Not by the Book: Observations of Delayed Oviposition and Re-Colonization of Human Remains by Blow Flies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Arthropod Observations and Collections
2.3. Hypothesis Testing
2.4. Entomological TOC Estimations
2.5. Anthropological TBS Estimations
3. Results
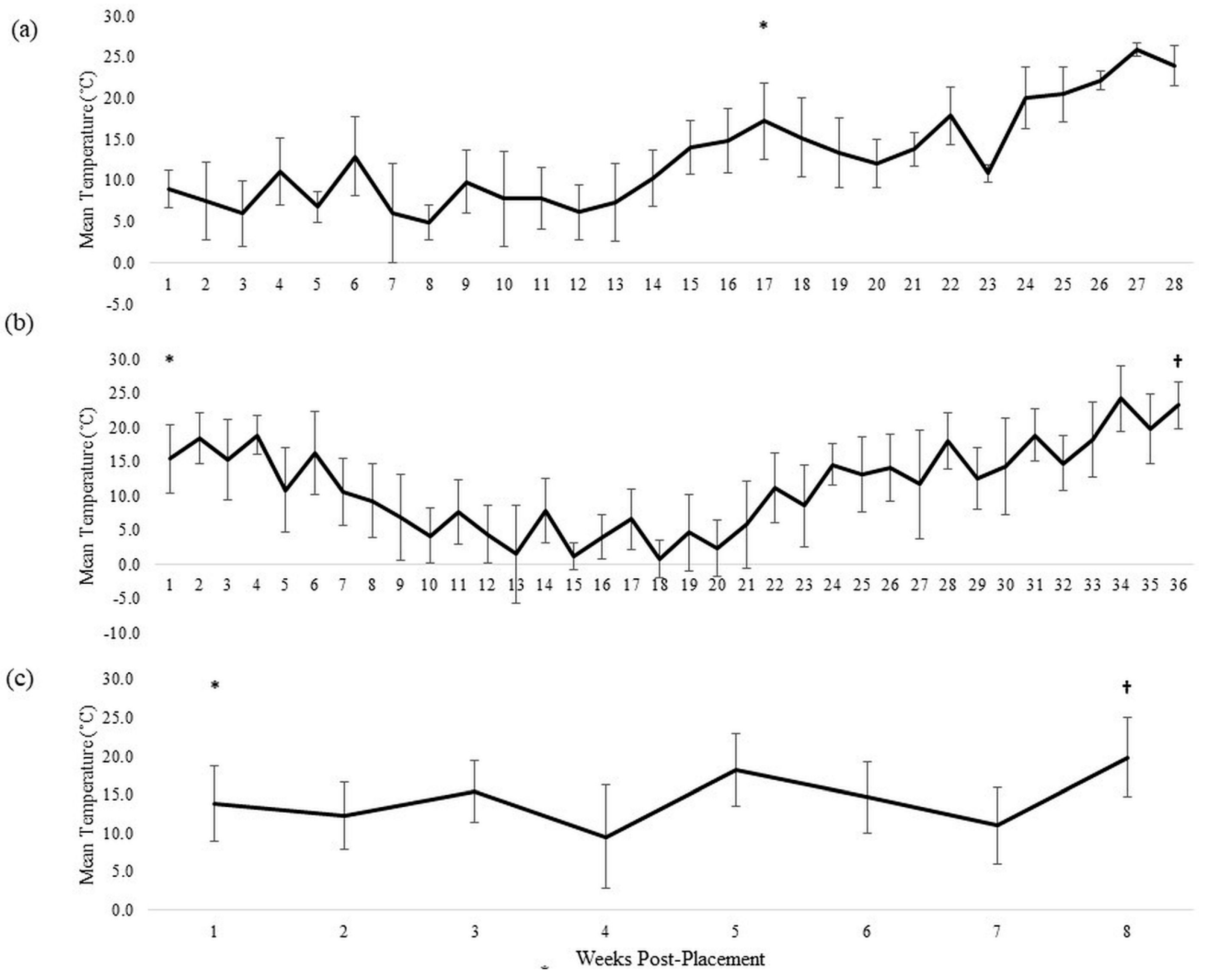
3.1. Donor 1
3.2. Donor 2
3.3. Donor 3
3.4. Accuracy of Estimation Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T. Post-mortem interval estimation: An overview of techniques. In Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment: Forensic Analysis of the Dead and the Depositional Environment; Wiley: Hoboken, NJ, USA, 2017; pp. 134–142. [Google Scholar]
- Metcalf, J.L. Estimating the postmortem interval using microbes: Knowledge gaps and a path to technology adoption. Forensic Sci. Int. Genet. 2019, 38, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.; Castner, J. Forensic Entomology: The Utility of Arthropods in Legal Investigations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B. Flies as forensic indicators. J. Med. Entomol. 1991, 28, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Villet, M.H.; Amendt, J. Advances in entomological methods for death time estimation. In Forensic Pathology Reviews; Springer: Berlin/Heidelberg, Germany, 2011; pp. 213–237. [Google Scholar]
- Tarone, A.M.; Sanford, M.R. Is PMI the hypothesis or the null hypothesis? J. Med. Entomol. 2017, 54, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- VanLaerhoven, S.L.; Anderson, G.S. Insect succession on buried carrion in two biogeoclimatic zones of British Columbia. J. Forensic Sci. 1999, 44, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.H.; Allen, J.C. The development of the black blow fly, Phormia regina (Meigen). Forensic Sci. Int. 2001, 120, 79–88. [Google Scholar] [CrossRef]
- Charabidze, D.; Hedouin, V. Temperature: The weak point of forensic entomology. Int. J. Leg. Med. 2019, 133, 633–639. [Google Scholar] [CrossRef]
- Higley, L.G.; Haskell, N.H. Insect development and forensic entomology. In Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: Boca Raton, FL, USA, 2001; pp. 287–302. [Google Scholar]
- Perez, A.E.; Haskell, N.H.; Wells, J.D. Evaluating the utility of hexapod species for calculating a confidence interval about a succession based postmortem interval estimate. Forensic Sci. Int. 2014, 241, 91–95. [Google Scholar] [CrossRef]
- LaMotte, L.R.; Wells, J.D. P-values for postmortem intervals from arthropod succession data. J. Agric. Biol. Environ. Stat. 2000, 5, 58–68. [Google Scholar] [CrossRef]
- Wells, J.D. A forensic entomological analysis can yield an estimate of postmortem interval, and not just a minimum postmortem interval: An explanation and illustration using a case. J. Forensic Sci. 2019, 64, 634–637. [Google Scholar] [CrossRef]
- Tomberlin, J.; Byrd, J.; Wallace, J.; Benbow, M. Assessment of decomposition studies indicates need for standardized and repeatable research methods in forensic entomology. J. Forensic Res. 2012, 3, 147. [Google Scholar] [CrossRef]
- Kotzé, Z.; Aimar, S.; Amendt, J.; Anderson, G.S.; Bourguignon, L.; Hall, M.J.; Tomberlin, J.K. The forensic entomology case report—A global perspective. Insects 2021, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Benbow, M.E.; Tarone, A.M.; Mohr, R.M. Basic research in evolution and ecology enhances forensics. Trends Ecol. Evol. 2011, 26, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Cuttiford, L.; Pimsler, M.L.; Heo, C.C.; Zheng, L.; Karunaratne, I.; Trissini, G.; Tarone, A.M.; Lambiase, S.; Cammack, J.A.; Tomberlin, J.K. Evaluation of development datasets for Hermetia illucens (L.) (Diptera: Stratiomyidae) for estimating the time of placement of human and swine remains in Texas, USA. J. Med. Entomol. 2021, 58, 1654–1662. [Google Scholar] [CrossRef]
- Amendt, J.; Krettek, R.; Zehner, R. Forensic entomology. Naturwissenschaften 2004, 91, 51–65. [Google Scholar] [CrossRef]
- Dourel, L.; Pasquerault, T.; Gaudry, E.; Vincent, B. Using estimated on-site ambient temperature has uncertain benefit when estimating postmortem interval. Psyche 2010, 2010, 610639. [Google Scholar] [CrossRef]
- Dabbs, G.R. Caution! All data are not created equal: The hazards of using National Weather Service data for calculating accumulated degree days. Forensic Sci. Int. 2010, 202, e49–e52. [Google Scholar] [CrossRef]
- Charabidze, D.; Bourel, B.; Gosset, D. Larval-mass effect: Characterisation of heat emission by necrophageous blowflies (Diptera: Calliphoridae) larval aggregates. Forensic Sci. Int. 2011, 211, 61–66. [Google Scholar] [CrossRef]
- Aubernon, C.; Hedouin, V.; Charabidze, D. The maggot, the ethologist and the forensic entomologist: Sociality and thermoregulation in necrophagous larvae. J. Adv. Res. 2019, 16, 67–73. [Google Scholar] [CrossRef]
- Chappell, T.M.; Rusch, T.W.; Tarone, A.M. A fly in the ointment: How to predict environmentally driven phenology of an organism that partially regulates its microclimate. Front. Ecol. Evol. 2022, 1–16. [Google Scholar] [CrossRef]
- Rusch, T.W.; Adutwumwaah, A.; Beebe, L.E.; Tomberlin, J.K.; Tarone, A.M. The upper thermal tolerance of the secondary screwworm, Cochliomyia macellaria Fabricius (Diptera: Calliphoridae). J. Therm. Biol. 2019, 85, 102405. [Google Scholar] [CrossRef] [PubMed]
- Rusch, T.W.; Faris, A.M.; Beebe, L.E.; Tomberlin, J.K.; Tarone, A.M. The upper thermal tolerance for a Texas population of the hairy maggot blow fly Chrysomya rufifacies Macquart (Diptera: Calliphoridae). Ecol. Entomol. 2020, 45, 1146–1157. [Google Scholar] [CrossRef]
- Ames, C.; Turner, B. Low temperature episodes in development of blowflies: Implications for postmortem interval estimation. Med. Vet. Entomol. 2003, 17, 178–186. [Google Scholar] [CrossRef]
- Flores, M.; Longnecker, M.; Tomberlin, J.K. Effects of temperature and tissue type on Chrysomya rufifacies (Diptera: Calliphoridae) (Macquart) development. Forensic Sci. Int. 2014, 245, 24–29. [Google Scholar] [CrossRef]
- Brundage, A.; Benbow, M.E.; Tomberlin, J.K. Priority effects on the life-history traits of two carrion blow fly (Diptera, Calliphoridae) species. Ecol. Entomol. 2014, 39, 539–547. [Google Scholar] [CrossRef]
- Shiao, S.-F.; Yeh, T.-C. Larval competition of Chrysomya megacephala and Chrysomya rufifacies (Diptera: Calliphoridae): Behavior and ecological studies of two blow fly species of forensic significance. J. Med. Entomol. 2008, 45, 785–799. [Google Scholar] [CrossRef]
- Goodbrod, J.R.; Goff, M.L. Effects of larval population density on rates of development and interactions between two species of Chrysomya (Diptera: Calliphoridae) in laboratory culture. J. Med. Entomol. 1990, 27, 338–343. [Google Scholar] [CrossRef]
- Andere, A.A.; Platt, R.N.; Ray, D.A.; Picard, C.J. Genome sequence of Phormia regina Meigen (Diptera: Calliphoridae): Implications for medical, veterinary and forensic research. BMC Genom. 2016, 17, 842. [Google Scholar] [CrossRef]
- Picard, C.J.; Deblois, K.; Tovar, F.; Bradley, J.L.; Johnston, J.S.; Tarone, A.M. Increasing precision in development-based postmortem interval estimates: What’s sex got to do with it? J. Med. Entomol. 2013, 50, 425–431. [Google Scholar] [CrossRef]
- Gallagher, M.B.; Sandhu, S.; Kimsey, R. Variation in developmental time for geographically distinct populations of the common green bottle fly, Lucilia sericata (Meigen). J. Forensic Sci. 2010, 55, 438–442. [Google Scholar] [CrossRef] [PubMed]
- VanLaerhoven, S. Blind validation of postmortem interval estimates using developmental rates of blow flies. Forensic Sci. Int. 2008, 180, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, E.; Dourel, L. Forensic entomology: Implementing quality assurance for expertise work. Int. J. Legal Med. 2013, 127, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.D.; Greenberg, B. Resource use by an introduced and native carrion flies. Oecologia 1994, 99, 181–187. [Google Scholar] [CrossRef]
- Payne, J.A. A summer carrion study of the baby pig Sus scrofa Linnaeus. Ecology 1965, 46, 592–602. [Google Scholar] [CrossRef]
- Watson, E.; Carlton, C. Spring succession of necrophilous insects on wildlife carcasses in Louisiana. J. Med. Entomol. 2003, 40, 338–347. [Google Scholar] [CrossRef]
- Sharanowski, B.J.; Walker, E.G.; Anderson, G.S. Insect succession and decomposition patterns on shaded and sunlit carrion in Saskatchewan in three different seasons. Forensic Sci. Int. 2008, 179, 219–240. [Google Scholar] [CrossRef]
- Watson, E.J.; Carlton, C.E. Insect succession and decomposition of wildlife carcasses during fall and winter in Louisiana. J. Med. Entomol. 2005, 42, 193–203. [Google Scholar] [CrossRef]
- Flores, M.; Crippen, T.L.; Longnecker, M.; Tomberlin, J.K. Nonconsumptive effects of predatory Chrysomya rufifacies (Diptera: Calliphoridae) larval cues on larval Cochliomyia macellaria (Diptera: Calliphoridae) growth and development. J. Med. Entomol. 2017, 54, 1167–1174. [Google Scholar] [CrossRef]
- Wells, J.D.; Greenberg, B. Interaction between Chrysomya rufifacies and Cochliomyia macellaria (Diptera: Calliphoridae): The possible consequences of an invasion. Bull. Entomol. Res. 1992, 82, 133–137. [Google Scholar] [CrossRef]
- Bauer, A.; Bauer, A.M.; Tomberlin, J.K. Impact of diet moisture on the development of the forensically important blow fly Cochliomyia macellaria (Fabricius) (Diptera: Calliphoridae). Forensic Sci. Int. 2020, 312, 110333. [Google Scholar] [CrossRef] [PubMed]
- Steadman, D.W.; DeBruyn, J.; Campagna, S.; Owings, C.G. The impact of drugs on human decomposition and the postmortem interval. In Proceedings of the American Academy of Forensic Sciences Annual Meeting, Virtual, 15–19 February 2021. [Google Scholar]
- Greenberg, B.; Tantawi, T.I. Different developmental strategies in two boreal blow flies (Diptera: Calliphoridae). J. Med. Entomol. 1993, 30, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, I.; Wall, R. Population dynamics of the sheep blowfly Lucilia sericata: Seasonal patterns and implications for control. J. Appl. Ecol. 2002, 39, 493–501. [Google Scholar] [CrossRef]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M. Best practice in forensic entomology—Standards and guidelines. Int. J. Legal Med. 2006, 121, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Wescott, D.J. Recent advances in forensic anthropology: Decomposition research. Forensic Sci. Res. 2018, 3, 278–293. [Google Scholar] [CrossRef]
- Wescott, D.J.; Steadman, D.W.; Miller, N.; Sauerwein, K.; Clemmons, C.M.; Gleiber, D.S.; McDaneld, C.; Meckel, L.; Bytheway, J.A. Validation of the total body score/accumulated degree-day model at three human decomposition facilities. Forensic Anthropol. 2018, 1, 143–149. [Google Scholar] [CrossRef]
- Megyesi, M.S.; Nawrocki, S.P.; Haskell, N.H. Using accumulated degree-days to estimate the postmortem interval from decomposed human remains. J. Forensic Sci. 2005, 50, 1–9. [Google Scholar] [CrossRef]
- Dabbs, G.R.; Connor, M.; Bytheway, J.A. Interobserver reliability of the total body score system for quantifying human decomposition. J. Forensic Sci. 2016, 61, 445–451. [Google Scholar] [CrossRef]
- Connor, M.; Baigent, C.; Hansen, E.S. Measuring desiccation using qualitative changes: A step toward determining regional decomposition sequences. J. Forensic Sci. 2019, 64, 1004–1011. [Google Scholar] [CrossRef]
- Suckling, J.K.; Spradley, M.K.; Godde, K. A longitudinal study on human outdoor decomposition in Central Texas. J. Forensic Sci. 2016, 61, 19–25. [Google Scholar] [CrossRef]
- Ceciliason, A.-S.; Andersson, M.G.; Lindström, A.; Sandler, H. Quantifying human decomposition in an indoor setting and implications for postmortem interval estimation. Forensic Sci. Int. 2018, 283, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Vidoli, G.M.; Steadman, D.W.; Devlin, J.B.; Jantz, L.M. History and development of the first anthropology research facility, Knoxville, Tennessee. In Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment; Schotsmans, E.M.J., Marquez-Grant, N., Forbes, S.L., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 461–475. [Google Scholar]
- Centers for Disease Control and Prevention. Adult BMI Calculator. Available online: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/english_bmi_calculator/bmi_calculator.html (accessed on 1 October 2021).
- Tomberlin, J.; Mohr, R.; Benbow, M.; Tarone, A.; Vanlaerhoven, S. A roadmap for bridging basic and applied research in forensic entomology. Annu. Rev. Entomol. 2011, 56, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.D.; Byrd, J.H.; Tantawi, T.I. Key to third-instar Chrysomyinae (Diptera: Calliphoridae) from carrion in the continental United States. J. Med. Entomol. 1999, 36, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Greenberg, B. Immature stages of some flies of forensic importance. Ann. Entomol. Soc. Am. 1989, 82, 80–93. [Google Scholar] [CrossRef]
- Jones, N.; Whitworth, T.; Marshall, S. Blow flies of North America: Keys to the subfamilies and genera of Calliphoridae, and to the species of the subfamilies Calliphorinae, Luciliinae and Chrysomyinae. Can. J. Arthropod Identif. 2019, 39, 1–191. [Google Scholar]
- NOAA. Climate at a Glance: U.S. Time Series. Available online: http://www.ncdc.noaa.gov/cag/ (accessed on 1 October 2021).
- Faris, A.M.; West, W.R.; Tomberlin, J.K.; Tarone, A.M. Field validation of a development data set for Cochliomyia macellaria (Diptera: Calliphoridae): Estimating insect age based on development stage. J. Med. Entomol. 2020, 57, 39–49. [Google Scholar] [CrossRef]
- Reed, H. A study of dog carcass communities in Tennessee, with special reference to the insects. Am. Midl. Nat. 1958, 59, 213–245. [Google Scholar] [CrossRef]
- Rodriguez, W.C.; Bass, W.M. Insect activity and its relationship to decay rates of human cadavers in East Tennessee. J. Forensic Sci. 1983, 28, 423–432. [Google Scholar] [CrossRef]
- Mądra, A.; Frątczak, K.; Grzywacz, A.; Matuszewski, S. Long-term study of pig carrion entomofauna. Forensic Sci. Int. 2015, 252, 1–10. [Google Scholar] [CrossRef]
- Anderson, G.S.; VanLaerhoven, S.L. Initial studies on insect succession on carrion in southwestern British Columbia. J. Forensic Sci. 1996, 41, 617–625. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Elliot, D.A. A preliminary investigation of insect colonisation and succession on remains in New Zealand. Forensic Sci. Int. 2008, 176, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Benbow, M.E. When entomological evidence crawls away: Phormia regina en masse larval dispersal. J. Med. Entomol. 2011, 48, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, A.; Hamilton, M.D.; Weaver, R. Moisture content in decomposing, desiccated, and mummified human tissue. Forensic Anthropol. 2020, 3, 1–16. [Google Scholar] [CrossRef]
- Sutherland, A.; Myburgh, J.; Steyn, M.; Becker, P.J. The effect of body size on the rate of decomposition in a temperate region of South Africa. Forensic Sci. Int. 2013, 231, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, A.; Rando, C.; Morgan, R.M.; Carter, D.O. The suitability of visual taphonomic methods for digital photographs: An experimental approach with pig carcasses in a tropical climate. Sci. Justice 2018, 58, 167–176. [Google Scholar] [CrossRef]
- Cockle, D.L.; Bell, L.S. The environmental variables that impact human decomposition in terrestrially exposed contexts within Canada. Sci. Justice 2017, 57, 107–117. [Google Scholar] [CrossRef]
- Steadman, D.W.; Dautartas, A.; Kenyhercz, M.W.; Jantz, L.M.; Mundorff, A.; Vidoli, G.M. Differential scavenging among pig, rabbit, and human subjects. J. Forensic Sci. 2018, 63, 1684–1691. [Google Scholar] [CrossRef]
- Schoenly, K.; Griest, K.; Rhine, S. An experimental field protocol for investigating the postmortem interval using multidisciplinary indicators. J. Forensic Sci. 1991, 36, 1395–1415. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Introna, F. The forensic entomologist in the context of the forensic pathologist’s role. Forensic Sci. Int. 2001, 120, 132–139. [Google Scholar] [CrossRef]
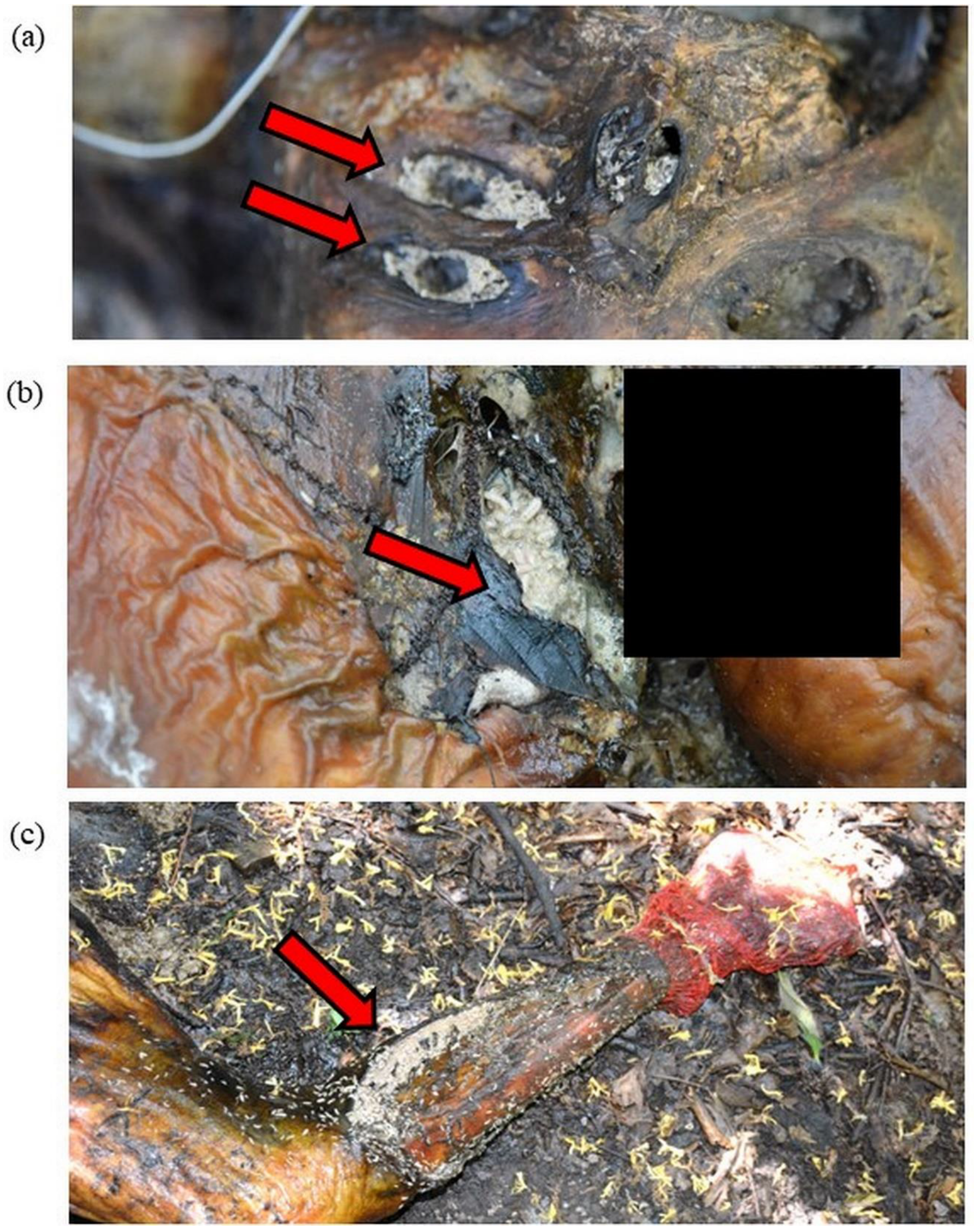
| Donor | Sex | SeasonP | SeasonC | Pre-CI (h) | Temp. (°C) |
|---|---|---|---|---|---|
| Donor 1 | F | Winter | Spring | 2760.0 | 9.1 ± 5.9 |
| Donor 2 | F | Fall | Fall | 47.8 | 13.3 ± 4.8 |
| Donor 3 | M | Spring | Spring | 95.4 | 13.6 ± 6.0 |
| Estimations (ADD) | ||||||
|---|---|---|---|---|---|---|
| Donor | Exposure Post-Placement (d) | Temp. (°C) | Site | TOP (ADD) | TOC | TBS |
| 1 | 122 | 9.4 ± 6.0 | Face | 266.3 | 29.6–145.0 | 0.0–728.6 * |
| 2 | 280 | 11.3 ± 8.0 | Groin | 1024.8 | 29.6–145.0 | 276.3–1052.7 * |
| 3 | 53 | 14.1 ± 5.9 | GBI, R. leg | 266.9 | 29.6–145.0 | 19.2–795.5 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owings, C.G.; McKee-Zech, H.S.; Schwing, S.T.; Bugajski, K.N.; Davis, M.C.; Steadman, D.W. Not by the Book: Observations of Delayed Oviposition and Re-Colonization of Human Remains by Blow Flies. Insects 2022, 13, 879. https://doi.org/10.3390/insects13100879
Owings CG, McKee-Zech HS, Schwing ST, Bugajski KN, Davis MC, Steadman DW. Not by the Book: Observations of Delayed Oviposition and Re-Colonization of Human Remains by Blow Flies. Insects. 2022; 13(10):879. https://doi.org/10.3390/insects13100879
Chicago/Turabian StyleOwings, Charity G., Hayden S. McKee-Zech, Sarah T. Schwing, Kristi N. Bugajski, Mary C. Davis, and Dawnie W. Steadman. 2022. "Not by the Book: Observations of Delayed Oviposition and Re-Colonization of Human Remains by Blow Flies" Insects 13, no. 10: 879. https://doi.org/10.3390/insects13100879





