Using Trait-Based Approaches to Assess the Response of Epedaphic Collembola to Organic Matter Management Practices: A Case Study in a Rubber Plantation in South-Eastern Côte d’Ivoire
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
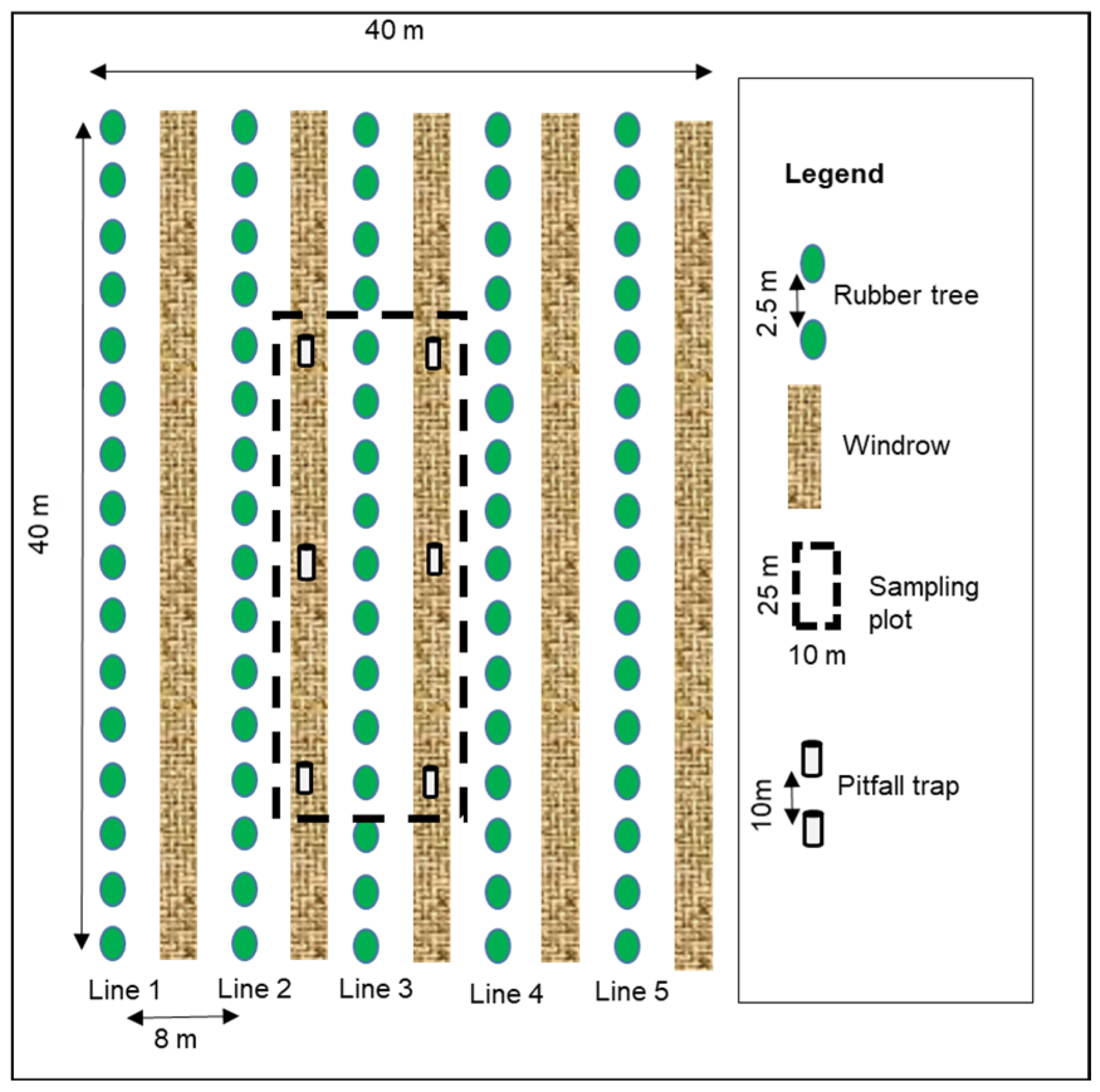
2.2. Experimental Design
- −
- R0L0: all rubber residues (R) removed from the plot, no legume (L);
- −
- R0L1: all rubber residues removed from the plot, legume (Pueraria phaseoloides);
- −
- R1L1: stumps, fine branches (less than 20 cm diameter) and leaves from the previous plantation left in the inter-row, legume (Pueraria phaseoloides);
- −
- R2L1: no rubber tree residues removed (leaves, trunk and stumps left), legume (Pueraria phaseoloides).
2.3. Sample Collection
2.4. Measurement of the Springtails Traits
- −
- The functional richness (FRic) corresponds to the volume of functional space occupied by species (abundance is not involved);
- −
- The functional divergence (FDiv) corresponds to a degree of niche differentiation among species within communities;
- −
- The functional evenness (FEve) measures the regularity of the distribution of abundance in functional space;
- −
- The functional dispersion (FDis) is a “pure” estimator of the dispersion in trait combination abundances.
2.5. Statistical Analysis
3. Results
3.1. Variation of Total Springtails Abundance with Amount of Organic Matter
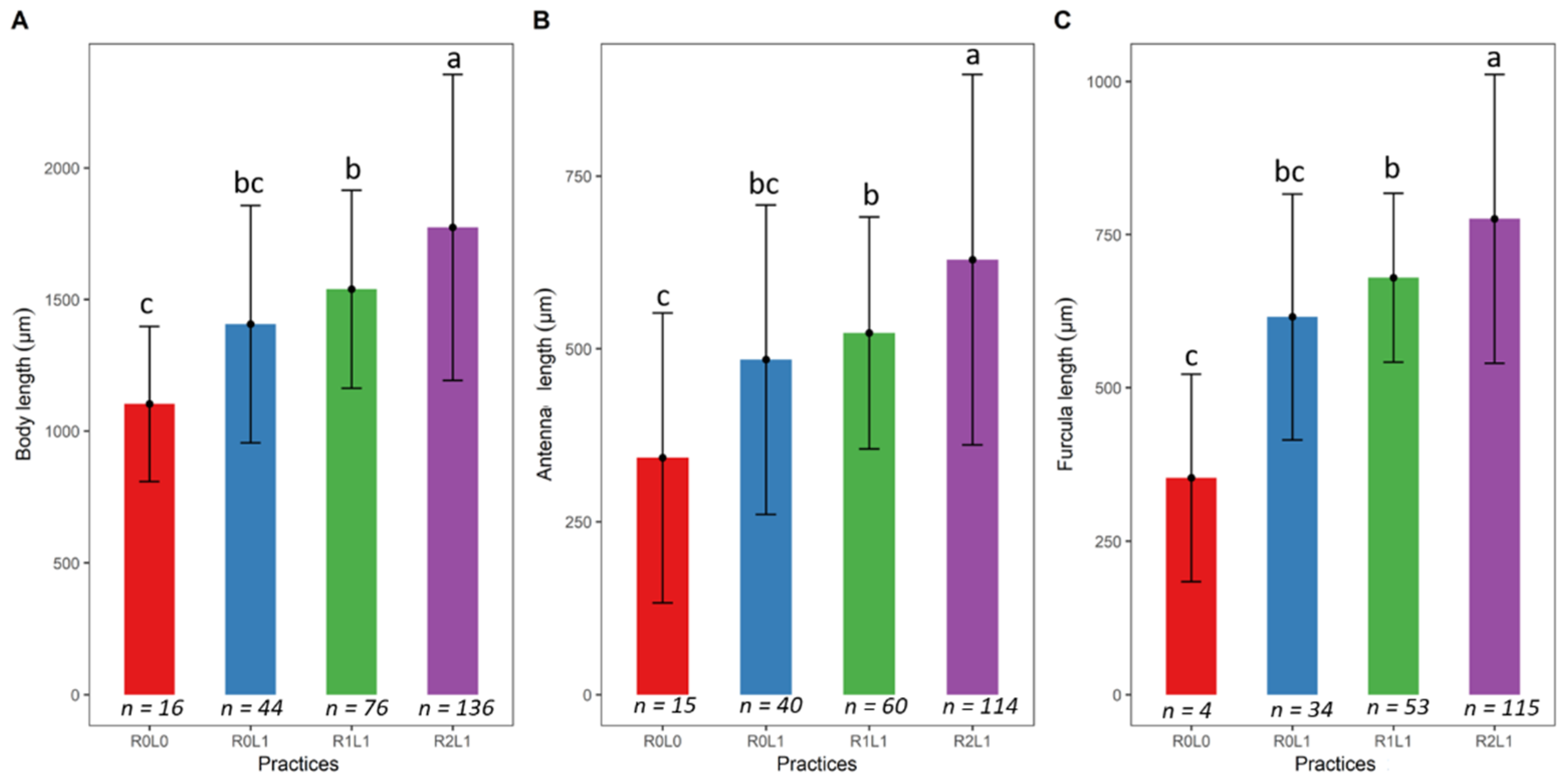
3.2. Springtails Size Response to Logging Residue and Legume Input
3.3. Response of the Functional Diversity Indices of Springtails to Logging Residues and Legumes
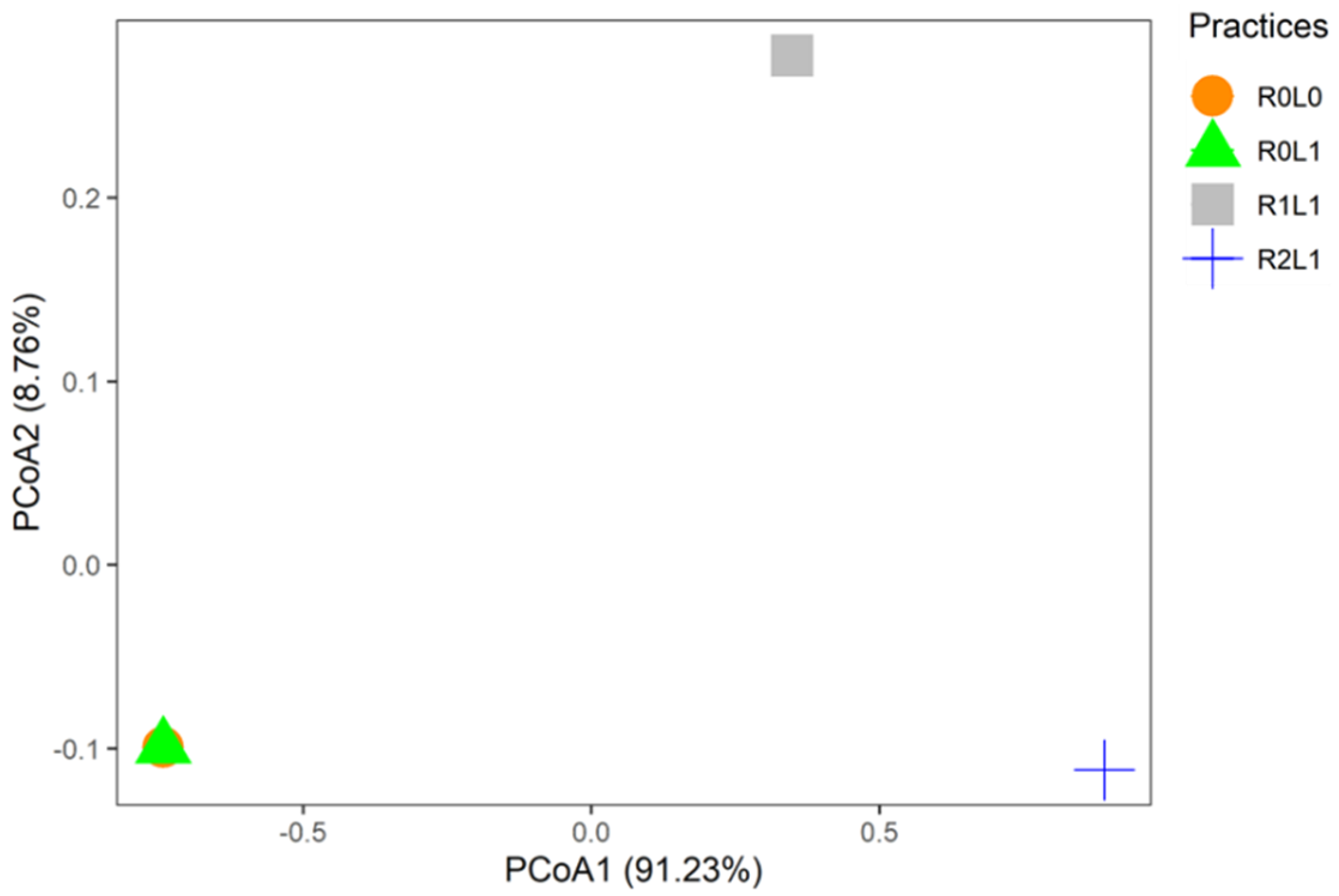
3.4. Response of the Functional Composition (CWM) of Springtails to the Organic Matter Gradient
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global Effects of Land Use on Local Terrestrial Biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Tolimir, M.; Kresović, B.; Životić, L.; Dragović, S.; Dragović, R.; Sredojević, Z.; Gajić, B. The Conversion of Forestland into Agricultural Land without Appropriate Measures to Conserve SOM Leads to the Degradation of Physical and Rheological Soil Properties. Sci. Rep. 2020, 10, 13668. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.K.; Angui, P.K.T.; Konaté, S.; Tondoh, J.E.; Tano, Y.; Abbadie, L.; Benest, D. Effects of Land Use Types on Soil Organic Carbon and Nitrogen Dynamics in Mid-West Côte d’Ivoire. Eur. J. Sci. Res. 2010, 40, 211–222. [Google Scholar]
- Kouadio, K.; Doumbia, M.; Jan, K.; Dagnogo, M.; Aidara, D. Soil/Litter Beetle Abundance and Diversity along a Land Use Gradient in Tropical Africa (Oumé, Ivory Coast). Sci. Nat. 2009, 6, 139–147. [Google Scholar] [CrossRef]
- Yeo, K.; Konaté, S.; Tiho, S.S.; Camara, S.K. Impacts of Land Use Types on Ant Communities in a Tropical Forest Margin (Oumé–Côte d’Ivoire). Afr. J. Agric. Res. 2011, 6, 260–274. [Google Scholar] [CrossRef]
- Gilot, C.; Lavelle, P.; Blanchart, E.; Keli, J.; Kouassi, P.; Guillaume, G. Biological Activity of Soil under Rubber Plantations in Côte d’Ivoire. Acta Zool. Fenn. 1995, 196, 186–189. [Google Scholar]
- N’Dri, J.K.; N’Guessan, K.K. Modification of Topsoil Physico-Chemical Characteristics and Macroinvertebrates Structure Consecutive to the Conversion of Secondary Forests into Rubber Plantations in Grand-Lahou, Côte d’Ivoire. J. Adv. Agric. 2018, 8, 1235–1255. [Google Scholar] [CrossRef]
- Tondoh, J.E.; Dimobe, K.; Guéi, A.M.; Adahe, L.; Baidai, Y.; N’Dri, J.K.; Forkuor, G. Soil Health Changes Over a 25-Year Chronosequence from Forest to Plantations in Rubber Tree (Hevea Brasiliensis) Landscapes in Southern Côte d’Ivoire: Do Earthworms Play a Role? Front. Environ. Sci. 2019, 7, 73. [Google Scholar] [CrossRef]
- Oku, E.; Iwara, A.; Ekukinam, E. Effects of Age of Rubber (Hevea Brasiliensis Muell Arg.) Plantations on PH, Organic Carbon, Organic Matter, Nitrogen and Micronutrient Status of Ultisols in the Humid Forest Zone of Nigeria. Kasetsart J. 2012, 46, 684–693. [Google Scholar]
- Panklang, P.; Thoumazeau, A.; Chiarawipa, R.; Sdoodee, S.; Sebag, D.; Gay, F.; Thaler, P.; Brauman, A. Rubber, Rubber and Rubber: How 75 Years of Successive Rubber Plantation Rotations Affect Topsoil Quality? Land Degrad. Dev. 2022, 33, 1159–1169. [Google Scholar] [CrossRef]
- Panklang, P.; Thaler, P.; Thoumazeau, A.; Chiarawipa, R.; Sdoodee, S.; Brauman, A. How 75 Years of Rubber Monocropping Affects Soil Fauna and Nematodes as the Bioindicators for Soil Biodiversity Quality Index. Acta Agric. Scand. Sect. B Soil Plant Sci. 2022, 72, 612–622. [Google Scholar] [CrossRef]
- Perron, T.; Kouakou, A.; Simon, C.; Mareschal, L.; Frédéric, G.; Soumahoro, M.; Kouassi, D.; Rakotondrazafy, N.; Rapidel, B.; Laclau, J.-P.; et al. Logging Residues Promote Rapid Restoration of Soil Health after Clear-Cutting of Rubber Plantations at Two Sites with Contrasting Soils in Africa. Sci. Total Environ. 2022, 816, 151526. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.D.; Allen, K.; Kreft, H.; Corre, M.D.; Jochum, M.; Veldkamp, E.; Clough, Y.; Daniel, R.; Darras, K.; Denmead, L.H.; et al. Direct and Cascading Impacts of Tropical Land-Use Change on Multi-Trophic Biodiversity. Nat. Ecol. Evol. 2017, 1, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.P.; Pierrat, M.; Snoeck, D.; Villenave, C.; Ribeyre, F.; Suhardi; Marichal, R.; Caliman, J.P. Temporal Variability in Soil Quality after Organic Residue Application in Mature Oil Palm Plantations. Soil Res. 2015, 53, 205. [Google Scholar] [CrossRef]
- George, P.B.L.; Keith, A.M.; Creer, S.; Barrett, G.L.; Lebron, I.; Emmett, B.A.; Robinson, D.A.; Jones, D.L. Evaluation of Mesofauna Communities as Soil Quality Indicators in a National-Level Monitoring Programme. Soil Biol. Biochem. 2017, 115, 537–546. [Google Scholar] [CrossRef]
- Kaneda, S.; Kaneko, N. Influence of Collembola on Nitrogen Mineralization Varies with Soil Moisture Content. Soil Sci. Plant Nutr. 2011, 57, 40–49. [Google Scholar] [CrossRef]
- Filser, J. The Role of Collembola in Carbon and Nitrogen Cycling in Soil: Proceedings of the Xth International Colloquium on Apterygota, České Budějovice 2000: Apterygota at the Beginning of the Third Millennium. Pedobiologia 2002, 46, 234–245. [Google Scholar] [CrossRef]
- Cortet, J.; Joffre, R.; Elmholt, S.; Krogh, P.H. Increasing Species and Trophic Diversity of Mesofauna Affects Fungal Biomass, Mesofauna Community Structure and Organic Matter Decomposition Processes. Biol. Fertil. Soils 2003, 37, 302–312. [Google Scholar] [CrossRef]
- Joimel, S.; Schwartz, C.; Hedde, M.; Kiyota, S.; Krogh, P.H.; Nahmani, J.; Pérès, G.; Vergnes, A.; Cortet, J. Urban and Industrial Land Uses Have a Higher Soil Biological Quality than Expected from Physicochemical Quality. Sci. Total Environ. 2017, 584–585, 614–621. [Google Scholar] [CrossRef]
- Joimel, S.; Schwartz, C.; Bonfanti, J.; Hedde, M.; Krogh, P.H.; Pérès, G.; Pernin, C.; Rakoto, A.; Salmon, S.; Santorufo, L.; et al. Functional and Taxonomic Diversity of Collembola as Complementary Tools to Assess Land Use Effects on Soils Biodiversity. Front. Ecol. Evol. 2021, 9, 630919. [Google Scholar] [CrossRef]
- de Filho, L.C.I.O.; Filho, O.K.; Baretta, D.; Tanaka, C.A.S.; Sousa, J.P. Collembola Community Structure as a Tool to Assess Land Use Effects on Soil Quality. Rev. Bras. Ciênc. Solo 2016, 40, e0150432. [Google Scholar] [CrossRef]
- Chang, L.; Wu, H.; Wu, D.; Sun, X. Effect of Tillage and Farming Management on Collembola in Marsh Soils. Appl. Soil Ecol. 2013, 64, 112–117. [Google Scholar] [CrossRef]
- Coulibaly, S.F.M.; Coudrain, V.; Hedde, M.; Brunet, N.; Mary, B.; Recous, S.; Chauvat, M. Effect of Different Crop Management Practices on Soil Collembola Assemblages: A 4-Year Follow-Up. Appl. Soil Ecol. 2017, 119, 354–366. [Google Scholar] [CrossRef]
- de Oliveira Filho, L.C.I.; Zeppelini, D.; Sousa, J.P.; Baretta, D.; Klauberg-Filho, O. Collembola Community Structure under Different Land Management in Subtropical Brazil. Ann. Appl. Biol. 2020, 177, 294–307. [Google Scholar] [CrossRef]
- Kontschán, J. A Second Species of the Family Eutrachytidae (Acari: Uropodina) in Africa: Mahnertellina Paradoxa Gen. Nov., Sp. Nov. from the Ivory Coast. Rev. Suisse Zool. 2020, 127, 75–81. [Google Scholar] [CrossRef]
- UNEP-WCMC. The State of Biodiversity in Africa: A Mid-Term Review of Progress towards the Aichi Biodiversity Targets; United Nations Environment Programme; UNEP-WCMC: Cambridge, UK, 2016; ISBN 978-92-807-3508-6. [Google Scholar]
- Korb, J.; Kasseney, B.D.; Cakpo, Y.T.; Casalla Daza, R.H.; Gbenyedji, J.N.K.B.; Ilboudo, M.E.; Josens, G.; Koné, N.A.; Meusemann, K.; Ndiaye, A.B.; et al. Termite Taxonomy, Challenges and Prospects: West Africa, A Case Example. Insects 2019, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, I.C.; Adam, C.; Yao, P.K.; Hilare, Y.-B.; Chișamera, G.B.; D’Amico, G.; Gherman, C.M.; Mihalca, A.D.; Sándor, A.D. Descriptions of Two New Species of Feather Mites (Acarina: Psoroptidia: Pteronyssidae) from Ivory Coast. Syst. Parasitol. 2018, 95, 281–292. [Google Scholar] [CrossRef]
- Gómez, K.; Kouakou, L.M.; Fischer, G.; Hita-Garcia, F.; Katzke, J.; Economo, E.P. Pheidole klaman Sp. Nov.: A New Addition from Ivory Coast to the Afrotropical Pulchella Species Group (Hymenoptera, Formicidae, Myrmicinae). ZooKeys 2022, 1104, 129–157. [Google Scholar] [CrossRef]
- Kontschán, J.; Ermilov, S.G. The Second Species of the Genus Ivoria Kontschán, 2019: Description of Ivoriaalourouai Sp. Nov. from Ivory Coast (Acari, Mesostigmata, Urodinychidae). Zookeys 2022, 1082, 63–71. [Google Scholar] [CrossRef]
- Kontschán, J. Ivoria taiensis Gen. Nov., Sp. Nov., a Remarkable New Mite Genus from West Africa (Acari: Mesostigmata: Urodinychidae). Syst. Appl. Acarol. 2019, 24, 1063–1070. [Google Scholar] [CrossRef]
- Zon, D.S.; Thibaud, J.-M.; Yao, T. Stages of the Knowledge of the Collembolans of Ivory Coast (Western Africa) (Collembola). Russ. Entomol. J. 2013, 22, 91–96. [Google Scholar]
- Zon, S.D.; Bedos, A.; D’Haese, C.A. Phylogeny of the Genus Willemia (Collembola: Hypogastruridae) and Biogeography of the W. Buddenbrocki-Group with Description of a New Species from Ivory Coast (Western Africa). Zootaxa 2015, 3980, 230. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Pey, B.; Nahmani, J.; Auclerc, A.; Capowiez, Y.; Cluzeau, D.; Cortet, J.; Decaëns, T.; Deharveng, L.; Dubs, F.; Joimel, S.; et al. Current Use of and Future Needs for Soil Invertebrate Functional Traits in Community Ecology. Basic Appl. Ecol. 2014, 15, 194–206. [Google Scholar] [CrossRef] [Green Version]
- Moretti, M.; Dias, A.T.C.; de Bello, F.; Altermatt, F.; Chown, S.L.; Azcárate, F.M.; Bell, J.R.; Fournier, B.; Hedde, M.; Hortal, J.; et al. Handbook of Protocols for Standardized Measurement of Terrestrial Invertebrate Functional Traits. Funct. Ecol. 2017, 31, 558–567. [Google Scholar] [CrossRef]
- Reis, F.; Carvalho, F.; Martins da Silva, P.; Mendes, S.; Santos, S.A.P.; Sousa, J.P. The Use of a Functional Approach as Surrogate of Collembola Species Richness in European Perennial Crops and Forests. Ecol. Indic. 2016, 61, 676–682. [Google Scholar] [CrossRef]
- Sanabria, C.; Barot, S.; Fonte, S.J.; Dubs, F. Do Morphological Traits of Ground-Dwelling Ants Respond to Land Use Changes in a Neotropical Landscape? Geoderma 2022, 418, 115841. [Google Scholar] [CrossRef]
- Querner, P.; Bruckner, A. Combining Pitfall Traps and Soil Samples to Collect Collembola for Site Scale Biodiversity Assessments. Appl. Soil Ecol. 2010, 45, 293–297. [Google Scholar] [CrossRef]
- Driessen, M.M.; Greenslade, P. Effect of Season, Location and Fire on Collembola Communities in Buttongrass Moorlands, Tasmania. Pedobiologia 2004, 48, 631–642. [Google Scholar] [CrossRef]
- Potapov, A.A.; Semenina, E.E.; Korotkevich, A.Y.; Kuznetsova, N.A.; Tiunov, A.V. Connecting Taxonomy and Ecology: Trophic Niches of Collembolans as Related to Taxonomic Identity and Life Forms. Soil Biol. Biochem. 2016, 101, 20–31. [Google Scholar] [CrossRef]
- Widenfalk, L.A.; Bengtsson, J.; Berggren, Å.; Zwiggelaar, K.; Spijkman, E.; Huyer-Brugman, F.; Berg, M.P. Spatially Structured Environmental Filtering of Collembolan Traits in Late Successional Salt Marsh Vegetation. Oecologia 2015, 179, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Salmon, S.; Ponge, J.F. Species Traits and Habitats in Springtail Communities: A Regional Scale Study. Pedobiologia 2012, 55, 295–301. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 27 July 2022).
- Laliberté, E.; Legendre, P. A Distance-Based Framework for Measuring Functional Diversity from Multiple Traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-444-53869-7. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; Wagner, H. Community Ecology Package; Version 2.5–4, R Package; Vegan, 2019. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Chauvat, M.; Wolters, V.; Dauber, J. Response of Collembolan Communities to Land-Use Change and Grassland Succession. Ecography 2007, 30, 183–192. [Google Scholar] [CrossRef]
- Potapov, A.M.; Goncharov, A.A.; Semenina, E.E.; Korotkevich, A.Y.; Tsurikov, S.M.; Rozanova, O.L.; Anichkin, A.E.; Zuev, A.G.; Samoylova, E.S.; Semenyuk, I.I.; et al. Arthropods in the Subsoil: Abundance and Vertical Distribution as Related to Soil Organic Matter, Microbial Biomass and Plant Roots. Eur. J. Soil Biol. 2017, 82, 88–97. [Google Scholar] [CrossRef]
- Harta, I.; Simon, B.; Vinogradov, S.; Winkler, D. Collembola Communities and Soil Conditions in Forest Plantations Established in an Intensively Managed Agricultural Area. J. For. Res. 2021, 32, 1819–1832. [Google Scholar] [CrossRef]
- Trentini, C.P.; Villagra, M.; Gómez Pámies, D.; Bernava Laborde, V.; Bedano, J.C.; Campanello, P.I. Effect of Nitrogen Addition and Litter Removal on Understory Vegetation, Soil Mesofauna, and Litter Decomposition in Loblolly Pine Plantations in Subtropical Argentina. For. Ecol. Manag. 2018, 429, 133–142. [Google Scholar] [CrossRef]
- Trentini, C.P.; Campanello, P.I.; Villagra, M.; Ritter, L.; Ares, A.; Goldstein, G. Thinning of Loblolly Pine Plantations in Subtropical Argentina: Impact on Microclimate and Understory Vegetation. For. Ecol. Manag. 2017, 384, 236–247. [Google Scholar] [CrossRef]
- Yu, D.; Yao, J.; Chen, X.; Sun, J.; Wei, Y.; Cheng, Y.; Hu, F.; Liu, M. Ecological Intensification Alters the Trait-Based Responses of Soil Microarthropods to Extreme Precipitation in Agroecosystem. Geoderma 2022, 422, 115956. [Google Scholar] [CrossRef]
- Tao, H.-H.; Snaddon, J.L.; Slade, E.M.; Henneron, L.; Caliman, J.-P.; Willis, K.J. Application of Oil Palm Empty Fruit Bunch Effects on Soil Biota and Functions: A Case Study in Sumatra, Indonesia. Agric. Ecosyst. Environ. 2018, 256, 105–113. [Google Scholar] [CrossRef]
- Jureková, N.; Raschmanová, N.; Miklisová, D.; Kováč, Ľ. A Comparison of Collecting Methods in Relation to the Diversity of Collembola in Scree Habitats. Subterr. Biol. 2021, 40, 1–26. [Google Scholar] [CrossRef]
- Woodward, G.; Hildrew, A.G. Body-Size Determinants of Niche Overlap and Intraguild Predation within a Complex Food Web. J. Anim. Ecol. 2002, 71, 1063–1074. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional Richness, Functional Evenness and Functional Divergence: The Primary Components of Functional Diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Susanti, W.I.; Widyastuti, R.; Scheu, S.; Potapov, A. Trophic Niche Differentiation and Utilisation of Food Resources in Collembola Is Altered by Rainforest Conversion to Plantation Systems. PeerJ 2021, 9, e10971. [Google Scholar] [CrossRef]
- Susanti, W.I.; Bartels, T.; Krashevska, V.; Widyastuti, R.; Deharveng, L.; Scheu, S.; Potapov, A. Conversion of Rainforest into Oil Palm and Rubber Plantations Affects the Functional Composition of Litter and Soil Collembola. Ecol. Evol. 2021, 11, 10686–10708. [Google Scholar] [CrossRef]
- Bellino, A.; Baldantoni, D.; Milano, V.; Santorufo, L.; Cortet, J.; Maisto, G. Spatial Patterns and Scales of Collembola Taxonomic and Functional Diversity in Urban Parks. Sustainability 2021, 13, 13029. [Google Scholar] [CrossRef]
- Fujii, S.; Berg, M.P.; Cornelissen, J.H.C. Living Litter: Dynamic Trait Spectra Predict Fauna Composition. Trends Ecol. Evol. 2020, 35, 886–896. [Google Scholar] [CrossRef]
- Sayer, E.J.; Tanner, E.V.J.; Lacey, A.L. Effects of Litter Manipulation on Early-Stage Decomposition and Meso-Arthropod Abundance in a Tropical Moist Forest. For. Ecol. Manag. 2006, 229, 285–293. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroğlu, T.; Roy, R.; Seleiman, M.F.; Ozlu, E.; Battaglia, M.L.; Uslu, Ö.S. Chapter 9—Relationship between Organic Matter and Microbial Biomass in Different Vegetation Types. In Microbial Syntrophy-Mediated Eco-enterprising; Developments in Applied Microbiology and Biotechnology; Pratap Singh, R., Manchanda, G., Bhattacharjee, K., Panosyan, H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 225–245. ISBN 978-0-323-99900-7. [Google Scholar]
- Coulibaly, S.F.M.; Winck, B.R.; Akpa-Vinceslas, M.; Mignot, L.; Legras, M.; Forey, E.; Chauvat, M. Functional Assemblages of Collembola Determine Soil Microbial Communities and Associated Functions. Front. Environ. Sci. 2019, 7, 52. [Google Scholar] [CrossRef] [Green Version]


| Trait | Attribute |
|---|---|
| Body length | µm |
| Body modification | Body not modified |
| Abdomen IV elongated | |
| Spherical body | |
| Furca length | µm |
| Dens | Short |
| Whip-shaped | |
| Long cylindric | |
| Mucro | Very small straight |
| Blade-like straight Bidentate Tridentate | |
| Antenna length | µm |
| Ocelli | 4 or 5 pairs of ocelli |
| 6, 7 or 8 pairs of ocelli | |
| Post Antennal Organ | Absent |
| Present | |
| Pigmentation | Absent |
| Diffuse | |
| Intense | |
| Pattern | |
| Scales | Absent |
| Present | |
| Empodial appendage | Absent |
| Present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouakou, A.K.; Cortet, J.; Kolo, Y.; Brauman, A. Using Trait-Based Approaches to Assess the Response of Epedaphic Collembola to Organic Matter Management Practices: A Case Study in a Rubber Plantation in South-Eastern Côte d’Ivoire. Insects 2022, 13, 892. https://doi.org/10.3390/insects13100892
Kouakou AK, Cortet J, Kolo Y, Brauman A. Using Trait-Based Approaches to Assess the Response of Epedaphic Collembola to Organic Matter Management Practices: A Case Study in a Rubber Plantation in South-Eastern Côte d’Ivoire. Insects. 2022; 13(10):892. https://doi.org/10.3390/insects13100892
Chicago/Turabian StyleKouakou, Aymard Kouakou, Jérôme Cortet, Yeo Kolo, and Alain Brauman. 2022. "Using Trait-Based Approaches to Assess the Response of Epedaphic Collembola to Organic Matter Management Practices: A Case Study in a Rubber Plantation in South-Eastern Côte d’Ivoire" Insects 13, no. 10: 892. https://doi.org/10.3390/insects13100892
APA StyleKouakou, A. K., Cortet, J., Kolo, Y., & Brauman, A. (2022). Using Trait-Based Approaches to Assess the Response of Epedaphic Collembola to Organic Matter Management Practices: A Case Study in a Rubber Plantation in South-Eastern Côte d’Ivoire. Insects, 13(10), 892. https://doi.org/10.3390/insects13100892






