Characterization of Resistance to the Mexican Rice Borer (Lepidoptera: Crambidae) among Sugarcane Cultivars
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Screenings for Resistance
2.2. Oviposition Preference
2.3. Neonate Establishment
2.4. Diet Incorporation Assay
3. Results
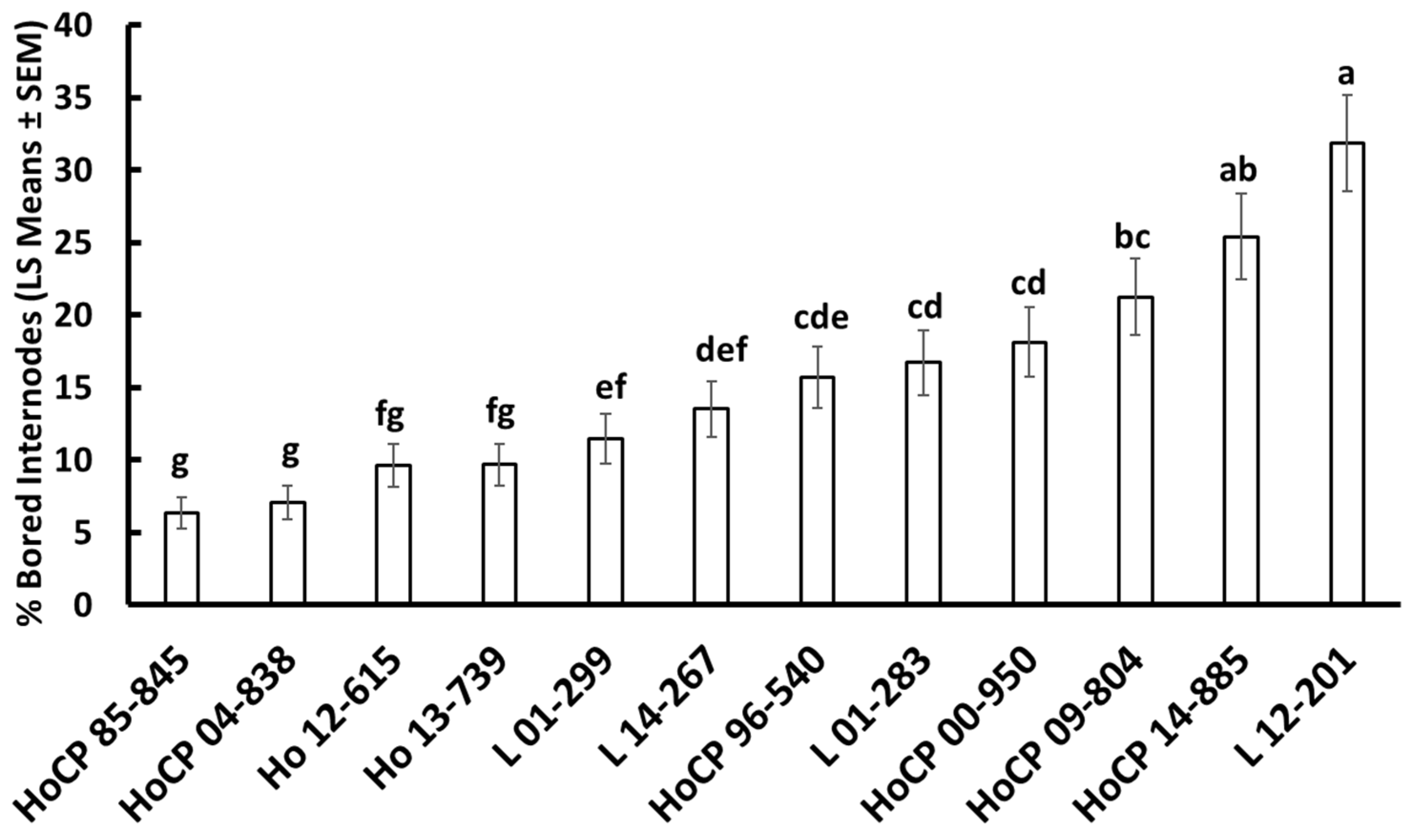
3.1. Field Screenings for Resistance
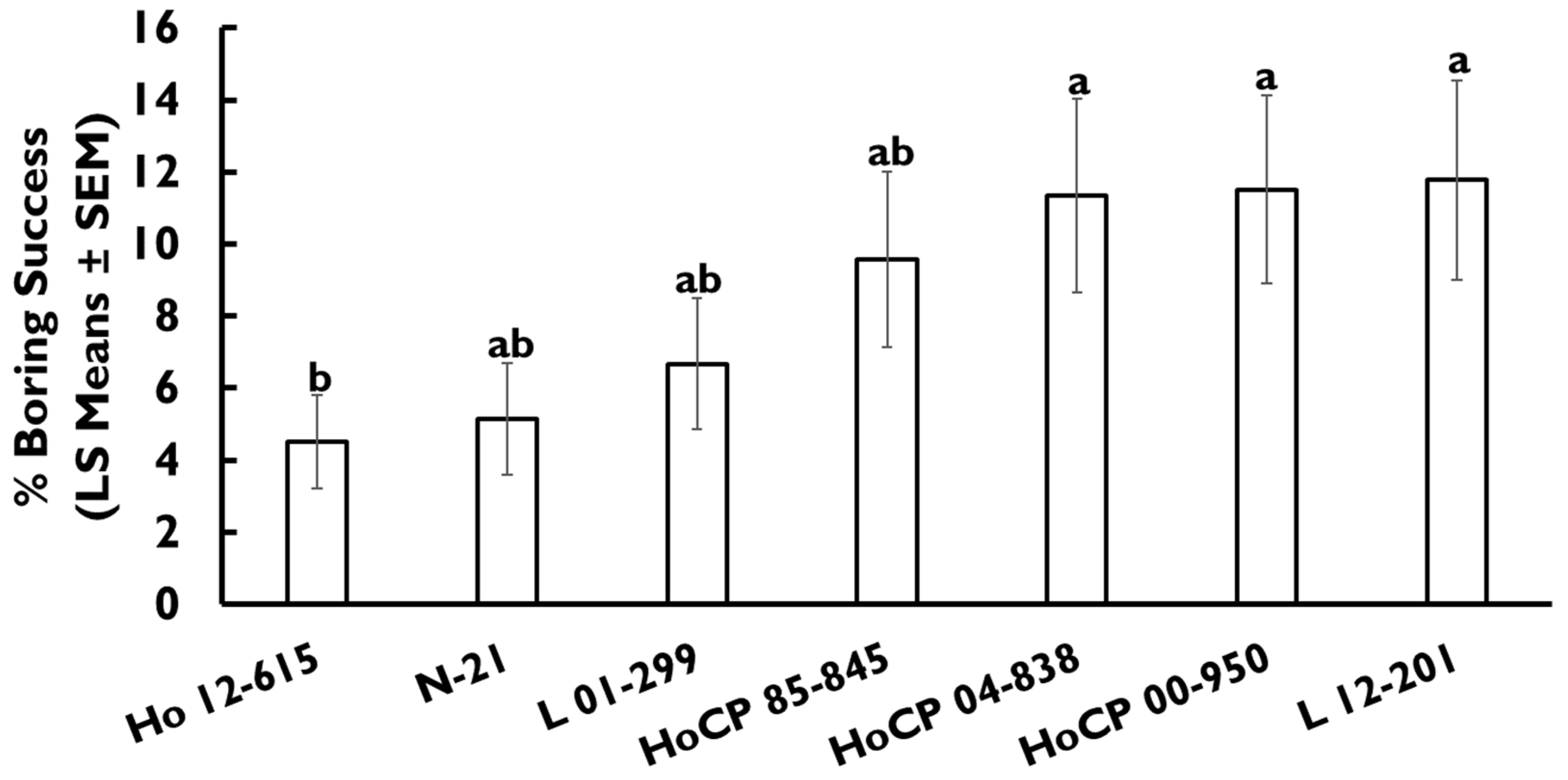
3.2. Oviposition Preference
3.3. Neonate Establishment
3.4. Diet Incorporation Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA-ERS. Sugar & Sweeteners: Background. Available online: https://www.ers.usda.gov/topics/crops/sugar-sweeteners/background.aspx (accessed on 14 June 2020).
- Reagan, T.E.; Mulcahy, M.M. Interaction of cultural, biological, and varietal controls for management of stalk borers in Louisiana sugarcane. Insects 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Showler, A.T.; Reagan, T.E. Chapter 1: Ecology and Tactics of Control for Three Sugarcane Stalkboring Species in the Western Hemisphere and Africa; Goncalves, J., Correia, K.D., Eds.; Nova Science Publishers: New York, NY, USA, 2012; Volume 4882, pp. 1–39. ISBN 9781619422131. [Google Scholar]
- Wilson, B.E.; White, W.H.; Richard, R.T.; Johnson, R.M. Population trends of the sugarcane borer (Lepidoptera: Crambidae) in Louisiana sugarcane. Environ. Entomol. 2020, 49, 1455–1461. [Google Scholar] [CrossRef]
- Showler, A.T. Mexican rice borer control tactics in United States sugarcane. Insects 2019, 10, 160. [Google Scholar] [CrossRef]
- Reay-Jones, F.P.F.; Wilson, L.T.; Reagan, T.E.; Legendre, B.L.; Way, M.O. Predicting economic losses from the continued spread of the Mexican rice borer (Lepidoptera: Crambidae). J. Econ. Entomol. 2008, 101, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-del-Bosque, L.A.; Reyes-Méndez, C.A. Eoreuma loftini displaced Diatraea lineolata and D. saccharalis (Lepidoptera: Crambidae) as the main corn stalkborer in northern Tamaulipas, México. Southwest. Entomol. 2013, 38, 75–78. [Google Scholar] [CrossRef]
- Wilson, B.E.; Hardy, T.N.; Beuzelin, J.M.; VanWeelden, M.T.; Reagan, T.E.; Miller, R.; Meaux, J.; Stout, M.J.; Carlton, C.E. Expansion of the Mexican rice borer (Lepidoptera: Crambidae) into rice and sugarcane in Louisiana. Environ. Entomol. 2015, 44, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.E.; Beuzelin, J.M.; Reagan, T.E. Population distribution and range expansion of the invasive Mexican rice borer (Lepidoptera: Crambidae) in Louisiana. Environ. Entomol. 2017, 46, 175–182. [Google Scholar] [CrossRef]
- Kang, I.; Wilson, B.; Carter, B.; Diaz, R. A new detection of the invasive Mexican rice borer (Lepidoptera: Crambidae) from Georgia in the United States based on morphological and molecular data. J. Integr. Pest Manag. 2022, 13, 17. [Google Scholar] [CrossRef]
- Meagher, R.L.; Smith, J.W.; Johnson, K.J.R. Insecticidal management of Eoreuma loftini (Lepidoptera: Pyralidae) on Texas sugarcane: A critical review. J. Econ. Entomol. 1994, 87, 1332–1344. [Google Scholar] [CrossRef]
- Reay-Jones, F.P.F.; Showler, A.T.; Reagan, T.E.; Legendre, B.L.; Way, M.O.; Moser, E.B. Integrated tactics for managing the Mexican rice borer (Lepidoptera: Crambidae) in sugarcane. Environ. Entomol. 2005, 34, 1558–1565. [Google Scholar] [CrossRef]
- Meagher, R.L.; Smith, J.W.; Browning, H.W.; Saldana, R.R. Sugarcane stemborers and their parasites in southern Texas. Environ. Entomol. 1998, 27, 759–766. [Google Scholar] [CrossRef]
- Wilson, B.E.; Showler, A.T.; Reagan, T.E.; Beuzelin, J.M. Improved chemical control for the Mexican rice borer (Lepidoptera: Crambidae) in sugarcane: Larval exposure, a novel scouting method, and efficacy of a single aerial insecticide application. J. Econ. Entomol. 2012, 105, 1998–2006. [Google Scholar] [CrossRef]
- Wilson, B.E.; VanWeelden, M.T.; Beuzelin, J.M.; Reagan, T.E.; Prado, J.A. Efficacy of insect growth regulators and diamide insecticides for control of stem borers (Lepidoptera: Crambidae) in sugarcane. J. Econ. Entomol. 2017, 110, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.E.; White, W.H.; Richard, R.T.; Johnson, R.M. Evaluation of sugarcane borer, Diatraea saccharalis, resistance among commercial and experimental cultivars in the Louisiana sugarcane cultivar development program. Int. Sugar J. 2021, 123, 256–261. [Google Scholar]
- Salgado, L.D.; Wilson, B.E.; Villegas, J.M.; Richard, R.T.; Penn, H.J. Resistance to the sugarcane borer (Lepidoptera: Crambidae) in Louisiana sugarcane cultivars. Environ. Entomol. 2022, 51, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Pfannenstiel, R.S.; Meagher, R.L. Sugarcane resistance to stalkborers (Lepidoptera: Pyralidae) in south Texas. Fla. Entomol. 1991, 74, 300. [Google Scholar] [CrossRef]
- Meagher, R.L.; Irvine, J.E.; Breene, R.G.; Pfannenstiel, R.S.; Gallo-Meagher, M. Resistance mechanisms of sugarcane to Mexican rice borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 1996, 89, 536–543. [Google Scholar] [CrossRef]
- Reay-Jones, F.P.F.; Way, M.O.; Sétamou, M.; Legendre, B.L.; Reagan, T.E. Resistance to the Mexican rice borer (Lepidoptera: Crambidae) among Louisiana and Texas sugarcane cultivars. J. Econ. Entomol. 2003, 96, 1929–1934. [Google Scholar] [CrossRef]
- VanWeelden, M.T.; Wilson, B.E.; Beuzelin, J.M.; Reagan, T.E.; Way, M.O. Oviposition preference and survival of the Mexican rice borer (Lepidoptera: Crambidae) in bioenergy and conventional sugarcane and sorghum. Environ. Entomol. 2017, 46, 855–863. [Google Scholar] [CrossRef]
- Wilson, B.E.; VanWeelden, M.T.; Beuzelin, J.M.; Reagan, T.E.; Way, M.O.; White, W.H.; Wilson, L.T.; Showler, A.T. A relative resistance ratio for evaluation of Mexican rice borer (Lepidoptera: Crambidae) susceptibility among sugarcane cultivars. J. Econ. Entomol. 2015, 108, 1363–1370. [Google Scholar] [CrossRef]
- Reay-Jones, F.P.F.; Wilson, L.T.; Showler, A.T.; Reagan, T.E.; Way, M.O. Role of oviposition preference in an invasive crambid impacting two graminaceous host crops. Environ. Entomol. 2007, 36, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Showler, A.T.; Castro, B.A. Influence of drought stress on Mexican rice borer (Lepidoptera: Crambidae) oviposition preference in sugarcane. Crop Prot. 2010, 29, 415–421. [Google Scholar] [CrossRef]
- Beuzelin, J.M.; Wilson, L.T.; Showler, A.T.; Mészáros, A.; Wilson, B.E.; Way, M.O.; Reagan, T.E. Oviposition and larval development of a stem borer, Eoreuma loftini, on rice and non-crop grass hosts. Entomol. Exp. Appl. 2013, 146, 332–346. [Google Scholar] [CrossRef]
- Martin, F.A.; Richard, C.A.; Hensley, S.D. Host resistance to Diatraea saccharalis (F.): Relationship of sugarcane internode hardness to larval damage. Environ. Entomol. 1975, 4, 687–688. [Google Scholar] [CrossRef]
- Coburn, G.E.; Hensley, S.D. Differential survival of Diatraea saccharalis (F.) larvae on two varieties of sugarcane. Proc. Int. Soc. Sugar Cane. Technol. 1972, 14, 440–444. [Google Scholar]
- Lama, L.; Wilson, B.E.; Richard, R.T.; Way, M.O.; Reagan, T.E. Evaluation of resistance to the Mexican rice borer among commercial and experimental sugarcane cultivars, Beaumont, TX, 2016. Arthropod Manag. Tests 2018, 43, 2018. [Google Scholar] [CrossRef]
- VanWeelden, M.T.; Wilson, B.E.; Beuzelin, J.M.; Reagan, T.E.; Way, M.O. Yield response to Mexican rice borer (Lepidoptera: Crambidae) injury in bioenergy and conventional sugarcane and sorghum. J. Econ. Entomol. 2015, 108, 2296–2304. [Google Scholar] [CrossRef]
- VanWeelden, M.T.; Wilson, B.E.; Beuzelin, J.M.; Reagan, T.E.; Way, M.O. Impact of nitrogen fertilization on Mexican rice borer (Lepidoptera: Crambidae) injury and yield in bioenergy sorghum. Crop Prot. 2016, 84, 37–43. [Google Scholar] [CrossRef]
- Gravois, K.; Viator, H.; Reagan, T.E.; Beuzelin, J.M.; Griffin, J.; Tubana, B.; Hoy, J.W. Sugarcane Production Handbook 2014; Louisiana State University AgCenter publication #2859: Baton Rouge, LA, USA, 2014. [Google Scholar]
- Bessin, R.T.; Moser, E.B.; Reagan, T.E.; White, W.H. Analysis of percent bored internode data collected from sugarcane borer varietal resistance evaluations. Proc. Int. Soc. Sugar Cane Technol. 1990, 10, 8–22. [Google Scholar]
- Kenward, M.G.; Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 53, 983. [Google Scholar] [CrossRef]
- Martinez, A.J.; Bard, J.; Hollard, T.A. Mass Rearing Sugarcane Borer and Mexican Rice Borer for Production of Parasites Allorhogas pyphagus and Rhacontus rosilensis; USDA-APHIS-PPQ, APHIS 83-1: Riverdale Park, MD, USA, 1988.
- Sileshi, G. Selecting the right statistical model for analysis of insect count data by using information theoretic measures. Bull. Entomol. Res. 2006, 96, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Showler, A.T.; Castro, B.A. Mexican rice borer (Lepidoptera: Crambidae) oviposition site selection stimuli on sugarcane, and potential field applications. J. Econ. Entomol. 2010, 103, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- White, W.H. Movement and establishment of sugarcane borer (Lepidoptera: Pyralidae) larvae on resistant and susceptible sugarcane. Florida Entomol. 1993, 76, 465. [Google Scholar] [CrossRef]
- Butt, B.; Cantu, E. Sex Determination of Lepidopterous Pupae; Agricultural Research Service, U.S. Dept. of Agriculture: Washington, DC, USA, 1962.
- Pontif, M.; Kimbeng, C.; Gravois, K.; Bischoff, K.; Sexton, D.; LaBorde, C.; Hawkins, G.; Hoy, J.; Baisakh, N.; Wilson, B.; et al. Registration of ‘L 12-201’ sugarcane. J. Plant Regist. 2022, 16, 363–377. [Google Scholar] [CrossRef]
- Sosa, O. Pubescence in sugarcane as a plant resistance character affecting oviposition and mobility by the sugarcane borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 1988, 81, 663–667. [Google Scholar] [CrossRef]
- Sosa, O. Oviposition preference by the sugarcane borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 1990, 83, 866–868. [Google Scholar] [CrossRef]
- Vanderzant, E.S. The amino acid requirements of the pink bollworm. J. Econ. Entomol. 1958, 51, 309–311. [Google Scholar] [CrossRef]
- Moerman, R.; Vanderplanck, M.; Roger, N.; Declèves, S.; Wathelet, B.; Rasmont, P.; Fournier, D.; Michez, D. Growth rate of bumblebee larvae is related to pollen amino acids. J. Econ. Entomol. 2015, 109, 25–30. [Google Scholar] [CrossRef]
- Vanderzant, E.S. Nutrition of the adult boll weevil: Oviposition on defined diets and amino acid requirements. J. Ins. Phys. 1963, 9, 683–691. [Google Scholar] [CrossRef]
- Van Leerdam, M.B. Bionomics of Eoreuma loftini, a Pyralid Stalk Borer of Sugarcane. Ph.D. Dissertation, Texas A&M University, College Station, TX, USA, 1986. Available online: https://www.proquest.com/dissertations-theses/bionomics-eoreuma-loftini-pyralid-stalk-borer/docview/303562008/se-2 (accessed on 31 August 2022).
- Leuck, D.B.; Perkins, W.D. A method of estimating fall armyworm progeny reduction when evaluating control achieved by host-plant resistance. J. Econ. Entomol. 1972, 65, 482–483. [Google Scholar] [CrossRef]
- Jallow, M.F.A.; Zalucki, M.P. Relationship between oviposition preference and offspring performance in Australian Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Austr. J. Ent. 2003, 42, 343–348. [Google Scholar] [CrossRef]
- Zhou, M.M.; Kimbeng, C.A.; da Silva, J.A.; White, W.H. Cross-resistance between the Mexican rice borer and the sugarcane borer (Lepidoptera: Crambidae): A case study using sugarcane breeding populations. Crop Sci. 2010, 50, 861–869. [Google Scholar] [CrossRef] [Green Version]


| Cultivar | Percentage of Bored Internodes (LS Means ± SEM) a,* | Emergence Holes per Stalk (LS Means ± 0.10 [SE]) | Relative Survival (LS Means ± 0.584 [SE]) | Relative Resistance Ratio (LS Means ± 0.115 [SE]) | Resistance Category b |
|---|---|---|---|---|---|
| HoCP 09-840 | 5.7 ± 1.0 a | 0.09 | 0.096 | 0.675 | Susceptible |
| HoCP 04-838 | 3.5 ± 0.7 ab | 0.16 | 0.199 | 0.667 | Susceptible |
| HoCP 91-555 | 3.4 ± 0.7 ab | 0.12 | 0.144 | 0.600 | Intermediate |
| HoCP 00-950 | 3.8 ± 0.8 ab | 0.28 | 0.222 | 0.600 | Intermediate |
| HoCP 96-540 | 2.7 ± 0.6 bc | 0.13 | 0.232 | 0.533 | Intermediate |
| Ho 07-613 | 2.4 ± 0.5 bc | 0.16 | 0.172 | 0.458 | Intermediate |
| Ho 95-988 | 3.2 ± 0.7 ab | 0.07 | 0.085 | 0.492 | Intermediate |
| L 01-226 | 3.2 ± 0.7 ab | 0.08 | 0.067 | 0.450 | Intermediate |
| N-21 | 2.4 ± 0.6 bcd | 0.03 | 0.073 | 0.433 | Intermediate |
| L 01-299 | 1.0 ± 0.3 cd | 0.04 | 0.200 | 0.442 | Intermediate |
| HoCP 09-804 | 1.8 ± 0.4 bcd | 0.08 | 0.096 | 0.358 | Resistant |
| HoCP 85-845 | 0.7 ± 0.3 d | 0.01 | 0.100 | 0.242 | Resistant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado, L.D.; Wilson, B.E.; Penn, H.J.; Richard, R.T.; Way, M.O. Characterization of Resistance to the Mexican Rice Borer (Lepidoptera: Crambidae) among Sugarcane Cultivars. Insects 2022, 13, 890. https://doi.org/10.3390/insects13100890
Salgado LD, Wilson BE, Penn HJ, Richard RT, Way MO. Characterization of Resistance to the Mexican Rice Borer (Lepidoptera: Crambidae) among Sugarcane Cultivars. Insects. 2022; 13(10):890. https://doi.org/10.3390/insects13100890
Chicago/Turabian StyleSalgado, Leonardo D., Blake E. Wilson, Hannah J. Penn, Randy T. Richard, and Michael O. Way. 2022. "Characterization of Resistance to the Mexican Rice Borer (Lepidoptera: Crambidae) among Sugarcane Cultivars" Insects 13, no. 10: 890. https://doi.org/10.3390/insects13100890





