Isolates on Zeugodacus cucurbitae Puparia Eclosion
2.5. Compatibility of Metarhizium anisopliae ICIPE 69 with Cuelure
2.6. Horizontal Transmission of Metarhizium anisopliae ICIPE 69 among Zeugodacus cucurbitae Adults
2.7. Data Analysis
3. Results
3.1. Pathogenicity and Virulence of Entomopathogenic Fungal Isolates against Zeugodacus cucurbitae Adults
3.2. Effect of Selected Metarhizium anisopliae Isolates on Zeugodacus cucurbitae Puparia Eclosion
3.3. Mortality of Zeugodacus cucurbitae Adults Eclosed from Inoculated Soil
3.4. Compatibility of Metarhizium anisopliae ICIPE 69 with Cuelure
3.5. Horizontal Transmission of Metarhizium anisopliae Inoculum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaefer, H.; Renner, S. Phylogenetic relationships in the order cucurbitales and a new classification of the gourd family (Cucurbitaceae). Taxon 2011, 60, 122–138. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. (FAOSTAT) Statistical Database; FAO: Rome, Italy, 2020; Available online: https://www.fao.org/faostat/en/ (accessed on 5 September 2022).
- Karrar, E.; Sheth, S.; Navicha, W.B.; Wei, W.; Hassanin, H.; Abdalla, M.; Wang, X. A potential new source: Nutritional and antioxidant properties of edible oils from cucurbit seeds and their impact on human health. J. Food Biochem. 2019, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae family and their products: Positive effect on human health. Nutrition 2020, 78, 110788. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A. Edible seeds from Cucurbitaceae family as potential functional foods: Immense promises, few concerns. Biomed. Pharmacother. 2017, 91, 330. [Google Scholar] [CrossRef]
- Njoroge, G.N. A Survey of Some Cucurbitaceae Species in Kenya with an Analysis of Cucurbitacin Content and an Identification Guide to Poisonous and Safe Species. Master’s Thesis, Kenyatta University, Nairobi, Kenya, 2012. [Google Scholar]
- Leblanc, L.; Vueti, E.T.; Drew, R.A.I.; Allwood, A.J. Host plant records for fruit flies (Diptera: Tephritidae: Dacini) in the Pacific Islands. Proc. Hawaii. Entomol. Soc. 2012, 44, 11–53. [Google Scholar]
- Shafiq Ansari, M.; Hasan, F.; Ahmad, N. Threats to fruit and vegetable crops: Fruit flies (Tephritidae)-ecology, behaviour, and management. J. Crop Sci. Biotechnol. 2012, 15, 169–188. [Google Scholar] [CrossRef]
- Kakar, M.Q.; Ullah, F.; Salioqi, A.R.; Ahmad, S.; Ali, I. Determination of fruit flies (Diptera: Tephritidae) infestation in guava, peach and bitter gourd orchards in Khyber. Sarhad J. Agric. 2014, 30, 241–246. [Google Scholar]
- Badii, K.B.; Billah, M.K.; Afreh Nuamah, K.; Obeng Ofori, D.; Nyarko, G. Review of the pest status, economic impact and management of fruit-infesting flies (Diptera: Tephritidae) in Africa. Afr. J. Agric. Res. 2015, 10, 1488–1498. [Google Scholar] [CrossRef]
- De Meyer, M.; Delatte, H.; Mwatawala, M.; Quilici, S.; Vayssières, J.F.; Virgilio, M. A review of the current knowledge on Zeugodacus cucurbitae (Coquillett) (Diptera, Tephritidae) in Africa, with a list of species included in Zeugodacus. ZooKeys 2015, 540, 539–557. [Google Scholar] [CrossRef]
- Mwatawala, M.W.; De Meyer, M.; Makundi, R.H.; Maerere, A.P. Host range and distribution of fruit-infesting pestiferous fruit flies (Diptera, Tephritidae) in selected areas of Central Tanzania. Bull. Entomol. Res. 2009, 99, 629–641. [Google Scholar] [CrossRef]
- Dhillon, M.; Singh, R.; Naresh, J.; Sharma, H. The melon fruit fly, Bactrocera cucurbitae: A review of its biology and management. J. Insect Sci. 2005, 5, 1–16. [Google Scholar] [CrossRef]
- Weems, H.V.; Heppner, J.B.; Fasulo, T.R. IFAS Extension EENY199. Melon Fly, Bactrocera cucurbitae (Coquillett) (Insecta: Diptera: Tephritidae); University of Florida: Gainesville, FL, USA, 2018. [Google Scholar]
- Magagula, C.N.; Nzima, B.A. Diversity and distribution of fruit flies (Diptera: Tephritidae) across agroecological zones in Swaziland: On the lookout for the invasive fruit fly Bactrocera invadens. J. Sustain. Agric. 2013, 8, 100–109. [Google Scholar]
- Horticultural Crops Directorate (HCD). Horticulture Validated Report 2019–2020; Ministry of Agriculture, Livestock and Fisheries: Nairobi, Kenya, 2020.
- Onsongo, S.K.; Gichimu, B.M.; Akutse, K.S.; Dubois, T.; Mohamed, S.A. Performance of three isolates of Metarhizium anisopliae and their virulence against Zeugodacus cucurbitae under different temperature regimes, with global extrapolation of their efficiency. Insects 2019, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Lin, A.; Wen, B.; Peng, Q. Population susceptibility to insecticides and the development of resistance in Bactrocera cucurbitae (Diptera: Tephritidae). J. Econ. Entomol. 2016, 109, 837–846. [Google Scholar] [CrossRef]
- Ryckewaert, P.; Deguine, J.P.; Brévault, T.; Vayssières, J.F. Fruit flies (Diptera: Tephritidae) on vegetable crops in Reunion Island (Indian Ocean): State of knowledge, control methods and prospects for management. Fruits 2010, 65, 113–130. [Google Scholar] [CrossRef]
- Vontas, J.; Hernández-Crespo, P.; Margaritopoulos, J.T.; Ortego, F.; Feng, H.T.; Mathiopoulos, K.D.; Hsu, J.C. Insecticide resistance in tephritid flies. Pestic. Biochem. Physiol. 2011, 100, 199–205. [Google Scholar] [CrossRef]
- Akutse, K.S.; Subramanian, S.; Maniania, N.K.; Dubois, T.; Ekesi, S. Biopesticide research and product development in Africa for sustainable agriculture and food security–experiences from the International Centre of Insect Physiology and Ecology (icipe). Front. Sustain. Food Syst. 2020, 4, 563016. [Google Scholar] [CrossRef]
- Garcia, F.R.M.; Ricalde, M.P. Augmentative biological control using parasitoids for fruit fly management in Brazil. Insects 2013, 4, 55–70. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Ekesi, S.; Hanna, R. Old and new host-parasitoid associations: Parasitization of the invasive fruit fly Bactrocera invadens (Diptera: Tephritidae) and five other African fruit fly species by Fopius arisanus, an Asian opine parasitoid. Biocontrol Sci. Technol. 2010, 10, 183–196. [Google Scholar] [CrossRef]
- Ovruski, S.; Aluja, M.; Sivinski, J.; Wharton, R. Hymenopteran parasitoids on fruit-infesting Tephritidae (Diptera) in Latin America and the southern United States: Diversity, distribution, taxonomic status and their use in fruit fly biological control. Int. J. Pest Manag. 2000, 5, 81–107. [Google Scholar]
- Rendon, P.; Sivinski, J.; Holler, T.; Bloem, K.; Lopez, M.; Martinez, A.; Aluja, M. The effects of sterile males and two braconid parasitoids, Fopius arisanus (Sonan) and Diachasmimorpha krausii (Fullaway) (Hymenoptera), on caged populations of Mediterranean fruit flies, Ceratitis capitata (Wied.) (Diptera: Tephritidae) at various sites. Biol. Control 2006, 36, 224–231. [Google Scholar] [CrossRef]
- Vayssieres, J.F.; Sinzogan, A.; Korie, S.; Ouagoussounon, I.; Thomas-Odjo, A. Effectiveness of spinosad bait sprays (GF-120) in controlling mango-infesting fruit flies (Diptera: Tephritidae) in Benin. J. Econ. Entomol. 2009, 102, 515–521. [Google Scholar] [CrossRef]
- Vega, F.E.; Dowd, P.F.; Lacey, L.A.; Pell, J.K.; Jackson, D.M.; Klein, M.G. Dissemination of beneficial microbial agents by insects. In Field Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Kaya, H.K., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2007; pp. 127–146. [Google Scholar]
- Hafsi, A.; Abbes, K.; Harbi, A.; Duyck, P.F.; Chermiti, B. Attract-and-kill systems efficiency against Ceratitis capitata (Diptera: Tephritidae) and effects on non-target insects in peach orchards. J. Appl. Entomol. 2016, 140, 28–36. [Google Scholar] [CrossRef]
- Faye, P.D.; Bal, A.B.; Ndiaye, N.M.; Diop, F.; Sangaré, Y.K.; Haddad, C.; Coly, E.V.; Dieng, E.O.; Niassy, S. Field efficacy of Metarhizium acridum (Hypocreales: Clavicipitaceae) in the control of Bactrocera dorsalis (Diptera: Tephritidae) in citrus orchards in Senegal. Int. J. Trop. Insect Sci. 2021, 41, 1185–1195. [Google Scholar] [CrossRef]
- Navarro-Llopis, V.; Ayala, I.; Sanchis, J.; Primo, J.; Moya, P. Field Efficacy of a Metarhizium anisopliae-based attractant-contaminant device to control Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 2015, 108, 1570–1578. [Google Scholar] [CrossRef]
- Dimbi, S.; Maniania, N.K.; Lux, S.A.; Ekesi, S.; Mueke, J.K. Pathogenicity of Metarhizium anisopliae (Metsch.) Sorokin and Beauveria bassiana (Balsamo) Vuillemin, to three adult fruit fly species: Ceratitis capitata (Weidemann), C. rosa var. fasciventris Karsch and C. cosyra (Walker) (Diptera: Tephritidae). Mycopathologia 2003, 156, 375–382. [Google Scholar] [CrossRef]
- Ekesi, S.; Maniania, N.K.; Lux, S.A. Mortality in three African tephritid fruit fly puparia and adults caused by the entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana. Biocontrol Sci. Technol. 2002, 12, 7–17. [Google Scholar] [CrossRef]
- Chang, C.L.; Caceres, C.; Jang, E.B. A novel liquid diet and its rearing system for melon fly, Bactrocera cucurbitae (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2004, 97, 524–528. [Google Scholar] [CrossRef]
- Mfuti, D.K.; Subramanian, S.; Niassy, S.; Salifu, D.; Du Plessis, H.; Ekesi, S.; Maniania, N.K. Screening for attractants compatible with entomopathogenic fungus Metarhizium anisopliae for use in thrips management. Afr. J. Biotechnol. 2016, 15, 714–721. [Google Scholar]
- Goettel, M.S.; Inglis, G.D. Fungi: Hyphomycetes. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 213–248. [Google Scholar]
- Migiro, L.N.; Maniania, N.K.; Chabi-Olaye, A.; Vandenberg, J. Pathogenicity of entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana (Hypocreales: Clavicipitaceae) isolates to the adult pea leafminer (Diptera: Agromyzidae) and prospects of an autoinoculation device for infection in the Field. Environ. Entomol. 2010, 39, 468–475. [Google Scholar] [CrossRef]
- Qazzaz, F.O.; Al-Masri, M.I.; Barakat, R.M. Effectiveness of Beauveria bassiana native isolates in the biological control of the Mediterranean fruit fly (Ceratitis capitata). Adv. Entomol. 2015, 3, 44–55. [Google Scholar] [CrossRef]
- Niassy, S.; Maniania, N.K.; Subramanian, S.; Gitonga, L.M.; Mburu, D.M.; Masiga, D.; Ekesi, S. Selection of promising fungal biological control agent of the western flower thrips Frankliniella occidentalis (Pergande). Lett. Appl. Microbiol. 2012, 54, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Opisa, S.; Du Plessis, H.; Akutse, K.S.; Fiaboe, K.K.M.; Ekesi, S. Horizontal transmission of Metarhizium anisopliae between Spoladea recurvalis (Lepidoptera: Crambidae) adults and compatibility of the fungus with the attractant phenylacetaldehyde. Microb. Pathog. 2019, 131, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Nana, P.; Maniania, N.K.; Maranga, R.O.; Boga, H.I.; Kutima, H.L.; Eloff, J.N. Compatibility between Calpurnia aurea leaf extract, attraction aggregation, and attachment pheromone and entomopathogenic fungus Metarhizium anisopliae on viability, growth, and virulence of the pathogen. J. Pest Sci. 2012, 85, 109–115. [Google Scholar] [CrossRef]
- Dimbi, S.; Maniania, N.K.; Ekesi, S. Horizontal transmission of Metarhizium anisopliae in fruit flies and effect of fungal infection on egg laying and fertility. Insects 2013, 4, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Oreste, M.; Baser, N.; Bubici, G.; Tarasco, E. Effect of Beauveria bassiana strains on the Ceratitis capitata-Psyttalia concolor system. Bull. Insectology 2015, 68, 265–272. [Google Scholar]
- Ortu, S.; Cocco, A.; Duu, R. Evaluation of the entomopathogenic fungus Beauveria bassiana strain ATCC 74040 for the management of Ceratitis capitata. Bull. Insectology 2009, 62, 245–252. [Google Scholar]
- Marri, D.; Gomez, D.A.M.A.; Wilson, D.D.; Billah, M.; Yeboah, S.; Osae, M. Evaluation of the efficacy of a commercial formulation of Beauveria bassiana for the control of the invasive fruit fly Bactrocera dorsalis (Diptera: Tephritidae). Biopestic. Int. 2016, 12, 9–19. [Google Scholar]
- Gul, H.T.; Freed, S.; Akmal, M.; Malik, M.N. Vulnerability of different life stages of Bactrocera zonata (Tephritidae: Diptera) against entomogenous fungi. Pak. J. Zool. 2015, 47, 307–317. [Google Scholar]
- Beris, E.I.; Papachristos, D.P. Pathogenicity of three entomopathogenic fungi on pupae and adults of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). J. Pest Sci. 2013, 86, 275–284. [Google Scholar] [CrossRef]
- Sookar, P.; Bhagwant, S.; Ouna, E.A. Isolation of entomopathogenic fungi from the soil and their pathogenicity to two fruit fly species (Diptera: Tephritidae). J. Appl. Entomol. 2008, 132, 778–788. [Google Scholar] [CrossRef]
- Bayissa, W.; Ekesi, S.; Mohamed, S.A.; Kaaya, G.P.; Wagacha, J.M.; Hanna, R.; Maniania, N.K. Selection of fungal isolates for virulence against three aphid pest species of crucifers and okra. J. Pest Sci. 2017, 90, 355–368. [Google Scholar] [CrossRef]
- Mweke, A.; Ulrichs, C.; Nana, P.; Akutse, K.S.; Kouma, K.; Fiaboe, M.; Ekesi, S. Biological and microbial control evaluation of the entomopathogenic fungi Metarhizium anisopliae, Beauveria bassiana and Isaria sp. for the management of Aphis craccivora (Hemiptera: Aphididdae). J. Econ. Entomol. 2018, 111, 1587–1594. [Google Scholar] [CrossRef]
- Ekesi, S.; Maniania, N.K.; Mohamed, S.A.; Lux, S.A. Effect of soil application of different formulations of Metarhizium anisopliae on African tephritid fruit flies and their associated endoparasitoids. Biol. Control 2005, 35, 83–91. [Google Scholar] [CrossRef]
- Inglis, G.D.; Goettel, M.S.; Butt, T.M.; Strasser, H. Use of hyphomycetous fungi for managing insect pests. In Fungi as Biocontrol Agents: Progress, Problems and Potential; Butt, T.M., Jackson, C., Magan, N., Eds.; CAB International: Wallingford, UK, 2001; pp. 23–29. [Google Scholar]
- Khlaywi, S.A.; Khudhair, M.W.; Alrubeai, H.F.; Shbar, A.K.; Hadi, S.A. Efficacy of Beauveria bassiana and Metarhizium anisopliae to control mediterranean fruit fly, Ceratitis capitata. Int. J. Entomol. Res. 2014, 2, 169–173. [Google Scholar]
- Mar, T.T.; Lumyong, S. Evaluation of effective entomopathogenic fungi to fruit fly pupa, Bactrocera spp. and their antimicrobial activity. Nat. Sci. 2012, 39, 464–477. [Google Scholar]
- Ekesi, S.; Maniania, N.K. Susceptibility of Megalurothrips sjostedti developmental stages to Metarhizium anisopliae and the effects of infection on feeding, adult fecundity, egg fertility and longevity. Entomol. Exp. Appl. 2000, 94, 229–236. [Google Scholar] [CrossRef]
- Chambers, A.H.; Evans, S.A.; Folta, K.M. Methyl anthranilate and γ-decalactone inhibit strawberry pathogen growth and achene germination. J. Agric. Food Chem. 2013, 61, 12625–12633. [Google Scholar] [CrossRef]
- Erdemgil, F.Z.; Ilhan, S.; Korkmaz, F.; Kaplan, C.; Mercangoz, A.; Arfan, M.; Ahmad, S. Chemical composition and biological activity of the essential oil of Perovskia atriplicifolia from Pakistan. Pharm. Biol. 2007, 45, 324–331. [Google Scholar] [CrossRef]
- Halim, V.A.; Vess, A.; Scheel, D.; Rosahl, S. The role of salicylic acid and jasmonic acid in pathogen defence. Plant Biol. 2006, 8, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Akutse, K.S.; Subramanian, S.; Khamis, F.M.; Ekesi, S.; Mohamed, S.A. Entomopathogenic fungus isolates for adult Tuta absoluta (Lepidoptera: Gelechiidae) management and their compatibility with Tuta pheromone. J. Appl. Entomol. 2020, 144, 777–787. [Google Scholar] [CrossRef]
- Mkiga, A.M.; Mohamed, S.A.; Du Plessis, H.; Khamis, F.M.; Akutse, K.S.; Ekesi, S. Metarhizium anisopliae and Beauveria bassiana: Pathogenicity, horizontal transmission, and their effects on reproductive potential of Thaumatotibia leucotreta (Lepidoptera: Tortricidae). J. Econ. Entomol. 2020, 113, 660–668. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Martin-Carballo, I.; Garrido-Jurado, I.; Santiago-Álvarez, C. Horizontal transmission of Metarhizium anisopliae among laboratory populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Biol. Control 2008, 47, 115–124. [Google Scholar] [CrossRef]
- Forlani, L.; Pedrini, N. Contribution of the horizontal transmission of the entomopathogenic fungus Beauveria bassiana to the overall performance of a fungal powder formulation against Triatoma infestans. Res. Rep. Trop. Med. 2011, 2, 135–140. [Google Scholar]
- Toledo, J.; Campos, S.E.; Flores, S.; Liedo, P.; Barrera, J.F.; Villaseñor, A.; Montoya, P. Horizontal transmission of Beauveria bassiana in Anastrepha ludens (Diptera: Tephritidae) under laboratory and field cage conditions. J. Econ. Entomol. 2007, 100, 291–297. [Google Scholar] [CrossRef]
- Dimbi, S.; Maniania, N.K.; Ekesi, S. Effect of Metarhizium anisopliae inoculation on the mating behavior of three species of African tephritid fruit flies, Ceratitis capitata, Ceratitis cosyra and Ceratitis fasciventris. Biol. Control 2009, 50, 111–116. [Google Scholar] [CrossRef]

| Fungal Species | Fungal Isolate | Source | Place of Origin (Country) | Year of Isolation |
|---|---|---|---|---|
| M. anisopliae | ICIPE 7 | Rhipicephalus appendiculatus Neumann (Ixodida: Ixodidae) | Rusinga Island (Kenya) | 1996 |
| ICIPE 18 | Soil | Mbita (Kenya) | 1989 | |
| ICIPE 20 | Soil | Migori (Kenya) | 1989 | |
| ICIPE 30 | Busseola fusca (Fuller) (Lepidoptera: Noctuidae) | Kendubay (Kenya) | 1989 | |
| ICIPE 62 | Soil | Matete (DR Congo) | 1990 | |
| ICIPE 69 | Soil | Matete (DR Congo) | 1990 | |
| ICIPE 78 | Tomobrachyta nigroplagiata Fairmaire (Coleoptera: Cerambycidae) | Ungoe (Kenya) | 1990 | |
| ICIPE 81 | Kraussaria angulifera (Krauss) (Orthoptera: Acrididae) | Kaffrine (Senegal) | 2003 | |
| ICIPE 315 | Tetranychus urticae K. (Trombidiformes: Tetranychidae) | Kerugoya (Kenya) | 2006 | |
| ICIPE 655 | Soil | Kabuti (Kenya) | 2008 | |
| ICIPE 656 | Soil | Kapiti (Kenya) | 2008 | |
| ICIPE 674 | Soil | Mariakani (Kenya) | 2008 | |
| ICIPE 690 | Lepidoptera Larvae | Kenya | 2010 | |
| B. bassiana | ICIPE 279 | Coleopteran larvae | Kericho (Kenya) | 2005 |
| ICIPE 603 | Hymenoptera | Taita (Kenya) | 2007 |
| Fungal Species | Isolates | Germination ± SE (%) | Mortality ± SE (%) | LT50 1 (Days) (95% FL 2) |
|---|---|---|---|---|
| Metarhizium anisopliae | ICIPE 7 | 90.32 ± 3.84cd | 56.58 ± 3.63f | 4.83 (4.79–4.86) |
| ICIPE 18 | 94.97 ± 0.67abcd | 85.56 ± 2.57b | 3.96 (3.94–3.98) | |
| ICIPE 20 | 95.28 ± 0.82abcd | 75.63 ± 4.77c | 4.39 (4.37–4.41) | |
| ICIPE 30 | 97.20 ± 0.45abc | 75.87 ± 3.30c | 4.12 (4.09–4.15) | |
| ICIPE 315 | 94.21 ± 1.75abcd | 55.74 ± 4.34f | 4.83 (4.80–4.87) | |
| ICIPE 690 | 94.28 ± 1.65abcd | 42.53 ± 2.71g | – | |
| ICIPE 62 | 90.23 ± 0.56cd | 71.24 ± 4.19cd | 4.40 (4.37–4.43) | |
| ICIPE 655 | 92.17 ± 2.98abcd | 56.66 ± 3.94f | 4.87 (4.83–4.91) | |
| ICIPE 656 | 90.46 ± 1.33abcd | 62.74 ± 5.98ef | 4.73 (4.70–4.77) | |
| ICIPE 674 | 95.56 ± 1.18abc | 29.08 ± 3.33h | – | |
| ICIPE 69 | 97.44 ± 0.72ab | 91.42 ± 2.71a | 3.84 (3.82–3.86) | |
| ICIPE 78 | 95.00 ± 3.00abcd | 74.24 ± 4.07c | 4.36 (4.33–4.39) | |
| ICIPE 81 | 97.82 ± 0.75a | 65.84 ± 4.01de | 4.52 (4.49–4.55) | |
| Beauveria bassiana | ICIPE 279 | 87.25 ± 3.00d | 13.5 ± 2.89i | – |
| ICIPE 603 | 90.43 ± 3.30bcd | 24.5 ± 3.61hi | – |
| Concentration | Fungal Isolates | ||
|---|---|---|---|
| ICIPE 18 | ICIPE 30 | ICIPE 69 | |
| Control | 91.00 ± 2.54 | ||
| 1 × 106 | 51.50 ± 13.19aA | 39.00 ± 7.01aB | 13.00 ± 2.39aC |
| 1 × 107 | 31.50 ± 12.09bA | 25.00 ± 3.80bB | 22.00 ± 5.03aB |
| 1 × 108 | 12.50 ± 1.76cA | 10.00 ± 1.13cAB | 8.00 ± 1.96bB |
| Conidial Concentration | Fungal Isolates | ||
|---|---|---|---|
| ICIPE 18 | ICIPE 30 | ICIPE 69 | |
| Control | 06.68 ± 1.53 | ||
| 1 × 106 | 22.50 ± 3.33bA | 34.38 ± 6.30bA | 22.74 ± 14.95bA |
| 1 × 107 | 20.23 ± 8.17bA | 51.22 ± 6.49aB | 24.91 ± 8.76bA |
| 1 × 108 | 53.39 ± 6.07aAB | 45.00 ± 2.89aB | 74.29 ± 15.25aA |
| Temperature | Days after Exposure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 6 | 8 | ||||||
| Cuelure | No Cuelure | Cuelure | No Cuelure | Cuelure | No Cuelure | Cuelure | No Cuelure | Cuelure | No Cuelure | |
| 18 °C | 80.60 ± 0.79c | 98.10 ± 0.80a | 80.42 ± 1.41c | 97.90 ± 0.37a | 82.29 ± 1.42b | 92.11 ± 0.62b | 81.75 ± 2.95a | 72.27 ± 2.95b | 71.05 ± 2.85b | 69.69 ± 1.49b |
| 25 °C | 92.09 ± 1.65b | 98.46 ± 0.25a | 89.69 ± 0.82b | 96.26 ± 0.52a | 83.44 ± 0.66b | 95.81 ± 1.62a | 77.19 ± 0.81a | 93.88 ± 0.99a | 68.91 ± 0.6b | 88.93 ± 2.05a |
| 30 °C | 97.56 ± 0.96a | 99.12 ± 0.08a | 96.13 ± 0.92a | 98.64 ± 0.17a | 93.51 ± 0.46a | 96.32 ± 0.45a | 76.90 ± 2.88a | 93.44 ± 0.27a | 78.19 ± 1.51a | 87.84 ± 0.38a |
| Temperature | Days after Exposure | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 6 | 8 | ||||||
| Cuelure | No Cuelure | Cuelure | No Cuelure | Cuelure | No Cuelure | Cuelure | No Cuelure | Cuelure | No Cuelure | |
| 18 °C | 102.65 ± 5.13b | 147.16 ± 12.82a | 89.56 ±7.22b | 119.78 ± 6.63b | 56.53 ± 3.80b | 110.61 ± 6.42a | 37.92 ± 0.9c | 83.05 ± 19.01a | 27.15 ± 1.59b | 75.52 ± 4.8b |
| 25 °C | 122.38 ± 2.88a | 113.26 ± 2.28b | 88.62 ± 6.05b | 103.09 ± 0.92c | 62.47 ± 4.64b | 96.30 ± 3.02a | 56.73 ± 1.43b | 84.80 ± 2.04a | 35.28 ± 3.52b | 74.91 ± 4.79b |
| 30 °C | 123.19 ± 3.48a | 145.33 ± 5.93a | 119.59 ± 4.62a | 140.64 ± 0.86a | 103.13 ± 3.97a | 97.25 ± 10.96a | 91.55 ± 2.21a | 96.78 ± 2.4a | 85.59 ± 1.38a | 91.48 ± 1.56a |
| Mortality ± SE (%) | LT50 1 (Days) (95% FL 2) | |
|---|---|---|
| Male donor | 100.00 ± 0.00a | 2.93 (2.89–2.96) |
| Female donor | 100.00 ± 0.00a | 2.64 (2.61–2.68) |
| Male recipient | 59.25 ± 5.92b | 4.46 (4.38–4.53) |
| Female recipient | 67.00 ± 4.49b | 4.30 (4.24–4.53) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onsongo, S.K.; Mohamed, S.A.; Akutse, K.S.; Gichimu, B.M.; Dubois, T. The Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana for Management of the Melon Fly Zeugodacus cucurbitae: Pathogenicity, Horizontal Transmission, and Compatability with Cuelure. Insects 2022, 13, 859. https://doi.org/10.3390/insects13100859
Onsongo SK, Mohamed SA, Akutse KS, Gichimu BM, Dubois T. The Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana for Management of the Melon Fly Zeugodacus cucurbitae: Pathogenicity, Horizontal Transmission, and Compatability with Cuelure. Insects. 2022; 13(10):859. https://doi.org/10.3390/insects13100859
Chicago/Turabian StyleOnsongo, Susan K., Samira A. Mohamed, Komivi S. Akutse, Bernard M. Gichimu, and Thomas Dubois. 2022. "The Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana for Management of the Melon Fly Zeugodacus cucurbitae: Pathogenicity, Horizontal Transmission, and Compatability with Cuelure" Insects 13, no. 10: 859. https://doi.org/10.3390/insects13100859
APA StyleOnsongo, S. K., Mohamed, S. A., Akutse, K. S., Gichimu, B. M., & Dubois, T. (2022). The Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana for Management of the Melon Fly Zeugodacus cucurbitae: Pathogenicity, Horizontal Transmission, and Compatability with Cuelure. Insects, 13(10), 859. https://doi.org/10.3390/insects13100859