Human Consumption of Insects in Sub-Saharan Africa: Lepidoptera and Potential Species for Breeding
Abstract
Simple Summary
Abstract
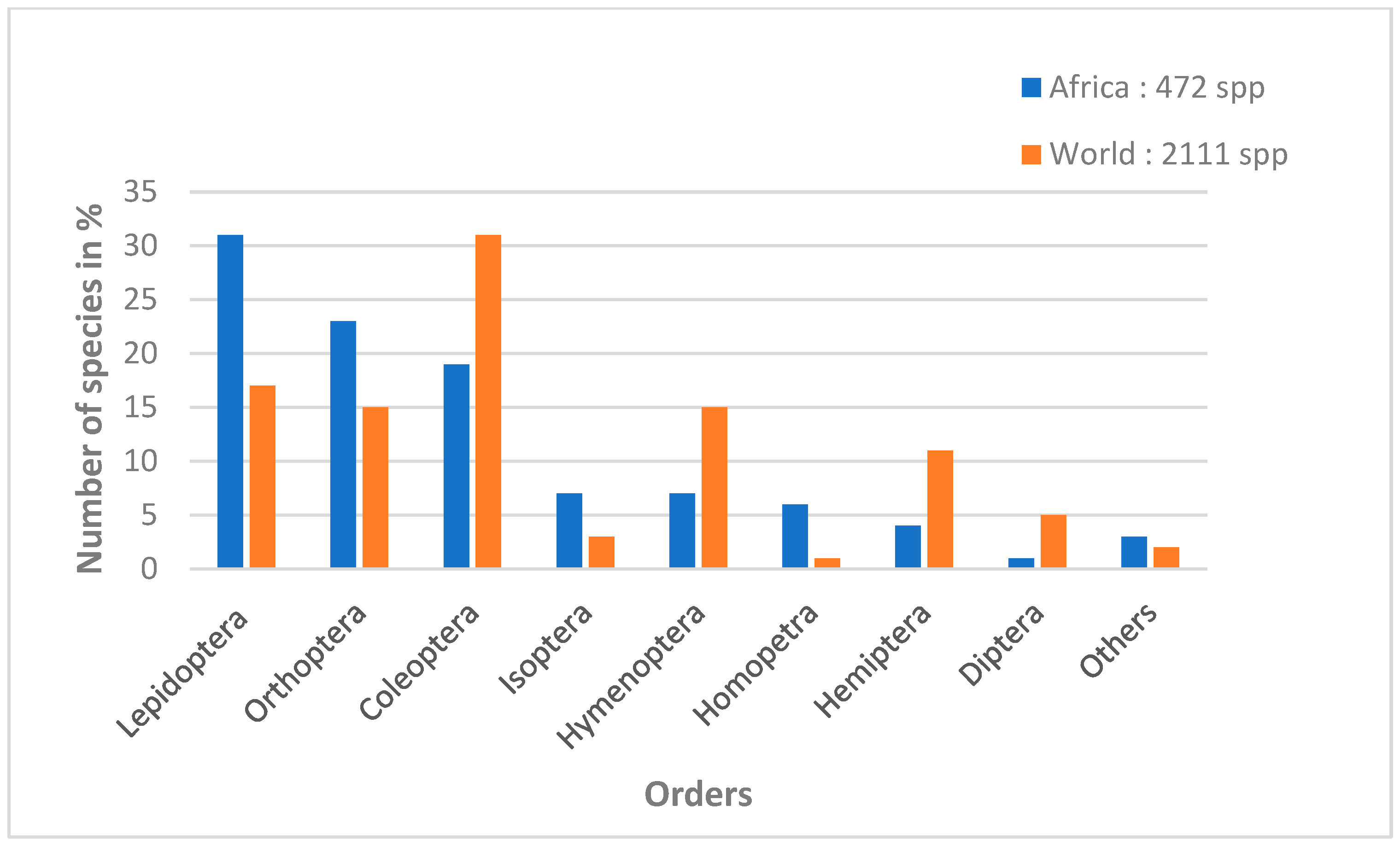
1. Introduction
2. Life Cycle of Lepidoptera
3. Nutritional Composition of Edible Caterpillars
| Family | Species | Protein (g/100 g) | Carbohydrates (g/100 g) | Fat (g/100 g) | Ash % | Energy (kcal/100 g) | Fiber % | Location |
|---|---|---|---|---|---|---|---|---|
| Notodontidae | Anaphe infracta | 20 | / | 15.2 | 1.6 | / | 2.4 | Guinea-Conakry, Cameroun, DRC, Equatorial Africa |
| Elaphrodes lactea | 53.6 | 1.4 | 21.9 | 3.9–4.2 | 417 | / | Zambia, DRC | |
| Saturnidae | Bunaea alcinoe | 74.3 | / | 14.1 | 2.9 | / | / | Mali, Burkina Faso, Nigeria, Ghana, Cameroon, South Africa, Zambia, DRC, Congo, RCA, Zimbabwe, Nigeria, Tanzania |
| Cirina butyrospermi | 63 | 13 | 14.5 | 5.1 | 432 | 5 | Togo, Mali, Burkina Faso, Nigeria, Ghana | |
| Cirina forda | 12–74.4 | 1.7–7 | 5.3–20.2 | 1.5–11.5 | 359–410 | 5.2–9.4 | Nigeria, DRC, CAR, Zambia, South Africa, Botswana, Burkina Faso, Mozambique, Namibia, Ghana, Togo, Tchad, Cameroon | |
| Imbrasia epimethea | 57.8–73 | 0.9–5.3 | 12.4–23 | 3.3–7.5 | 419–501 | / | Guinea-Conakry, DRC, Zambia, South Africa, Cameroon, Congo, CAR, Zimbabwe | |
| Imbrasi belina | 35.2–56.9 | 7.8–10.9 | 10–23.3 | 6.9–11.3 | 402 | 27.8 | DCR, Zambia, South Africa, Zimbabwe, Botswana, Malawi | |
| Imbrasia oyemensis | 23.7–61.6 | 1.5–11 | 19.1–57.7 | 2.6–5.5 | 384–477 | Guinea-Conakry, Cameroon, DRC | ||
| Imbrasia obscura | 62.3 | / | 12.2 | / | / | / | DRC, South Africa, Zimbabwe, Botswana, Gabon, Mozambique, Namibia | |
| Imbrasia truncata | 54.5–73 | 1.2–11 | 15.2–27.8 | 2.7–5.5 | 418–499 | / | DRC |
4. Availability, Host Plants, Harvesting, and Storage
Availability and Relationship of Lepidoptera with Host Plant(s)
5. Harvest
6. Conservation
7. Semi-Domestication and Farming
7.1. Semi-Domestication
7.2. Farming
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naumovski, N. Plenary lecture: Technology and food safety: Facts and myths of genetically modified food and organic food. In Proceedings of the Annual Scientific Sessions of the Nutrition Society of Sri Lanka, Colombo, Sri Lanka, 22–23 January 2022; pp. 40–42. [Google Scholar]
- Caparros Megido, R.; Alabi, T.; Larreché, S.; Alexandra, L.; Haubruge, E.; Francis, F. Risques et valorisation Des insectes dans l’alimentation humaine et animale. Ann. Société Entomol. De Fr. 2015, 51, 215–258. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellntienchess foods: A Review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Tiencheu, B.; Womeni, H.M. Entomophagy: Insects as food. In Insect Physiology and Ecology; InTech: London, UK, 2017; pp. 267–322. [Google Scholar]
- Kouame, K.; Koko, A.; Diomande, M.; Konate, I.; Assidjo, N. Caractérisation physicochimique de trois espèces de champignons sauvages comestibles couramment rencontrées dans la région du Haut-Sassandra (Côte d’Ivoire). J. Appl. Biosci. 2018, 121, 12110–12120. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Insectes Comestibles Perspectives Pour la Sécurité Alimentaire et L’alimentation Animale; FAO Forestry Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107595-1. [Google Scholar]
- van Huis, A. Insects as food in Sub-Saharan Africa. Insect Sci. Its Appl. 2003, 23, 163–185. [Google Scholar] [CrossRef]
- Gustafsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: “Global Food Losses and Food Waste—Extent, Causes and Prevention”; FAO: Rome, Italy, 2013; ISBN 978-91-7290-3234. [Google Scholar]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible Insects in a food safety and nutritional Perspective: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Lavalette, M. Les Insectes: Une Nouvelle Ressource en Protéines Pour L’alimentation Humaine. Ph.D. Thesis, Université de Lorraine, Nancy, France, 2013. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Enwemiwe, V.N.; Popoola, K.O.K. Edible Insects: Rearing methods and incorporation into commercial food products–A Critical Review. Int. J. Adv. Res. Publ. 2018, 2, 9. [Google Scholar]
- Baiano, A. Edible Insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Musundire, R.; Ngonyama, D.; Chemura, A.; Ngadze, R.T.; Jackson, J.; Matanda, M.J.; Tarakini, T.; Langton, M.; Chiwona-Karltun, L. Stewardship of wild and farmed edible insects as food and feed in Sub-Saharan Africa: A perspective. Front. Vet. Sci. 2021, 8, 601386. [Google Scholar] [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the development of edible insect-based foods in Europe. Foods 2021, 10, 766. [Google Scholar] [CrossRef]
- Van Huis, A.; Oonincx, D.G.A.B. The Environmental sustainability of insects as food and feed. A Review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Rothman, J.M. Nutritional ecology of entomophagy in humans and other primates. Annu. Rev. Entomol. 2013, 58, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef] [PubMed]
- Babarinde, S.A.; Mvumi, B.M.; Babarinde, G.O.; Manditsera, F.A.; Akande, T.O.; Adepoju, A.A. Insects in food and feed systems in Sub-Saharan Africa: The untapped potentials. Int. J. Trop. Insect Sci. 2021, 41, 1923–1951. [Google Scholar] [CrossRef]
- Balinga, M.P.; Mapunzu, P.M.; Moussa, J.B.; N’gasse, G. Contribution des Insectes de la Forêt à la Sécurité Alimentaire: L’exemple des Chenilles d’Afrique Centrale; FAO: Rome, Italy, 2004; pp. 66–81. [Google Scholar]
- Niassy, S.; Fiaboe, K.K.M.; Affognon, H.D.; Akutse, K.S.; Tanga, M.C.; Ekesi, S. African indigenous knowledge on edible insects to guide research and policy. J. Insects Food Feed 2016, 2, 161–170. [Google Scholar] [CrossRef]
- Vantomme, P.; Göhler, D.; Deckere-ziangba, F.N. Contribution of forest insects to food security and forest conservation: The example of caterpillars in central Africa. Wildl. Policy Brief. Number 2004, 3, 1–4. [Google Scholar]
- Gahukar, R.T. Edible Insects Collected from Forests for Family Livelihood and Wellness of Rural Communities: A Review. Glob. Food Secur. 2020, 25, 100348. [Google Scholar] [CrossRef]
- Raheem, D.; Carrascosa, C.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Millán, R.; Raposo, A. Traditional consumption of and rearing edible insects in Africa, Asia and Europe. Crit. Rev. Food Sci. Nutr. 2019, 59, 2169–2188. [Google Scholar] [CrossRef]
- Vantomme, P. Way forward to bring insects in the human food chain. J. Insects Food Feed 2015, 1, 121–129. [Google Scholar] [CrossRef]
- Spiegel, M.V.D.; Noordam, M.Y. Safety of novel protein sources (Insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Ngute, A.S.K.; Dongmo, M.A.K.; Effa, J.A.M.; Ambombo Onguene, E.M.; Fomekong Lontchi, J.; Cuni-Sanchez, A. Edible caterpillars in central Cameroon: Host plants, value, harvesting, and availability. For. Trees Livelihoods 2020, 29, 16–33. [Google Scholar] [CrossRef]
- Mariod, A.A. African Edible Insects as Alternative Source of Food, Oil, Protein and Bioactive Components; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-32952-5. [Google Scholar]
- Ramos-elorduy, J. Anthropo-Entomophagy: Cultures, evolution and sustainability. Entomol. Res. 2009, 39, 271–288. [Google Scholar] [CrossRef]
- Kusia, E.S.; Borgemeister, C.; Khamis, F.M.; Copeland, R.S.; Tanga, C.M.; Ombura, F.L.; Subramanian, S. Diversity, host plants and potential distribution of edible Saturniid caterpillars in Kenya. Insects 2021, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, F.; Latham, P. Human consumption of Lepidoptera in Africa: An updated chronological list of references (370 Quoted!) with their ethnozoological analysis. Geo-Eco-Trop 2014, 38, 339–372. [Google Scholar]
- Bara, G.T.; Sithole, R.; Macheka, L. The mopane worm (Gonimbrasia Belina Westwood): A review of its biology, ecology and utilisation in Zimbabwe. J. Insects Food Feed 2022, 1–14. [Google Scholar] [CrossRef]
- Ande, A.T.; Fasoranti, J.O. Some aspects of the biology, foraging and defensive behaviour of the emperor moth caterpillar, Cirina Forda (Westwood). Int. J. Trop. Insect Sci. 1998, 18, 177–181. [Google Scholar] [CrossRef]
- Wongsorn, D.; Sirimungkararat, S.; Saksirirat, W. Improvement of Eri silkworm (Samia Ricini, D.) tolerance to high temperature and low humidity conditions by discontinuous regime. Songklanakarin J. Sci. Technol. 2015, 37, 401–408. [Google Scholar]
- Sekonya, J.G.; McClure, N.J.; Wynberg, R.P. New pressures, old foodways: Governance and access to edible mopane caterpillars, Imbrasia (Gonimbrasia) Belina, in the context of commercialization and environmental change in South Africa. Int. J. Commons 2020, 14, 139–153. [Google Scholar] [CrossRef]
- Cloutier, J. Insectes Comestibles En Afrique: Introduction à la Collecte, au Mode de Preparation et à la Consommation des Insectes; Agromisa, CTA Editions: Wageningen, Belgium, 2015; ISBN 978-90-8573-147-4. [Google Scholar]
- Rémy, D.A.; Hervé, B.B.; Sylvain, O.N. Study of some biological parameters of Cirina Butyrospermi Vuillet (Lepidoptera, Attacidae), an edible insect and shea Caterpillar (Butyrospermum Paradoxum Gaertn. F.) in a Context of climate change in Burkina Faso. Adv. Entomol. 2017, 6, 1–8. [Google Scholar] [CrossRef][Green Version]
- Shuey, J.A.; Butte, J.V.C.; Ohio, O. Life history notes for the pallid emperor moth, Cirina Forda (Saturniidae) in Nigeria. J. Lejiidopterists Soc. 1997, 51, 269–273. [Google Scholar]
- Munyuli, T. Etude préliminaire orientée vers la production des chenilles consommables par l’élevage Des Papillons Anaphe Infracta (Thaumetopoeidae) à Lwiro, Sud-Kivu, République Démocratique Du Congo. Tropicultura 2000, 18, 208–211. [Google Scholar]
- Amadi, E.; Kiin Kabari, D. Nutritional composition and microbiology of some edible insects commonly eaten in Africa, hurdles and future prospects: A Critical Review. J. Food Microbiol. Saf. Hyg. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Mabossy-Mobouna, G.; Malaisse, F.; Richel, A.; Maesen, P.; Latham, P.; Roulon-Doko, P.; Madamo, F.; Lognay, G. Imbrasia Obscura, an edible caterpillar of tropical Africa: Chemical composition and Nutritional Value. Tropicultura 2018, 36, 798–811. [Google Scholar]
- Muya, G.M.N.; Kambashi, B.M.; Bindelle, J.; Frédéric, F.; Megido, R.C. Description of the development Cycle of Aegocera Rectilinea (Lepidoptera: Noctuidae), a caterpillar consumed in western Democratic Republic of Congo. J. Insects Food Feed 2021, 8, 439–446. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, F. Se Nourrir en forêt claire africaine. Approche écologique et nutritionnelle. In Nature Sciences Société; CTA Editions: Gembloux, Belgium, 1997; Volume 88, p. 1007. [Google Scholar]
- Womeni, H.M.; Tiencheu, B.; Mbiapo, F.T.; Linder, M.; Fanni, J.; Parmentier, M.; Villeneuve, P. Oils of insects and larvae consumed in Africa: Potential sources of polyunsaturated fatty acids. OCL Ol. Corps Gras Lipides 2009, 16, 230–235. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Y.; Chen, Z. Common edible insects and their utilization in China. Entomol. Res. 2009, 39, 299–303. [Google Scholar] [CrossRef]
- Bukkens, S.G.F. The Nutritional value of Edible Insects. Ecol. Food Nutr. 1997, 36, 287–319. [Google Scholar] [CrossRef]
- Oibiokpa, F.I.; Akanya, H.O.; Jigam, A.A.; Saidu, A.N.; Egwim, E.C. Protein quality of four indigenous edible insect species in Nigeria. Food Sci. Hum. Wellness 2018, 7, 175–183. [Google Scholar] [CrossRef]
- Moyo, S.; Masika, P.J.; Muchenje, V. The potential of Imbrasia belina worm as a poultry and fish feed. A Review. J. Anim. Feed Sci. 2019, 28, 209–219. [Google Scholar] [CrossRef]
- Akinnawo, O.; Ketiku, A.O. Chemical Composition and Fatty Acid Profile of Edible Larva of Cirina Forda (Westwood). Afr. J. Biomed. Res. 2000, 3, 93–96. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O.; Borkovcova, M.; Bednarova, M. A Comprehensive look at the possibilities of edible insects as food in Europe—A Review. Pol. J. Food Nutr. Sci. 2014, 64, 147–157. [Google Scholar] [CrossRef]
- Anvo, P.M.; Toguyéni, A.; Otchoumou, A.K.; Zoungrana-Kaboré, C.Y.; Koumelan, E.P. Nutritional qualities of edible caterpillars Cirina butyrospermi in southwestern of Burkina Faso. Int. J. Innov. Appl. Stud. 2016, 18, 639–645. [Google Scholar]
- Langrenez, L.; Messier, J. Insectes Comestibles; Privateley Published: Paris, France, 2017. [Google Scholar]
- Nantanga, K.K.M.; Amakali, T. Diversification of mopane caterpillars (Gonimbrasia Belina) Edible forms for improved livelihoods and food security. J. Arid Environ. 2020, 177, 104148. [Google Scholar] [CrossRef]
- Millward, D.J.; Layman, D.K.; Tomé, D.; Schaafsma, G. Protein quality assessment: Impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 2008, 87, 1576S–1581S. [Google Scholar] [CrossRef] [PubMed]
- Banjo, A.D.; Lawal, O.A.; Songonuga, E.A. The nutritional value of fourteen species of edible insects in Southwestern Nigeria. Afr. J. Biotechnol. 2006, 5, 298–301. [Google Scholar] [CrossRef]
- Okangola, E.; Solomo, E.; Tchatchambe, W.B.; Mate, M.; Upoki, A.; Dudu, A.; Asimonyio, J.A.; Bongo, G.N.; Pius, T. Valeurs nutritionnelles des chenilles comestibles de la ville de Kisangani et ses environs (Province de la Tshopo, République Démocratique Du Congo). Int. J. Inov. Sci. Res. 2016, 25, 10. [Google Scholar]
- Lautenschläger, T.; Neinhuis, C.; Kikongo, E.; Henle, T.; Förster, A. Impact of different Preparations on the Nutritional value of the edible caterpillar Imbrasia Epimethea from northern Angola. Eur. Food Res. Technol. 2017, 243, 1–10. [Google Scholar] [CrossRef]
- Kanga-Kanga, M.R.; Mulungu-Lungu, N.D.; Mpanda, G.N.; L’kisaten, H.M.; Kiyula, F.M.; Kalaka, C.; Kasumpa, D.B.; Tshovu, D.N.; Sumba, J.K. Valeur nutritionnelle des chenilles comestibles de la ville de Lubumbashi (Province du Haut-Katanga, R.D.C.). Int. J. Innov. Appl. Stud. 2018, 24, 6. [Google Scholar]
- Justin, B. Nutritional quality evaluation of complementary foods flour based on edible caterpillars: Bunaeopsis aurantiaca, Imbrasia oyemensis and Cirina forda eaten in south Kivu province, eastern D.R. Ccongo. Anal. Food Sci. Technol. 2019, 20, 18. [Google Scholar]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible insects as a protein source: A review of public perception, processing technology, and research trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Hlongwane, Z.T.; Slotow, R.; Munyai, T.C. Nutritional Composition of Edible Insects Consumed in Africa: A Systematic Review. Nutrients 2020, 12, 2786. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, L.; Sauer, W.C.; Kozikowski, V.; Ryan, J.K.; Jørgensen, H.; Jelen, P. Nutritive value of protein extracted from honey Bees. J. Food Sci. 1985, 50, 1327–1329. [Google Scholar] [CrossRef]
- Dušková, J.; Tishchenko, G.; Ponomareva, E.; Šimůnek, J.; Koppová, I.; Skálová, T.; Štěpánková, A.; Hašek, J.; Dohnálek, J. Chitinolytic enzymes from bacterium inhabiting human gastrointestinal Tract—Critical parameters of protein isolation from anaerobic culture. Acta Biochim. Pol. 2011, 58, 2275. [Google Scholar] [CrossRef]
- Jantzen da Silva Lucas, A.; Menegon de Oliveira, L.; da Rocha, M.; Prentice, C. Edible Insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Ladrón de Guevara, O.; Padilla, P.; García, L.; Pino, J.M.; Ramos-Elorduy, J. Amino acid determination in some edible mexican insects. Amino Acids 1995, 9, 161–173. [Google Scholar] [CrossRef]
- DeFoliart, G.R. Insects as Human Food: Gene DeFoliart discusses some nutritional and economic aspects. Crop Prot. 1992, 11, 395–399. [Google Scholar] [CrossRef]
- Moyo, S.; Jaja, I.F.; Mopipi, K.; Masika, P.; Muchenje, V. Effect of graded levels of Imbrasia belina meal on blood lipid profile, bone morphometric and mineral content of broiler chickens. Anim. Feed Sci. Technol. 2021, 271, 114736. [Google Scholar] [CrossRef]
- Li-Chan, E.C. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review: Bioactive peptides effects. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef]
- Vercruysse, L.; Smagghe, G.; Beckers, T.; Camp, J.V. Antioxidative and ACE inhibitory activities in enzymatic hydrolysates of the cotton leafworm, Spodoptera littoralis. Food Chem. 2009, 114, 38–43. [Google Scholar] [CrossRef]
- Lemes, A.; Sala, L.; Ores, J.; Braga, A.; Egea, M.; Fernandes, K. A Review of the latest advances in encrypted bioactive peptides from protein-rich Waste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current trends of bioactive peptides—new sources and therapeutic effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Fogang Mba, A.R.; Kansci, G.; Viau, M.; Rougerie, R.; Genot, C. Edible Caterpillars of Imbrasia truncata and Imbrasia epimethea contain lipids and proteins of high potential for nutrition. J. Food Compos. Anal. 2019, 79, 70–79. [Google Scholar] [CrossRef]
- Mabossy-Mobouna, G.; Kinkela, T.; Lenga, A.; Malaisse, F. Imbrasia truncata Aurivillius (Saturniidae): Importance en afrique centrale, commercialisation et valorisation à Brazzaville. Geo-Eco-Trop 2013, 37, 313–330. [Google Scholar]
- Atowa, C.O.; Okoro, B.C.; Umego, E.C.; Atowa, A.O.; Emmanuel, O.; Ude, V.C.; Ugbogu, E.A. Nutritional values of Zonocerus variegatus, Macrotermes bellicosus and Cirina Forda insects: Mineral composition, fatty acids and amino acid profiles. Sci. Afr. 2021, 12, e00798. [Google Scholar] [CrossRef]
- Rapatsa, M.M.; Moyo, N.A.G. Evaluation of Imbrasia belina meal as a fishmeal substitute in Oreochromis mossambicus diets: Growth performance, histological analysis and enzyme activity. Aquac. Rep. 2017, 5, 18–26. [Google Scholar] [CrossRef]
- Foua Bi, F.; Meite, A.; Dally, T.; Ouattara, H.; Kouame, K.; Kati-Coulibaly, S. Étude de la qualité biochimique et nutritionnelle de la poudre séchée d’Embrasai oyemensis, chenilles consommées au Centre-Ouest de la Côte d’Ivoire. J. App. Biosci. 2015, 96, 9039. [Google Scholar] [CrossRef]
- Anvo, M.P.M.; Aboua, B.R.D.; Compaoré, I.; Sissao, R.; Zoungrana-Kaboré, C.Y.; Kouamelan, E.P.; Toguyéni, A. Fish meal replacement by Cirina butyrospermi caterpillar’s meal in practical diets for Clarias Gariepinus Fingerlings. Aquac. Res. 2017, 48, 5243–5250. [Google Scholar] [CrossRef]
- Dooshima Igbabul, B. Nutritional and microbial quality of dried larva of Cirina forda. Int. J. Nutr. Food Sci. 2014, 3, 602. [Google Scholar] [CrossRef]
- Olaleye, A.A. Amino acid profiles of five commonly consumed insects in southwestern Nigeria. Carpathian J. Food Sci. Technol. 2021, 12, 42–51. [Google Scholar] [CrossRef]
- Séré, A.; Bougma, A.; Bazié, B.S.R.; Traoré, E.; Parkouda, C.; Gnankiné, O.; Bassolé, I.H.N. Chemical composition, energy and nutritional values, digestibility and functional properties of defatted flour, protein concentrates and isolates from Carbula marginella (Hemiptera: Pentatomidae) and Cirina Butyrospermi (Lepidoptera: Saturniidae). BMC Chem. 2021, 15, 46. [Google Scholar] [CrossRef]
- Durst, P.B.; Johnson, D.V.; Leslie, R.N.; Shono, K. Forest Insects as Food: Humans Bite Back; RAP Publication: Rockville, MD, USA, 2010; ISBN 9789251064887. [Google Scholar]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr. 2021, 62, 3499–3508. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J.A. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2019, 27, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Motshegwe, S.M.; Holmback, J.; Yeboah, S.O. General properties and the fatty acid composition of the oil from the mophane caterpillar, Imbrasia Belina. JAOCS J. Am. Oil Chem. Soc. 1998, 75, 725–728. [Google Scholar] [CrossRef]
- Zinzombe, M.; Georges, S. Larval lipid quality of lepidoptera: Gonimbrasia Belina. Botsw. Notes Rec. 1994, 26, 167–173. [Google Scholar]
- Aiko, Y.B.; Jacob, J.O.; Salihu, S.O.; Dauda, B.E.N.; Suleiman, M.A.T.; Akanya, H.O. Fatty acid and amino acid profile of emperor moth caterpillar (Cirina Forda) in Paikoro Local Government Area of Niger State, Nigeria. Am. J. Biochem. 2014, 2014, 29–34. [Google Scholar] [CrossRef]
- Oriolowo, O.B.; Abubakar, D.S.; Bidda, R.D.; Masa’udu, S. Nutritional comparison of the pallid emperor moth, Cirina Forda and the atlantic mackerel, scombrus scomber. Acta Entomol. Zool. 2021, 2, 24–31. [Google Scholar] [CrossRef]
- Pennino, M.; Dierenfeld, E.S.; Behler, J.L. Retinol, α-Tocopherol and proximate nutrient composition of invertebrates used as feed. Int. Zoo Yearb. 1991, 30, 143–149. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Oonincx, D.G.A.B.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; van Loon, J.J.A. Insects as sources of iron and zinc in human nutrition. Nutr. Res. Rev. 2018, 31, 248–255. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Mabossy-Mobouna, G.; Bouyer, T.; Latham, P.; Roulondoko, P.; Konda Ku Mbuta, A.; Malaisse, F. Preliminary knowledge for breeding edible caterpillars in congo-brazzaville. Geo-Eco-Trop 2016, 40, 145–174. [Google Scholar]
- Mbata, K.J.; Chidumayo, E.N.; Lwatula, C.M. Traditional regulation of edible caterpillar exploitation in the kopa area of mpika district in northern zambia. J. Insect Conserv. 2002, 6, 16. [Google Scholar] [CrossRef]
- Lisingo, J.; Wetsi, J.L.; Ntahobavuka, H. Enquête sur les chenilles comestibles et les divers usages de leurs plantes hôtes dans les districts de Kisangani et de la Tshopo ( R.D.Congo ). Geo-Eco-Trop J. 2010, 34, 139–146. [Google Scholar]
- Benneker, C.; Assumani, D.M.; Maindo, A.; Kimbuani, G.; Lescuyer, G.; Esuka, J.C.; Kasongo, E. Exploitation artisanale de bois d’oeuvre En RD Congo: Secteur porteur d’espoir pour le développement des petites et moyennes entreprises; Tropenbos International RD Congo: Kisangani, Democratic Republic of the Congo, 2012; ISBN 978-90-5113-109-3. [Google Scholar]
- Bomolo, O.; Niassy, S.; Chocha, A.; Longanza, B.; Bugeme, D.M.; Ekesi, S.; Tanga, C.M. Ecological diversity of edible insects and their potential contribution to household food security in Haut-Katanga province, Democratic Republic of Congo. Afr. J. Ecol. 2017, 55, 640–653. [Google Scholar] [CrossRef]
- Latham, P. Les Chenilles Comestibles et Leurs Plantes Nourricières dans la Province du Bas-Congo, 3rd ed.; Privateley Publised: Paris, France, 2015; ISBN 9780955420863. [Google Scholar]
- Gowdey, C.C. On the utilisation of an indigenous african silk-worm ( Anaphe Infracta , Wlsm.) in Uganda. Bull. Entomol. Res. 1912, 3, 269–274. [Google Scholar] [CrossRef]
- Tchibozo, S.; Malaisse, F.; Mergen, P. Insectes consommés par l’homme en Afrique Occidentale Francophone. Geo-Eco-Trop 2016, 40, 105–114. [Google Scholar]
- Agbidye, F.S.; Nongo, N.N. Harvesting and processing techniques for the larvae of the pallid emperor moth, Cirina Forda Westwood (Lepidoptera: Saturniidae), among the Tiv people of Benue State, Nigeria. J. Res. For. Wildl. Environ. 2009, 1, 123–132. [Google Scholar]
- Bocquet, E.; Maniacky, J.; Vermeulen, C.; Malaisse, F. A Propos de quelques chenilles consommées par les mongo en province de l’Équateur (République Démocratique Du Congo). Geo-Eco-Trop 2020, 44, 109–130. [Google Scholar]
- Payne, C.; Badolo, A.; Sagnon, B.; Cox, S.; Pearson, S.; Sanon, A.; Bationo, F.; Balmford, A. Effects of defoliation by the edible caterpillar “Chitoumou” (Cirina Butyrospermi) on harvests of shea (Vitellaria Paradoxa) and growth of maize (Zea Mays). Agrofor. Syst. 2020, 94, 231–240. [Google Scholar] [CrossRef]
- Muvatsi, P.; Kahindo, J.M.; Snook, L.K. Can the production of wild forest foods be sustained in timber concessions? logging and the availability of edible caterpillars hosted by Sapelli (Entandrophragma Cylindricum) and Tali (Erythrophleum Suaveolens) trees in the Democratic Republic of Congo. For. Ecol. Manag. 2018, 410, 56–65. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; Moreno, J.M.P.; Vázquez, A.I.; Landero, I.; Oliva-Rivera, H.; Camacho, V.H.M. Edible Lepidoptera in Mexico: Geographic distribution, ethnicity, economic and nutritional importance for rural people. J. Ethnobiol. Ethnomed. 2011, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yen, A.L. Insects as food and feed in the asia pacific region: Current perspectives and future directions. J. Insects Food Feed 2015, 1, 33–55. [Google Scholar] [CrossRef]
- Akpalu, W.; Muchapondwa, E.; Zikhali, P. Can the restrictive harvest period policy conserve mopane worms in southern Africa? A bioeconomic modelling approach. Environ. Dev. Econ. 2009, 14, 587–600. [Google Scholar] [CrossRef]
- Illgner, P.; Nel, E. The geography of edible insects in Sub-Saharan Africa: A study of the mopane caterpillar. Geogr. J. 2000, 166, 336–351. [Google Scholar] [CrossRef]
- Vega, F.; Kaya, H. Insect Pathology; Academic Press: London, UK, 2012. [Google Scholar]
- Rothschild, M.; Reichstein, T.; Von Euw, J.; Aplin, R.; Harman, R.R.M. Toxic Lepidoptera. Toxicon 1970, 8, 293–296. [Google Scholar] [CrossRef]
- Nishida, R. Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 2002, 47, 57–92. [Google Scholar] [CrossRef]
- Mutungi, C.; Irungu, F.G.; Nduko, J.; Mutua, F.; Affognon, H.; Nakimbugwe, D.; Ekesi, S.; Fiaboe, K.K.M. Postharvest Processes of Edible Insects in Africa: A review of processing methods, and the implications for nutrition, safety and new products development. Crit. Rev. Food Sci. Nutr. 2019, 59, 276–298. [Google Scholar] [CrossRef]
- Omotoso, O.T. Nutritional quality, functional properties and anti-nutrient compositions of the larva of Cirina Forda (Westwood) (Lepidoptera: Saturniidae). J. Zhejiang Univ. Sci. B 2006, 7, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Dauda, B. Nutritive and anti-nutritive Composition of locust bean tree emperor moth larvae Bunaea Alcinoe (Lepidoptera-Saturniidae Stoll 1780) from Gurara Local Government Area, Niger State, Nigeria. JSRR 2014, 3, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Nishimune, T.; Watanabe, Y.; Okazaki, H.; Akai, H. Thiamin is decomposed due to anaphe spp. entomophagy in seasonal ataxia patients in Nigeria. J. Nutr. 2000, 130, 1625–1628. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E. Investigations of the Source, Distribution, Expression and Physiological Function of Thiaminase I; Cornell University: Ithaca, NY, USA, 2012. [Google Scholar]
- Zhou, J.; Han, D. Safety evaluation of protein of silkworm (Antheraea Pernyi) pupae. Food Chem. Toxicol. 2006, 44, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Amisi, M.F.; Héritier, U.S.; Paul, M.; Georges, A.L.; Innocent, B.K.; Pascal, I.M. Valorisation de la chenille comestible Bunaeopsis aurantiaca dans la gestion communautaire des forêts du Sud-Kivu (République Démocratique du Congo). VertigO Rev. Électronique Sci. L’environ. 2013. [Google Scholar] [CrossRef]
- Noutcheu, R.; Snook, L.K.; Tchatat, M.; Taedoumg, H.; Tchingsabe, O.; Tieguhong, J.C. Do Logging concessions decrease the availability to villagers of foods from timber trees? A quantitative analysis for moabi (Baillonella Toxisperma), Sapelli (Entandrophragma Cylindricum) and Tali (Erythrophleum Suaveolens) in Cameroon. For. Ecol. Manag. 2016, 381, 279–288. [Google Scholar] [CrossRef]
- McGregor, J. Gathered produce in zimbabwe’s communal areas changing resource availability and use. Ecol. Food Nutr. 1995, 33, 163–193. [Google Scholar] [CrossRef]
- Kenis, M.; Sileshi, G.; Mbata, K.; Chidumayo, E.; Meke, G.; Muatinte, B. Towards conservation and sustainable utilization of edible caterpillars of the Miombo. In Presentation to the SIL Annual Conference on Trees for Poverty Alleviation; Cabi: Paris, France, 2006; Volume 9. [Google Scholar]
- Eckebil, P.P.T.; Verheggen, F.; Doucet, J.-L.; Malaisse, F.; Daïnou, K.; Cerutti, P.O.; Vermeulen, C. Entandrophragma cylindricum (Sprague) Sprague (Meliaceae), une espèce ligneuse concurrentielle en Afrique centrale (synthèse bibliographique). Biotechnol. Agron. Soc. Environ. 2017, 18. [Google Scholar] [CrossRef]
- De Foliart, G.R. Edible Insects as minilivestock. Biodivers. Conserv. 1995, 4, 306–321. [Google Scholar] [CrossRef]
- De Foliart, G.R. An overview of the role of edible insects in preserving biodiversity. Ecol. Food Nutr. 1997, 36, 109–132. [Google Scholar] [CrossRef]
- Leleup, N.; Daems, H. Les chenilles alimentaires du Kwango. Causes de leur Raréfaction et mesures préconisées pour y remédier. J. D’agric. Trop. Bot. Appliquée 2014, 16, 1–21. [Google Scholar] [CrossRef]
- Syampungani, S.; Chirwa, P.W.; Akinnifesi, F.K.; Sileshi, G.; Ajayi, O.C. The Miombo woodlands at the cross roads: Potential threats, sustainable livelihoods, policy gaps and challenges. Nat. Resour. Forum 2009, 33, 150–159. [Google Scholar] [CrossRef]
- Govorushko, S. Global Status of insects as food and feed source: A review. Trends Food Sci. Technol. 2019, 91, 436–445. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.-J.; Salinas-Castro, A. Edible insects processing: Traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef] [PubMed]
- Birari, V.V.; Siddhapara, M.R.; Patel, D.H. Biology of eri silkworm, Samia Ricini ( Donovan ) on castor, Ricinus Communis L. Entomon 2019, 44, 229–234. [Google Scholar] [CrossRef]
- Van Itterbeeck, J.; van Huis, A. Environmental manipulation for edible insect procurement: A historical perspective. J Ethnobiol. Ethnomedicine 2012, 8, 3. [Google Scholar] [CrossRef]
- Ngoka, B.M.; Kioko, E.N.; Raina, S.K.; Mueke, J.M.; Kimbu, D.M. Semi-captive rearing of the african wild silkmoth Gonometa Postica (Lepidoptera: Lasiocampidae) on an indigenous and a non-indigenous host plant in Kenya. JTI 2007, 27, 183. [Google Scholar] [CrossRef]
- Hartland-Rowe, R. The biology of the wild silk moth Gonometa Rufobrunnea Aurivillius (Lasiocampidae) in northeastern Botswana, with some comments on its potential as a source of wild silk. Botsw. Notes Rec. 1992, 24, 11. [Google Scholar]
- Ghazoul, J. Mopane Woodlands and the Mopane Worm: Enhancing Rural Livelihoods and Resource Sustainability. Wild. Fish. Manag. 2006. [Google Scholar]
| Branch | Order | Family | Genus/Specie |
|---|---|---|---|
| Arthropoda | Coleoptera | Dryophthoridae | Rhynchophorus ferrugineus (Olivier 1791) |
| Rhynchophorus palmarum (Fabricius 1801) | |||
| Tenebrionidae | Tenebrio molitor (L. 1758) | ||
| Hymenoptera | Apidae | Apis melifera (L. 1758) | |
| Isoptera | Termitidae | Macrotermes natalensis (Haviland 1898) Macrotermes subhyalinus (Rambur 1842) | |
| Lepidoptera | Noctuidae | Aegocera rectilinea (Boisduval 1836) | |
| Bombycidae | Bombyx mori (L. 1758) | ||
| Sphingidae | Clanis bilineata (Walker, 1866) | ||
| Danainae | Danaus plexippus (L. 1758) | ||
| Notodontidae | Anaphe infracta (Boisduval 1847) | ||
| Anaphe venata (Butler 1878) | |||
| Antheua insignata (Gaede 1928) | |||
| Elaphrodes lactea (Gaede 1932) | |||
| Lasiocampidae | Gonometa postica (Walker 1855) | ||
| Gonometa rufobrunnea (Aurivillius 1992) | |||
| Noctuidae | Aegocera rectilinea (Boiduval 1836) | ||
| Heliothis zea (Boddie 1850) | |||
| Saturniidae | Bunaea alcinoe (Stoll 1780) | ||
| Cirina forda (Westwood 1849) | |||
| Cinabra hyperbius (Westwood 1881) | |||
| Cirina Butyrospermi (Vuillet 1911) | |||
| Gonimbrasia cocaulti (Darge and Terral 1993) | |||
| Gonimbrasia krucki (Hering 1930) | |||
| Gonimbrasia zambesina (Walker 1865) | |||
| Gynanisa nigra (Klug 1836) | |||
| Imbrasia ertli (Rebel 1904) | |||
| Imbrasia belina (Westwood 1849) | |||
| Imbrasia dione (Fabricius 1793) | |||
| Imbrasia epimethea (Drury 1773) | |||
| Imbrasia obscura (Butler 1878) | |||
| Imbrasia oyemensis (Rougeot 1955) | |||
| Imbrasia truncata (Aurivillius 1909) | |||
| Lobobunaea phaedusa (Drury 1782) | |||
| Samia ricini (Drury 1773) | |||
| Tagoropsis flavinata (Walker 1865) Usta terpsichore (Maassen and Weymer, 1885) | |||
| Odonate | Libellulidae | Orthetrum sabina (Drury 1770) | |
| Orthoptera | Acrididae | Locusta migratoria (L. 1758) | |
| Gryllidae | Acheta domesticus (L. 1758) | ||
| Brachytrupes membranaceus (Drury 1770) | |||
| Gryllus bimaculatus (De Geer 1773) | |||
| Tettigoniidae | Ruspolia differens (Serville 1838) | ||
| Cyanobacteria | Oscillatoriales | Phormidiaceae | Arthrospira platensis (Gomont 1892) |
| Plantae | Fabales | Fabaceae | Acacia auriculiformis (A. Cunn. Ex Benth. 1842) |
| Acacia. Mearnsii (De Wild 1925) | |||
| Albizia antunesiana (Harms) | |||
| Amphimas pterocarpoides (Harms) | |||
| Burkea africana (Hook) | |||
| Colophospermum spp | |||
| Erythrophleum africanum (Welw. ex Benth.) Harms | |||
| Erythrophleum suaveolens (Guill. and Perr.) Brenan | |||
| Isoberlinia (Craib and Stapf ex Holland) | |||
| Piptadeniastrum africanum (Hook.f.) Brenan | |||
| Ebenales | Sapotaceae | Autranella congoensis (De Wild.) A. Chev. en RCA | |
| Vitellaria paradoxa (C.F. Gaertn). | |||
| Caryophyllales | Nyctaginaceae | Boerhavia diffusa (L.) | |
| Malpighiales | Phyllanthaceae | Bridelia micrantha (Hochst.) Baill | |
| Gentianales | Rubiaceae | Crossopteryx febrifuga (Afzel. ex G. Don) Benth | |
| Apocynaceae | Funtumia africana (Benth.) Stapf | ||
| Sapindales | Burseraceae | Dacryodes edulis (G. Don) H.J. Lam | |
| Meliaceae | Entandrophragma cylindricum (Sprague and Hoyle) | ||
| Lecythidales | Lecythidaceae | Petertianthus macrocarpus (P. Beauv.) Liben | |
| Agaricales | Psathyrellaceae | Psathyrella tuberculata (Pat.) A.H. Sm | |
| Malpighiales | Phyllanthaceae | Uapaca guinensis (Müll.Arg) |
| Species | Duration (Days) | Total Cycle Time | Type of Development | |||
|---|---|---|---|---|---|---|
| Egg | Larva | Nymph | Adult | |||
| Anaphe infracta | / | 56 | 77 | 7 | 140 | Monovoltine |
| Aegocera rectilinea | ≈3 | ≈20 | ≈12 | 8 | ≈36 | Multivoltine |
| Bunea alcinoe | / | / | / | / | / | Multivoltine |
| Cirina forda | ≈35 | ≈50 | 270 | ≈2 | 357 | Monovoltine |
| Cirina butyrospermi | 30 | 33 | 330 | 5 | 398 | Monovoltine |
| Elaphrodes lactea | 60 | 60 | 30 | 210 | 360 | Monovoltine |
| Imbrasia belina | 10 | 42 | 210 | 3 | 265 | Bivoltine |
| Imbrasia obscura | / | / | / | / | / | Monovoltine |
| Amino Acids | Cirina butyrospermi | Cirina forda | Imbrasia epimethea | Imbrasia belina | Imbrasia oyemensis | Imbrasia obscura | Imbrasi truncata |
|---|---|---|---|---|---|---|---|
| Histidine | 23–41.5 | 32–54 | 20 | 17–31.1 | 2 | 20 | 17.4 |
| Lysine | 25.3–61.3 | 56–71 | 50–74.2 | 36–74.2 | 3.3 | 33 | 79 |
| Leucine | 40.8–59.3 | 63.1–74 | 48.2–81 | 35–82.2 | 3.3 | 33 | 73.1 |
| Isoleucine | 31.1–52 | 18–46.4 | 29–36 | 22–75.4 | 2.4 | 24 | 24.2 |
| Methionin | 16 | 18.2–23 | 12–22.4 | 9–22 | 1.1 | 11 | 22.2 |
| Phenylalanin | 34 | 43.4–55 | 45.1–65 | 25–52 | 3.2 | 32 | 62.2 |
| Proline | 32–78 | 46–75.4 | / | 44–51 | 4 | 35 | 21.4 |
| Serin | 17–126.1 | 42–53 | / | 44–56.2 | 3 | 28 | 49 |
| Threonin | 23.1–138.1 | 56.4–61 | 34.1–48 | 27–73 | 3 | 29 | 47 |
| Tyrosine | 16 | 43.1–75 | 75 | 37–64 | 4.1 | 41 | 77 |
| Tryptophan | / | 23–73.1 | 16 | 7–12 | 1 | 10 | 17 |
| Valine | 9–63.4 | 49.4–66 | 44–102 | 32–57 | 3 | 27 | 102 |
| Fatty Acids | Cirina butyrospermi | Cirina forda | Imbrasia epimethea | Imbrasia belina | Imbrasia oyemensis | Imbrasia obscura | Imbrasia truncata |
|---|---|---|---|---|---|---|---|
| Lauric acid (C12:0) SFA | 0.08 | <0.1 | 0.2 | <0.1 | 1.66 | / | trace |
| Myristic acid (C14:0) SFA | 0.3–0.6 | 0.7 | 0.6 | 0.3–1.2 | 0.5–1.9 | 0.2 | 0.2–0.3 |
| Pentadecanoic acid (C15:0) SFA | / | <0.1–1.9 | trace | 0.1 | / | 0.2 | trace |
| Palmitic acid (C16:0) SFA | 17.9–27.5 | 7.4–13 | 23.2 | 3.2–31.9 | 10.1–46 | 17.1 | 22.3–20.6 |
| Palmitoleic acid (C16:1) MUFA | 0.3 | 0.2–4.3 | 0.6 | 0.1–1.8 | / | 0.3 | 0.2–0.5 |
| Margaric acid (C17:0) SFA | 0.1–1.3 | 1.1–5.8 | / | 0.3–0.4 | / | 1.1 | 1.17 |
| Heptadecenoic acid (C17:1) SFA | / | / | / | 0.12 | / | / | / |
| Steric acid (C18:0) SFA | 35.4–8.9 | 6.9–16 | 22.1 | 1.7–13.5 | 7.2 | 18–38.5 | 16.4–22.3 |
| Oleic acids (C18:1) MUFA | 0.4–26.4 | 3.7–17.9 | 8.4 | 1.6–34.2 | 34.6 | 8–40.3 | 7.4–9.5 |
| Linoleic acid (C18:2 n6) MUFA | 4.5–30.2 | 8.1–29.2 | 7 | 1.6–10.9 | 11.2 | 6.6–9.2 | 7.1–7.6 |
| α-linolenic acid (C18:3 n3) MUFA | 0.8–35.8 | 4.9–45.3 | 35.1 | 19.6–29.4 | / | 0.8–41.1 | 28.6–42.6 |
| Arachidic acid (C20:0) MUFA | 0.4 | 2.4 | <0.1 | 0.3–0.4 | / | 0.3 | 0.3–8.7 |
| Eicosadienoic acid (C20:2) PUFA | / | 0.2 | / | / | / | / | 0.5 |
| Arachidonic acid (C20:4) PUFA | / | <0.1 | <0.1 | 0.5 | / | 0.3 | 0.3 |
| Eicosapentaenoic acid (C20:5) PUFA | / | <0.1 | / | / | / | / | |
| Docosenoic acid (C22:1) MUFA | / | / | / | / | / | / | 0.1 |
| Minerals | Ca | K | Mg | P | Na | Fe | Zn | Mn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| Cirina butyrospermi | 32–0.2 | 1160–1278 | 150–169 | 390 | 13 | 13–31 | 1.9–10 | 0.6–10 | 0.13 |
| Cirina forda | 7–634 | 48–2130 | 296–1180 | 46–1090 | 42–128 | 1.3–64 | 3.7–24.2 | 6.4–7.5 | / |
| Imbrasia epimethea | 225 | 1258 | 402 | 666 | 75 | 13 | 11.1 | 5.8 | 1.2 |
| Imbrasia belina | 174 | 1 032 | 160 | 543 | 1024 | 31 | 14 | 3.9 | 0.91 |
| Imbrasia oyemensis | 73 | 680 | 610 | 514.01 | 730 | 70.214 | 11.185 | 387.9 | 387.9 |
| Imbrasia obscura | 100 | 970 | 12–240 | 280 | 20 | / | / | / | / |
| Imbrasia truncata | 122–132 | 1250–1348 | 178–192 | 841 | 170–183 | 8.1–8.7 | 10.3–11.1 | 3.2 | 1.3–1.4 |
| Recommended Daily Intake | 900 | 420 | 420 | 750 | 1500 | 9 | 11 | / | 1.5 |
| Scientific Name | Availability | Food Behavior | Host Plants | References |
|---|---|---|---|---|
| Anaphe infracta | January to December, August to September | Polyphagous | Bridelia minrantha, Cynometra alexandri Triumfetta manrophylla Alhizzia fnstiyiata | [100] |
| Bunaea alcinoe | June to August | Oligophage | Pycnanthus angolensis, Dacryodes edulis | [99,101] |
| Cirina forda | June to August, September to December | Polyphagous | Albizia antunesiana, Erythrophleum suaveolens, Baillonella toxisperma, Burkea africana, Crossopteryx febrifuga, Erythrophleum africanum, Vitellaria paradoxa | [99,102,103] |
| Cirina butyrospermi | June to August | Monophage | Vitellaria paradoxa, | [37,101,104] |
| Elaphrodes lactea | December to January | Oligophage | Albizia ferruginea | [99] |
| Imbrasia belina | December to January, April to May | Monophage | Colophospermum mopane | [35] |
| Imbrasia obscura | October to February | Monophage | Pentaclethra macrophylla | [99] |
| Imbrasia epimethea | June to September, January to February | Oligophage | Funtumia africana, Dacryodes edulis | [99,101,103] |
| I. truncata | August | Oligophage | Amphimas pterocarpoides, Petertianthus macrocarpus, Piptadeniastrum africanum | [75,103] |
| I. oyemensis | June to August, August to September | Monophage | Entandrophragma cylindricum | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Numbi Muya, G.M.; Mutiaka, B.K.; Bindelle, J.; Francis, F.; Caparros Megido, R. Human Consumption of Insects in Sub-Saharan Africa: Lepidoptera and Potential Species for Breeding. Insects 2022, 13, 886. https://doi.org/10.3390/insects13100886
Numbi Muya GM, Mutiaka BK, Bindelle J, Francis F, Caparros Megido R. Human Consumption of Insects in Sub-Saharan Africa: Lepidoptera and Potential Species for Breeding. Insects. 2022; 13(10):886. https://doi.org/10.3390/insects13100886
Chicago/Turabian StyleNumbi Muya, Gloria Marceline, Bienvenu Kambashi Mutiaka, Jérôme Bindelle, Frédéric Francis, and Rudy Caparros Megido. 2022. "Human Consumption of Insects in Sub-Saharan Africa: Lepidoptera and Potential Species for Breeding" Insects 13, no. 10: 886. https://doi.org/10.3390/insects13100886
APA StyleNumbi Muya, G. M., Mutiaka, B. K., Bindelle, J., Francis, F., & Caparros Megido, R. (2022). Human Consumption of Insects in Sub-Saharan Africa: Lepidoptera and Potential Species for Breeding. Insects, 13(10), 886. https://doi.org/10.3390/insects13100886










