Phat Queens Emerge Fashionably Late: Body Size and Condition Predict Timing of Spring Emergence for Queen Bumble Bees
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
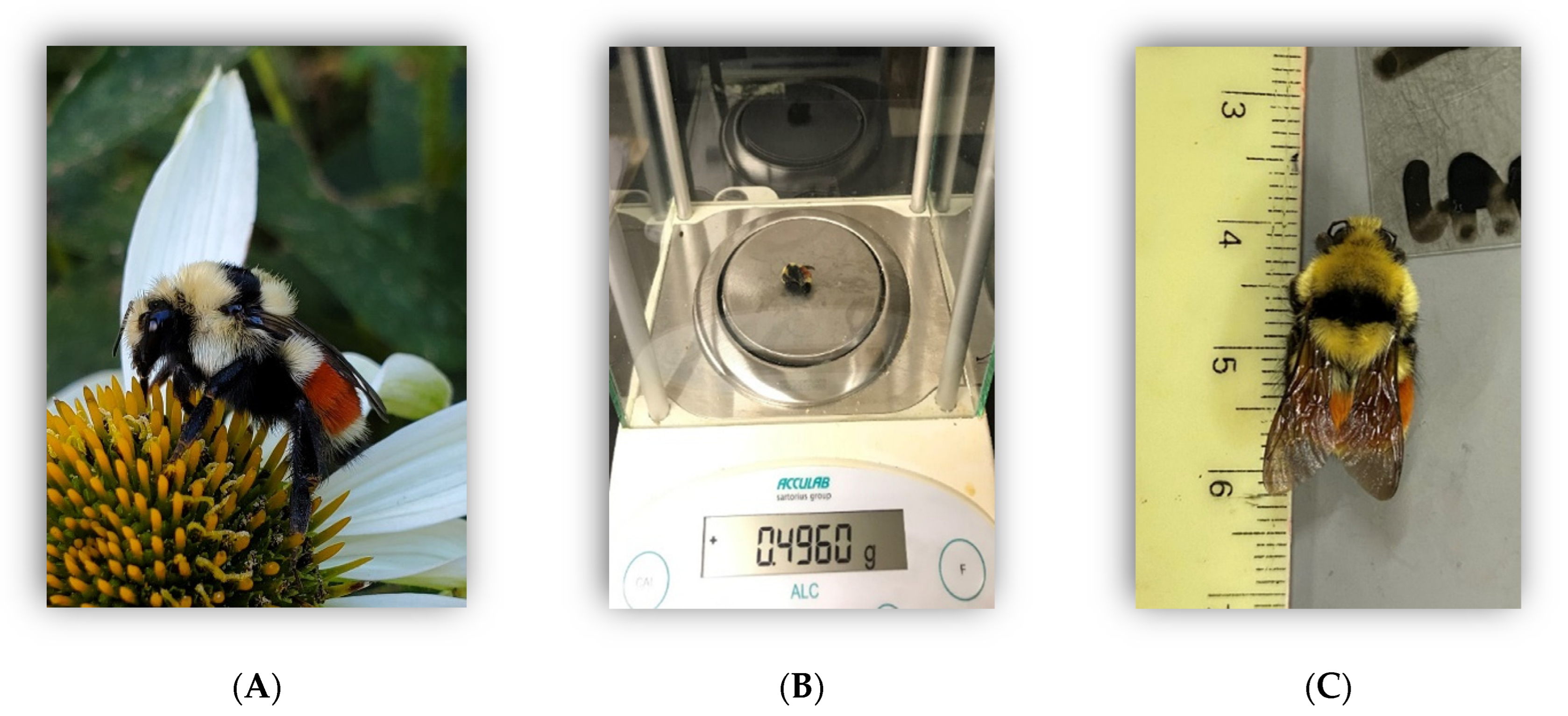
2.1. Animal Collection
2.2. Body Size and Condition
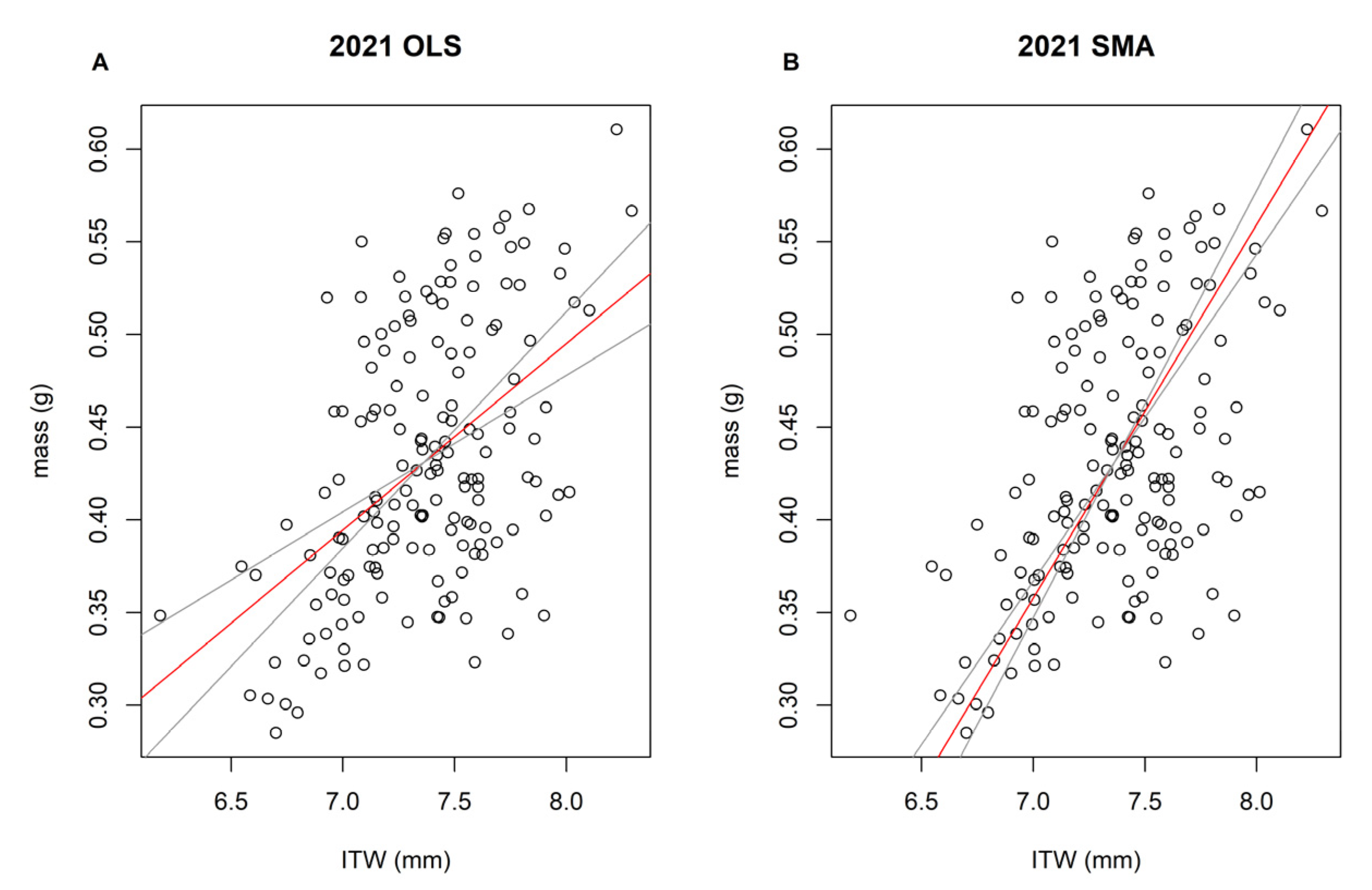
2.3. Statistical Analyses
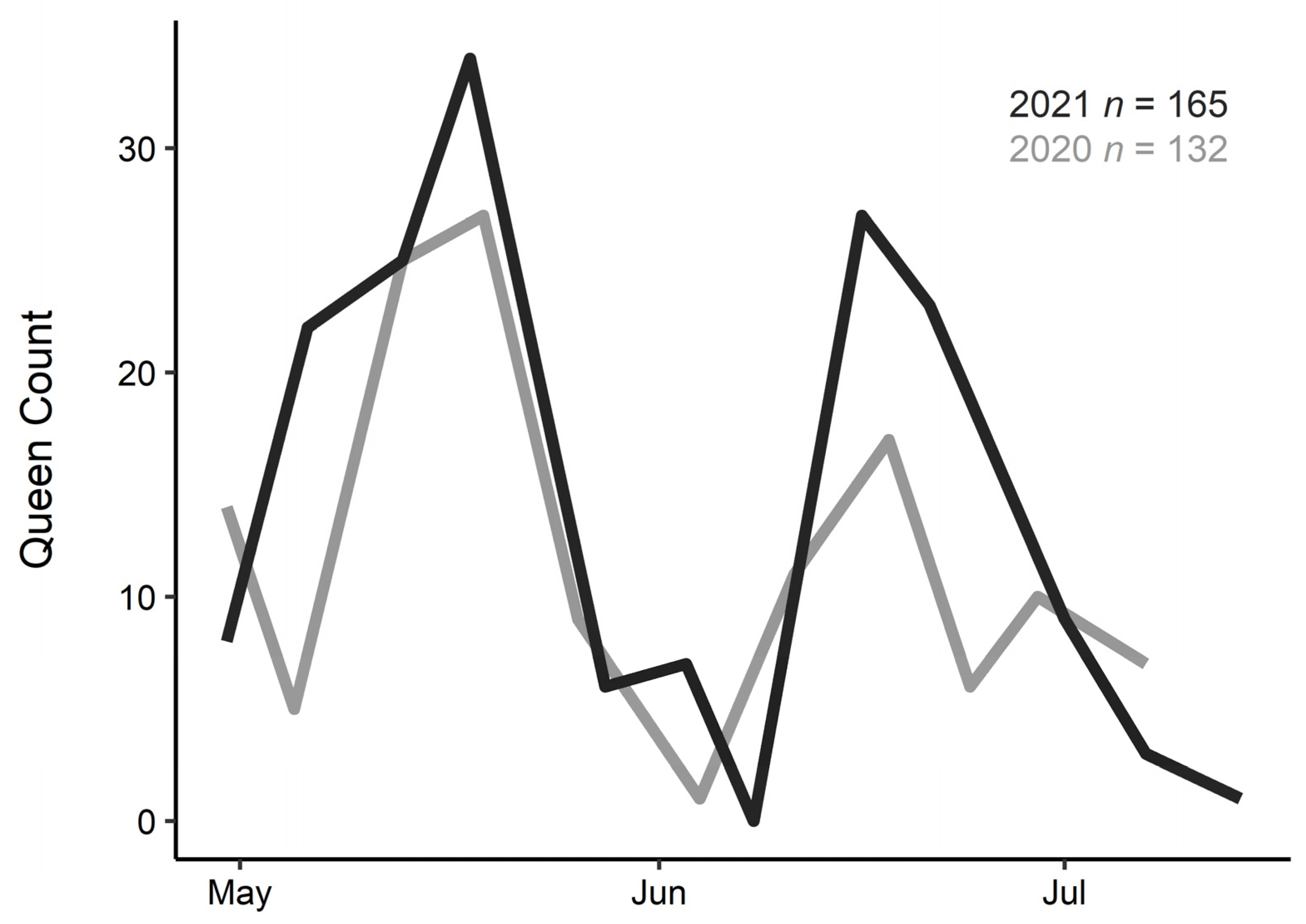
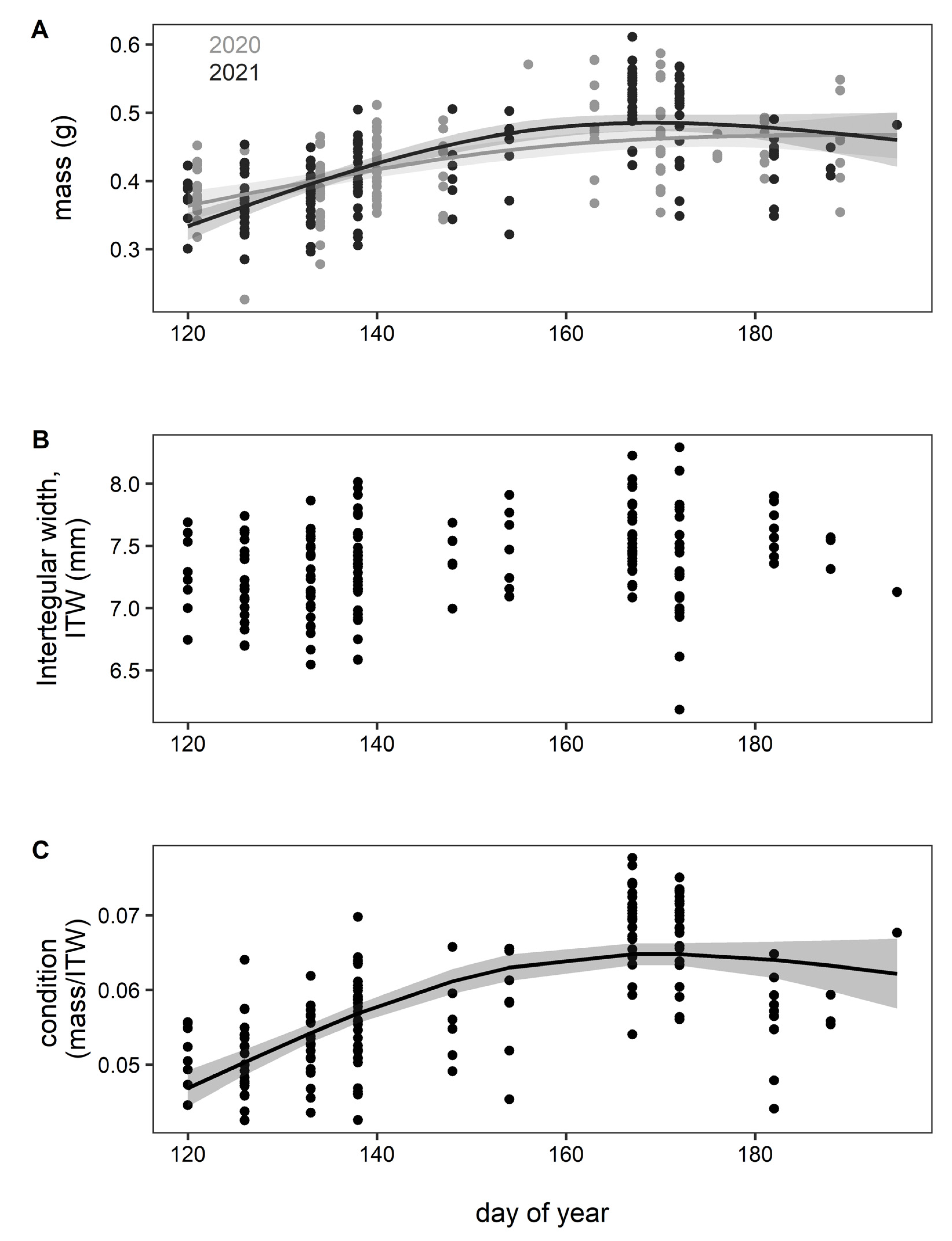
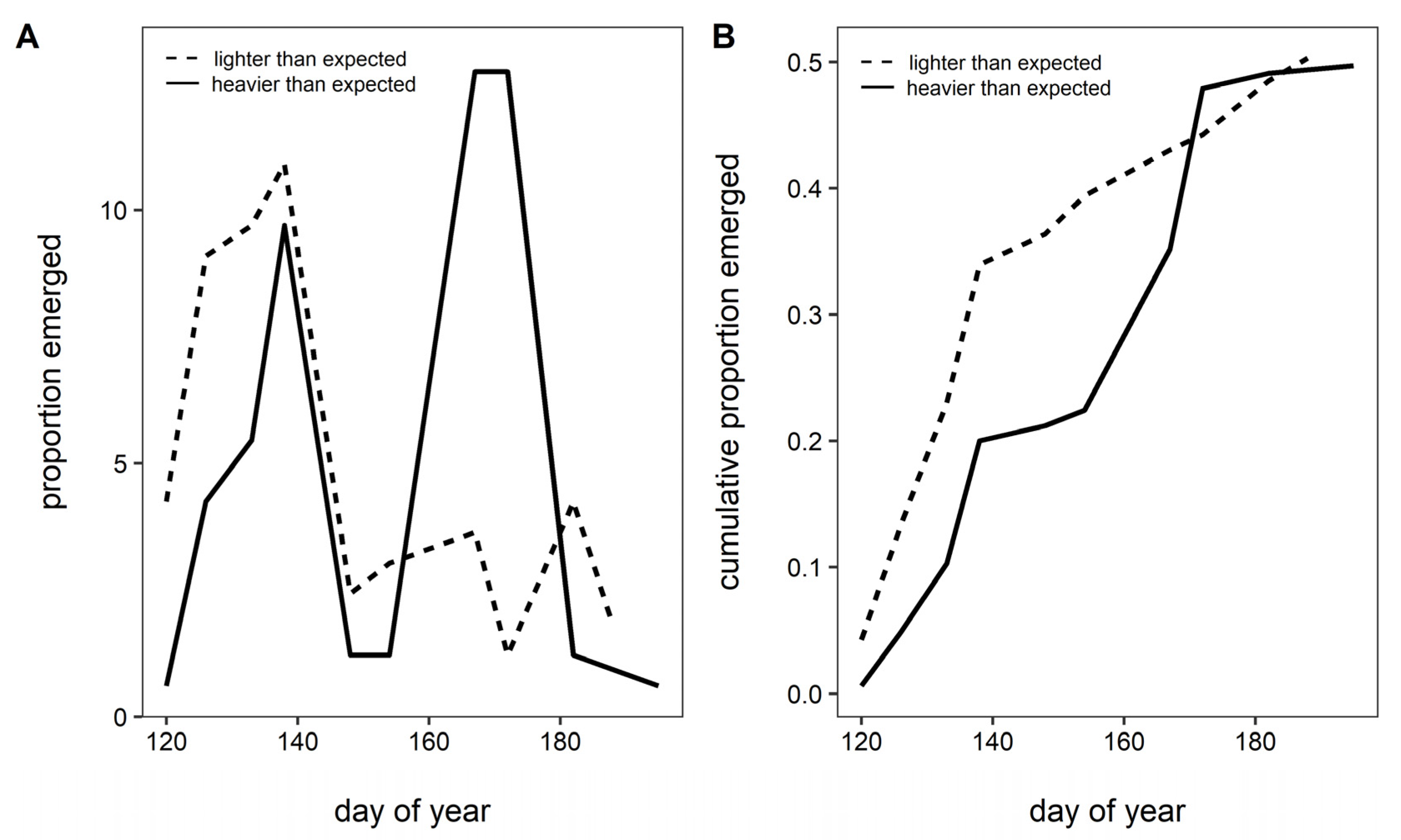
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Van Asch, M.; Visser, M.E. Phenology of Forest Caterpillars and Their Host Trees: The Importance of Synchrony. Annu. Rev. Entomol. 2007, 52, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.R.K.; Thomson, J.D. An Examination of Synchrony between Insect Emergence and Flowering in Rocky Mountain Meadows. Ecol. Monogr. 2011, 81, 469–491. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A. Insect Seasonality: Diapause Maintenance, Termination, and Postdiapause Development. Annu. Rev. Entomol. 1976, 21, 81–107. [Google Scholar] [CrossRef]
- Ovaskainen, O.; Skorokhodova, S.; Yakovleva, M.; Sukhov, A.; Kutenkov, A.; Kutenkova, N.; Shcherbakov, A.; Meyke, E.; Delgado, M.d.M. Community-Level Phenological Response to Climate Change. Proc. Natl. Acad. Sci. USA 2013, 110, 13434–13439. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, S.J.; Sparks, T.H.; Frederiksen, M.; Burthe, S.; Bacon, P.J.; Bell, J.R.; Botham, M.S.; Brereton, T.M.; Bright, P.W.; Carvalho, L.; et al. Trophic Level Asynchrony in Rates of Phenological Change for Marine, Freshwater and Terrestrial Environments: Phenological Change across Major Environments. Glob. Change Biol. 2010, 16, 3304–3313. [Google Scholar] [CrossRef]
- Cohen, J.M.; Lajeunesse, M.J.; Rohr, J.R. A Global Synthesis of Animal Phenological Responses to Climate Change. Nat. Clim. Change 2018, 8, 224–228. [Google Scholar] [CrossRef]
- Kudo, G.; Ida, T.Y. Early Onset of Spring Increases the Phenological Mismatch between Plants and Pollinators. Ecology 2013, 94, 2311–2320. [Google Scholar] [CrossRef]
- Inouye, D.W.; Barr, B.; Armitage, K.B.; Inouye, B.D. Climate Change Is Affecting Altitudinal Migrants and Hibernating Species. Proc. Natl. Acad. Sci. USA 2000, 97, 1630–1633. [Google Scholar] [CrossRef]
- Pincebourde, S.; Dillon, M.E.; Woods, H.A. Body Size Determines the Thermal Coupling between Insects and Plant Surfaces. Funct. Ecol. 2021, 35, 1424–1436. [Google Scholar] [CrossRef]
- Potter, K.A.; Woods, H.A.; Pincebourde, S. Microclimatic Challenges in Global Change Biology. Glob. Change Biol. 2013, 19, 2932–2939. [Google Scholar] [CrossRef] [PubMed]
- Woods, H.A.; Pincebourde, S.; Dillon, M.E.; Terblanche, J.S. Extended Phenotypes: Buffers or Amplifiers of Climate Change? Trends Ecol. Evol. 2021, 36, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Poethke, H.J.; Hovestadt, T.; Mitesser, O. The Evolution of Optimal Emergence Times: Bet Hedging and the Quest for an Ideal Free Temporal Distribution of Individuals. Oikos 2016, 125, 1647–1656. [Google Scholar] [CrossRef]
- Jackson, M.T. Effects of Microclimate on Spring Flowering Phenology. Ecology 1966, 47, 407–415. [Google Scholar] [CrossRef]
- Vermunt, B.; Cuddington, K.; Sobek-Swant, S.; Crosthwaite, J.C.; Barry Lyons, D.; Sinclair, B.J. Temperatures Experienced by Wood-Boring Beetles in the under-Bark Microclimate. For. Ecol. Manag. 2012, 269, 149–157. [Google Scholar] [CrossRef]
- Graves, B.M.; Duvall, D. Spring Emergence Patterns of Wandering Garter Snakes and Prairie Rattlesnakes in Wyoming. J. Herpetol. 1990, 24, 351–356. [Google Scholar] [CrossRef]
- Norquay, K.J.O.; Willis, C.K.R. Hibernation Phenology of Myotis Lucifugus. J. Zool. 2014, 294, 85–92. [Google Scholar] [CrossRef]
- Michener, G.R. Effect of Age and Parity on Weight Gain and Entry into Hibernation in Richardson’s Ground Squirrels. Can. J. Zool. 1978, 56, 2573–2577. [Google Scholar] [CrossRef]
- Armitage, K.B.; Downhower, J.F.; Svendsen, G.E. Seasonal Changes in Weights of Marmots. Am. Midl. Nat. 1976, 96, 36–51. [Google Scholar] [CrossRef]
- Holm, S.N. Weight and Life Length of Hibernating Bumble Bee Queens (Hymenoptera: Bombidae) under Controlled Conditions. Insect Syst. Evol. 1972, 3, 313–320. [Google Scholar] [CrossRef]
- Willis, Y.L.; Moyle, D.L.; Baskett, T.S. Emergence, Breeding, Hibernation, Movements and Transformation of the Bullfrog, Rana Catesbeiana, in Missouri. Copeia 1956, 1956, 30–41. [Google Scholar] [CrossRef]
- Ohlberger, J. Climate Warming and Ectotherm Body Size—From Individual Physiology to Community Ecology. Funct. Ecol. 2013, 27, 991–1001. [Google Scholar] [CrossRef]
- Chmura, H.E.; Kharouba, H.M.; Ashander, J.; Ehlman, S.M.; Rivest, E.B.; Yang, L.H. The Mechanisms of Phenology: The Patterns and Processes of Phenological Shifts. Ecol. Monogr. 2019, 89, e01337. [Google Scholar] [CrossRef]
- Ryan, R.A. Growth Rates of Some Ranids under Natural Conditions. Copeia 1953, 1953, 73–80. [Google Scholar] [CrossRef]
- Wonglersak, R.; Fenberg, P.B.; Langdon, P.G.; Brooks, S.J.; Price, B.W. Insect Body Size Changes under Future Warming Projections: A Case Study of Chironomidae (Insecta: Diptera). Hydrobiologia 2021, 848, 2785–2796. [Google Scholar] [CrossRef]
- Wonglersak, R.; Fenberg, P.B.; Langdon, P.G.; Brooks, S.J.; Price, B.W. Temperature-Body Size Responses in Insects: A Case Study of British Odonata. Ecol. Entomol. 2020, 45, 795–805. [Google Scholar] [CrossRef]
- Buck, C.L.; Barnes, B.M. Annual Cycle of Body Composition and Hibernation in Free-Living Arctic Ground Squirrels. J. Mammal. 1999, 80, 430–442. [Google Scholar] [CrossRef]
- Bosch, J.; Kemp, W.P. Effect of Pre-Wintering and Wintering Temperature Regimes on Weight Loss, Survival, and Emergence Time in the Mason Bee Osmia Cornuta (Hymenoptera: Megachilidae). Apidologie 2004, 35, 469–479. [Google Scholar] [CrossRef]
- Bols, J.H. Observations on Bombus and Psithyrus, Especially on Their Hibernation. Proc. R. Soc. A. 1937, 12, 47–50. [Google Scholar] [CrossRef]
- Frison, T.H. Contribution to the Knowledge of the Interrelations of the Bumblebees of Illinois with Their Animate Environment. Ann. Entomol. Soc. Am. 1926, 19, 203–235. [Google Scholar] [CrossRef][Green Version]
- Cumber, R.A. The Biology of Humble-Bees, with Special Reference to the Production of the Worker Caste. Trans. R. Entomol. Soc. Lond. 1949, 100, 1–45. [Google Scholar] [CrossRef]
- Bartomeus, I.; Ascher, J.S.; Wagner, D.; Danforth, B.N.; Colla, S.; Kornbluth, S.; Winfree, R. Climate-Associated Phenological Advances in Bee Pollinators and Bee-Pollinated Plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20645–20649. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.V. Experiments on the Domestication of Bumblebees (Bombus Latr.) in Particular B. lapidarius L. and B. terrestris L.; FAO: Québec, Canada, 1960; pp. 1–19. [Google Scholar]
- Lanterman, J.; Reeher, P.; Mitchell, R.J.; Goodell, K. Habitat Preference and Phenology of Nest Seeking and Foraging Spring Bumble Bee Queens in Northeastern North America (Hymenoptera: Apidae: Bombus). Am. Midl. Nat. 2019, 182, 131–159. [Google Scholar] [CrossRef]
- Skou, P.; Holm, S.N.; Haas, H. Preliminary Investigations on Diseases in Bumble-Bees (Bombus Latr.). In Yearbook 1963; Royal Veterinary and Agricultural College: Frederiksberg, Denmark, 1963; pp. 27–41. [Google Scholar]
- Holm, S. Problems of the Domestication of Bumble Bees. Bee World 1966, 47, 179–186. [Google Scholar]
- Heinrich, B. Bumblebee Economics; Harvard University Press: Cambridge, MA, USA, 2004; ISBN 978-0-674-01639-2. [Google Scholar]
- Wignall, V.R.; Brolly, M.; Uthoff, C.; Norton, K.E.; Chipperfield, H.M.; Balfour, N.J.; Ratnieks, F.L.W. Exploitative Competition and Displacement Mediated by Eusocial Bees: Experimental Evidence in a Wild Pollinator Community. Behav. Ecol. Sociobiol. 2020, 74, 152. [Google Scholar] [CrossRef]
- DeGregorio, B.A.; Tuberville, T.D.; Kennamer, R.A.; Harris, B.B.; Brisbin, I.L. Spring Emergence of Eastern Box Turtles (Terrapene Carolina): Influences of Individual Variation and Scale of Temperature Correlates. Can. J. Zool. 2017, 95, 23–30. [Google Scholar] [CrossRef]
- Fründ, J.; Zieger, S.L.; Tscharntke, T. Response Diversity of Wild Bees to Overwintering Temperatures. Oecologia 2013, 173, 1639–1648. [Google Scholar] [CrossRef]
- Schenk, M.; Mitesser, O.; Hovestadt, T.; Holzschuh, A. Overwintering Temperature and Body Condition Shift Emergence Dates of Spring-Emerging Solitary Bees. PeerJ 2018, 6, e4721. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Else, P.L. Mechanisms Underlying the Cost of Living in Animals. Annu. Rev. Physiol. 2000, 62, 207–235. [Google Scholar] [CrossRef]
- Kleiber, M. Body Size and Metabolic Rate. Physiol. Rev. 1947, 27, 511–541. [Google Scholar] [CrossRef]
- Kleiber, M. Body Size and Metabolism. J. Agric. Sci. 1932, 6, 313–353. [Google Scholar] [CrossRef]
- Cane, J.H. Estimation of Bee Size Using Intertegular Span (Apoidea). J. Kans. Entomol. Soc. 1987, 60, 145–147. [Google Scholar]
- Vogt, J.R.; Dillon, M.E. Allometric Scaling of Tracheal Morphology among Bumblebee Sisters (Apidae: Bombus): Compensation for Oxygen Limitation at Large Body Sizes? Physiol. Biochem. Zool. 2013, 86, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ 1997; National Institutes of Health: Bethesda, MD, USA, 1997.
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lozier, J.D.; Parsons, Z.M.; Rachoki, L.; Jackson, J.M.; Pimsler, M.L.; Oyen, K.J.; Strange, J.; Dillon, M.E. Divergence in Body Mass, Wing Loading, and Population Structure Reveals Species-Specific and Potentially Adaptive Trait Variation across Elevations in Montane Bumble Bees. Insect Conserv. Divers. 2021, 5, 3. [Google Scholar] [CrossRef]
- Pridal, A.; Hofbauer, J. Laboratory Rearing and Nutrition of Young Queens of Bumblebee (Bombus Terrestris L.) from Emergence to Diapause. Fruit Grow. Res. 1996, 14, 125–131. [Google Scholar]
- Treanore, E.D.; Kiner, J.M.; Kerner, M.E.; Amsalem, E. Shift in Worker Physiology and Gene Expression Pattern from Reproductive to Diapause-like with Colony Age in the Bumble Bee Bombus Impatiens. J. Exp. Biol. 2020, 223, jeb218768. [Google Scholar] [CrossRef]
- Woodard, S.H.; Duennes, M.A.; Watrous, K.M.; Jha, S. Diet and Nutritional Status during Early Adult Life Have Immediate and Persistent Effects on Queen Bumble Bees. Conserv. Physiol. 2019, 7, coz048. [Google Scholar] [CrossRef]
- Legendre, P. Lmodel2: Model II Regression 2018, R Package Version 1.7-3. Available online: https://CRAN (accessed on 14 June 2022).
- R Core Team. R: A Language and Environment for Statistical Computing 2021; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Statistics for Biology and Health; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Beekman, M.; van Stratum, P.; Lingeman, R. Diapause Survival and Post-Diapause Performance in Bumblebee Queens (Bombus terrestris). Entomol. Exp. Appl. 1998, 89, 207–214. [Google Scholar] [CrossRef]
- Tian, L.; Hines, H.M. Morphological Characterization and Staging of Bumble Bee Pupae. PeerJ 2018, 6, e6089. [Google Scholar] [CrossRef] [PubMed]
- Rytteri, S.; Kuussaari, M.; Saastamoinen, M. Microclimatic Variability Buffers Butterfly Populations against Increased Mortality Caused by Phenological Asynchrony between Larvae and Their Host Plants. Oikos 2021, 130, 753–765. [Google Scholar] [CrossRef]
- Liczner, A.R.; Colla, S.R. A Systematic Review of the Nesting and Overwintering Habitat of Bumble Bees Globally. J. Insect Conserv. 2019, 23, 787–801. [Google Scholar] [CrossRef]
- Huey, R.B.; Ma, L.; Levy, O.; Kearney, M.R. Three Questions about the Eco-Physiology of Overwintering Underground. Ecol. Lett. 2021, 24, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Pisanty, G.; Mandelik, Y. Profiling Crop Pollinators: Life History Traits Predict Habitat Use and Crop Visitation by Mediterranean Wild Bees. Ecol. Appl. 2015, 25, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Bommarco, R.; Biesmeijer, J.C.; Meyer, B.; Potts, S.G.; Pöyry, J.; Roberts, S.P.M.; Steffan-Dewenter, I.; Öckinger, E. Dispersal Capacity and Diet Breadth Modify the Response of Wild Bees to Habitat Loss. Proc. R. Soc. B 2010, 277, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Geslin, B.; Oddie, M.; Folschweiller, M.; Legras, G.; Seymour, C.L.; van Veen, F.J.F.; Thébault, E. Spatiotemporal Changes in Flying Insect Abundance and Their Functional Diversity as a Function of Distance to Natural Habitats in a Mass Flowering Crop. Agric. Ecosyst. Environ. 2016, 229, 21–29. [Google Scholar] [CrossRef]
- Nooten, S.S.; Rehan, S.M. Historical Changes in Bumble Bee Body Size and Range Shift of Declining Species. Biodivers. Conserv. 2020, 29, 451–467. [Google Scholar] [CrossRef]
- Podgaiski, L.R.; Cavalleri, A.; Ferrando, C.P.R.; Pillar, V.D.; Mendonça, M., Jr. de S. Prescribed Patch Burnings Increase Thrips Species Richness and Body Size in Grassland Communities. Insect Conserv. Divers. 2018, 11, 204–212. [Google Scholar] [CrossRef]
- Denlinger, D.L.; Hahn, D.A.; Merlin, C.; Holzapfel, C.M.; Bradshaw, W.E. Keeping Time without a Spine: What Can the Insect Clock Teach Us about Seasonal Adaptation? Philos. Trans. R. Soc. 2017, 372, 20160257. [Google Scholar] [CrossRef] [PubMed]
- Alford, D.V. Studies on the Fat-Body of Adult Bumble Bees. J. Apic. Res. 1969, 8, 37–48. [Google Scholar] [CrossRef]

| Response Variable | Model | AIC | ΔAIC | R2 | Deviance Explained | edf | df | n |
|---|---|---|---|---|---|---|---|---|
| mass | ~s (day of year, k = 3) + year + s (day of year) × year | −889.406 | 0 | 0.397 | 40.70% | 2020:1.821 2021:1.950 | 6.770464 | 295 |
| mass | ~s (day of year, k = 3) | −888.142 | 1.264 | 0.389 | 39.30% | 1.958 | 3.958283 | 295 |
| mass | ~s (dayofyear, k = 3) + year | −887.086 | 2.32 | 0.389 | 39.50% | 1.958 | 4.958068 | 295 |
| ITW | ~s (day of year, k = 3) | 116.9413 | 0 | 0.0794 | 8.76% | 1.448 | 3.447957 | 165 |
| condition | ~s (day of year, k = 3) | −1195.27 | 0 | 0.456 | 46.30% | 1.958 | 3.957785 | 165 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keaveny, E.C.; Dillon, M.E. Phat Queens Emerge Fashionably Late: Body Size and Condition Predict Timing of Spring Emergence for Queen Bumble Bees. Insects 2022, 13, 870. https://doi.org/10.3390/insects13100870
Keaveny EC, Dillon ME. Phat Queens Emerge Fashionably Late: Body Size and Condition Predict Timing of Spring Emergence for Queen Bumble Bees. Insects. 2022; 13(10):870. https://doi.org/10.3390/insects13100870
Chicago/Turabian StyleKeaveny, Ellen C., and Michael E. Dillon. 2022. "Phat Queens Emerge Fashionably Late: Body Size and Condition Predict Timing of Spring Emergence for Queen Bumble Bees" Insects 13, no. 10: 870. https://doi.org/10.3390/insects13100870
APA StyleKeaveny, E. C., & Dillon, M. E. (2022). Phat Queens Emerge Fashionably Late: Body Size and Condition Predict Timing of Spring Emergence for Queen Bumble Bees. Insects, 13(10), 870. https://doi.org/10.3390/insects13100870









