Effect of Male Body Size on Female Reproduction in Pyrrhocoris apterus (L.) (Heteroptera, Pyrrhocoridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
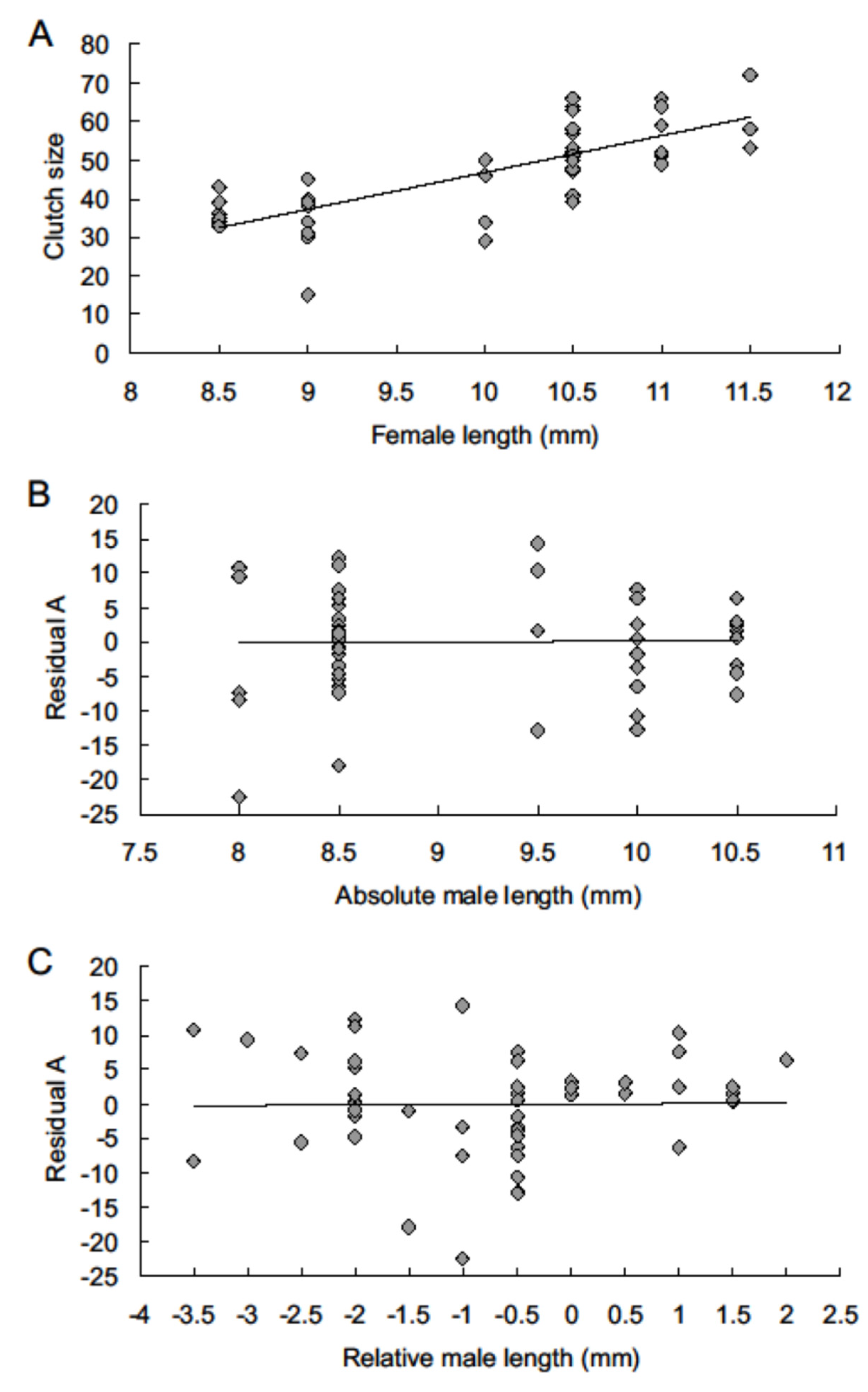
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Garcia-Barros, E. Body size, egg size, and their interspecific relationships with ecological and life history traits in butterflies (Lepidoptera : Papilionoidea, Hesperioidea). Biol. J. Linn. Soc. 2000, 70, 251–284. [Google Scholar] [CrossRef]
- Chown, S.L.; Gaston, K.J. Body size variation in insects: A macroecological perspective. Biol. Rev. 2010, 85, 139–169. [Google Scholar] [CrossRef] [PubMed]
- Quiring, D.T.; McNeill, J.N. Influence of intraspecific larval competition and mating on the longevity and reproductive performance of females of the leaf miner Agromyza frontella (Rondani) (Diptera, Agromyzidae). Can. J. Zool. 1984, 62, 2197–2200. [Google Scholar] [CrossRef]
- Nasci, R.S. The size of emerging and host seeking Aedes aegypti and the relation of size to blood feeding success in the field. J. Am. Mosq. Control Assoc. 1986, 2, 61–62. [Google Scholar]
- Lavadinho, A.M.P. Toxicological studies on adult Sitophilus granarius (L.)—Influence of indiidual body weight on susceptibility to DDT and Malathion. J. Stored Prod. Res. 1976, 12, 215–224. [Google Scholar] [CrossRef]
- Harvey, G.T. Egg weight as a factor in the overwintering survival of spruce budworm (Lepidoptera: Tortricidae) larvae. Can. Entomol. 1985, 117, 1451–1461. [Google Scholar] [CrossRef]
- Kidd, N.A.C.; Cleaver, A.M. The control of migratory urge in Aphis fabae Scopoli (Hemiptera, Aphididae). Bull. Entomol. Res. 1986, 76, 77–87. [Google Scholar] [CrossRef]
- Tsuji, J.S.; Kingsolver, J.G.; Watt, W.B. Thermal physiological ecology of Colias butterflies in flight. Oecologia 1986, 69, 161–170. [Google Scholar] [CrossRef]
- Bennettova, B.; Fraenkel, G. What determines the number of ovarioles in a fly ovary? J. Insect Physiol. 1981, 27, 403–410. [Google Scholar] [CrossRef]
- Injeyan, H.S.; Tobe, S.S. Phase polymorphism in Schistocerca gregaria: Reproductive parameters. J. Insect Physiol. 1981, 27, 97–102. [Google Scholar] [CrossRef]
- Leather, S.R.; Wellings, P.W. Ovariole number and fecundity in aphids. Entomol. Exp. Appl. 1981, 30, 128–133. [Google Scholar] [CrossRef]
- Schlein, Y. Ultraviolet light and puparial weight as factors in autogeny of fleshfly, Sarcophaga falculata. J. Insect Physiol. 1977, 23, 961–964. [Google Scholar] [CrossRef]
- Griffiths, D. Phenology and larval-adult size relations in the ant-lion Macroleon quinquemaculatus. J. Anim. Ecol. 1985, 54, 573–581. [Google Scholar] [CrossRef]
- Kimura, T.; Tsubaki, Y. Female size and age-specific fecundity in the small white butterfly, Pieris rapae crucivora Boisduval (Lepidoptera, Pieridae). Res. Popul. Ecol. 1986, 28, 295–304. [Google Scholar] [CrossRef]
- Iga, M. Mating preferences of Dacus dorsalis Hendel (Diptera: Tephritidae) with reference to individual variations in size. Japanese J. Appl. Entomol. 1981, 25, 292–294. [Google Scholar] [CrossRef]
- Honek, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- Murdie, G. The biological consequences of decreased size caused by crowding or rearing temperatures in apterae of the pea aphid, Acyrthosiphon pisum Harris. Trans. Ent. Soc. Lond. 1969, 121, 443–455. [Google Scholar] [CrossRef]
- Wedell, N. Ejaculate size in bushcrickets: The importance of being large. J. Evol. Biol. 1997, 10, 315–325. [Google Scholar] [CrossRef]
- Boivin, G.; Martel, V. Size-induced reproductive constraints in an egg parasitoid. J. Insect Physiol. 2012, 58, 1694–1700. [Google Scholar] [CrossRef]
- Hatala, A.J.; Harrington, L.C.; Degner, E.C. Age and body size influence sperm quantity in male Aedes albopictus (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 2018, 55, 1051–1054. [Google Scholar] [CrossRef] [Green Version]
- Brown, W.D. Female remating and the intensity of female choice in black-horned tree crickets, Oecanthus nigricornis. Behav. Ecol. 1997, 8, 66–74. [Google Scholar] [CrossRef]
- South, A.; Lewis, S.M. The influence of male ejaculate quantity on female fitness: A meta-analysis. Biol. Rev. 2011, 86, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Savalli, U.M.; Czesak, M.E.; Fox, C.W. Paternal investment in the seed beetle Callosobruchus maculatus (Coleoptera: Bruchidae): Variation among populations. Ann. Entomol. Soc. Am. 2000, 93, 1173–1178. [Google Scholar] [CrossRef]
- Singh, P.; Mishra, G.; Omkar. Influence of body size and familiarity on mating and reproductive parameters in the zig-zag ladybird beetle, Menochilus sexmaculatus (Coleoptera: Coccinellidae). Can. J. Zool. 2019, 97, 453–463. [Google Scholar] [CrossRef]
- McLain, D.K. Behavioral and morphological correlates of male dominance and courtship persistence in the blister beetle Epicauta pennsylvanica (Coleoptera: Meloidae). Am. Midl. Nat. 1982, 107, 396–403. [Google Scholar] [CrossRef]
- O’Neill, K.M. The significance of body size in territorial interactions of male beewolves (Hymenoptera:Sphecidae, Philanthus). Anim. Behav. 1983, 31, 404–411. [Google Scholar] [CrossRef]
- Burk, T. Male-male interactionsin Caribbean fruit flies, Anasterpha suspensa (Loew) (Diptera, Tephritidae)—Territorial fights and signaling stimulation. Fla. Entomol. 1984, 67, 542–547. [Google Scholar] [CrossRef]
- McLain, D.K.; Boromisa, R.D. Male choice, fighting ability, assortative mating and the intensity of sexual selection in the milkweed longhorn beetle, Tetraopes tetraophthalmus (Coleoptera, Cerambycidae). Behav. Ecol. Sociobiol. 1987, 20, 239–246. [Google Scholar] [CrossRef]
- Frey, D. Resistance to mating by female monarch butterflies. In The 1997 North American Conference on the Monarch Butterfly; Hoth, J., Merino, L., Oberhauser, K., Pisanty, I., Price, S., Wilkinson, T.P., Eds.; Intercept Commision for Environmental Cooperation: Montreal, QC, Canada, 1999; pp. 79–87. [Google Scholar]
- Jorge, A.S.; Lomonaco, C. Body size, symmetry and courtship behavior of Dysdercus maurus Distant (Hemiptera: Pyrrhocoridae). Neotrop. Entomol. 2011, 40, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Conroy, L.P.; Gray, D.A. Male armaments and reproductive behavior in "Nutcracker" Camel Crickets (Rhaphidophoridae, Pristoceuthophilus). Insects 2015, 6, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K. Alternative mating strategies in the water strider Gerris elongatus (Heteroptera, Gerridae). Behav. Ecol. Sociobiol. 1985, 16, 301–306. [Google Scholar] [CrossRef]
- Montiglio, P.O.; Wey, T.W.; Chang, A.T.; Fogarty, S.; Sih, A. Multiple mating reveals complex patterns of assortative mating by personality and body size. J. Anim. Ecol. 2016, 85, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrest, T.G.; Sylvester, J.L.; Testa, S.; Smith, S.W.; Dinep, A.; Cupit, T.L.; Huggins, J.M.; Atkins, K.L.; Eubanks, M. Mate choice in ground crickets (Gryllidae, Nemoniidae). Fla. Entomol. 1991, 74, 74–80. [Google Scholar] [CrossRef]
- Hingle, A.; Fowler, K.; Pomiankowski, A. Size-dependent mate preference in the stalk-eyed fly Cyrtodiopsis dalmanni. Anim. Behav. 2001, 61, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Beeler, A.E.; Rauter, C.M.; Moore, A.J. Mate discrimination by females in the burying beetle Nicrophorus orbicollis: The influence of male size on attractiveness to females. Ecol. Entomol. 2002, 27, 1–6. [Google Scholar] [CrossRef]
- Watson, N.L.; Simmons, L.W. Mate choice in the dung beetle Onthophagus sagittarius: Are female horns ornaments? Behav. Ecol. 2010, 2, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Kosal, E.F.; Niedzlek-Feave, M. Female preferences for large, heavy mates in Schistocerca americana (Orthoptera: Acrididae). J. Insect Behav. 1997, 10, 711–725. [Google Scholar] [CrossRef]
- Fukaya, M.; Yasuda, T.; Akino, T.; Yasui, H.; Wakamura, S.; Fukuda, T.; Ogawa, Y. Effects of male body size on mating behavior and female mate refusal in the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2004, 39, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Cordeir, E.M.C.; Correa, A.S.; Rosi-Denadai, C.A.; Tome, H.V.V.; Guedes, R.N.C. Insecticide resistance and size assortative mating in females of the maize weevil (Sitophilus zeamais). Pest Manag. Sci. 2017, 73, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Capone, T.A. Mutual preference for large mates in green stink bugs, Acrosternum hilare (Hemiptera, Pentatomidae). Anim. Behav. 1995, 49, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Kristenova, M.; Exnerova, A.; Stys, P. Seed preferences of Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae): A re there specialized trophic populations? Eur. J. Entomol. 2011, 108, 581–586. [Google Scholar] [CrossRef]
- Honek, A. Body size and mating success in Pyrrhocoris apterus (Heteroptera). Eur. J. Entomol. 2003, 100, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Socha, R. Wing morph- and age-related differences in fertilization success of adult males of a flightless bug, Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). Eur. J. Entomol. 2008, 108, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Bangham, J.; Chapman, T.; Partridge, L. Effects of body size, accessory gland and testis size on pre- and postcopulatory success in Drosophila melanogaster. Anim. Behav. 2002, 64, 915–921. [Google Scholar] [CrossRef] [Green Version]
- Fairn, E.R.; Schulte-Hostedde, A.I.; Alarie, Y. Sexual selection on accessory glands, genitalia and protarsal pads in the whirligig beetle Dineutus nigrior Roberts (Coleoptera: Gyrinidae). Ethology 2007, 113, 257–266. [Google Scholar] [CrossRef]
- Lemmen, J.; Keddie, B.A.; Evenden, M.L. Size and protein content of accessory glands in adult male Caloptilia fraxinella in different physiological states. Physiol. Entomol. 2016, 41, 74–82. [Google Scholar] [CrossRef]
- Socha, R. Endocrine control of wing morph-related differences in mating success and accessory gland size in male firebugs. Anim. Behav. 2006, 71, 1273–1281. [Google Scholar] [CrossRef]
- Systat Software Inc. SigmaStat 3.5; Systat Software, Inc.: Point Richmond, CA, USA, 2006. [Google Scholar]
- Zdarek, J. Le comportement d’accouplement á la fin de la diapause imaginale et son controle hormonal dans le cas de la punaise Pyrrhocoris apterus L. (Pyrrhocoridae, Heteroptera). Ann. Endocrin. 1968, 29, 703–707. [Google Scholar]
- Socha, R. Decreased mating propensity of macropterous morph in a flightless wing-polymorphic insect, Pyrrhocoris apterus (Heteroptera). Eur. J. Entomol. 2004, 101, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Socha, R.; Zemek, R. Mating behaviour and wing morp-related differences in the sexual activity of a flightless bug, Pyrrhocoris apterus (L.) (Heteroptera). Ethol. Ecol. Evol. 2004, 16, 217–229. [Google Scholar] [CrossRef]
- Honek, A.; Martinkova, Z.; Brabec, M. Mating activity of Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae) in nature. Eur. J. Entomol. 2019, 116, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Honek, A.; Martinkova, Z. Behavioural thermoregulation hastens spring mating activity in Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). J. Therm. Biol. 2019, 84, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Honek, A. Body size and fecundity in natural populations of Pyrrhocoris apterus L. (Heteroptera, Pyrrhocoridae). Zool. Jahrb., Abt. Syst. Geogr. Biol. Tiere. 1986, 113, 125–140. [Google Scholar]
- Honek, A.; Hodek, I. Diapause of Chrysopa carnea (Chrysopidae: Neuroptera) females in the field. Věstník Československé Společnosti Zoologické 1973, 37, 95–100. [Google Scholar]
- Honek, A. Regulation of body size in a heteropteran bug, Pyrrhocoris apterus. Entomol. Exp. Appl. 1987, 44, 257–262. [Google Scholar] [CrossRef]
| 2004 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Male Size (mm) | |||||||||
| 8 | 8.5 | 9 | 9.5 | 10 | 10.5 | 11 | Sum | ||
| Female size (mm) | 8.5 | 3 | 1 | 1 | 1 | 6 | |||
| 9 | 2 | 8 | 1 | 4 | 3 | 18 | |||
| 9.5 | 0 | ||||||||
| 10 | 2 | 1 | 1 | 4 | |||||
| 10.5 | 12 | 1 | 7 | 20 | |||||
| 11 | 1 | 2 | 3 | 6 | |||||
| 11.5 | 2 | 1 | 3 | ||||||
| Sum | 5 | 27 | 0 | 4 | 12 | 9 | 0 | 57 | |
| 2022 | |||||||||
| Male Size (mm) | |||||||||
| 8 | 8.5 | 9 | 9.5 | 10 | 10.5 | 11 | Sum | ||
| Female size (mm) | 8.5 | 2 | 1 | 2 | 1 | 6 | |||
| 9 | 9 | 9 | |||||||
| 9.5 | 1 | 1 | 3 | 3 | 8 | ||||
| 10 | 1 | 2 | 3 | ||||||
| 10.5 | 6 | 1 | 4 | 1 | 12 | ||||
| 11 | 1 | 2 | 1 | 1 | 5 | ||||
| 11.5 | 1 | 1 | 2 | ||||||
| Sum | 3 | 11 | 3 | 7 | 12 | 7 | 2 | 45 | |
| Regression | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clutch | n Eggs | (a) Female Size | (b) Male Size (abs) | (c) Male Size (rel) | |||||||||||
| N | Mean ± SE | a | Rsqr | F | P | a | Rsqr | F | P | a | Rsqr | F | P | ||
| 2004 | |||||||||||||||
| 1 | 57 | 45.9 ± 1.52 | 9.549 | 0.5970 | 81.44 | <0.001 | 0.174 | 0.0004 | 0.02 | 0.877 | 0.079 | 0.0002 | 0.01 | 0.917 | |
| 2 | 57 | 53.1 ± 1.38 | 7.717 | 0.4750 | 49.84 | <0.001 | −0.222 | 0.0007 | 0.04 | 0.848 | −0.101 | 0.0003 | 0.02 | 0.897 | |
| 3 | 57 | 46.8 ± 1.63 | 6.522 | 0.2420 | 17.56 | <0.001 | 0.799 | 0.0043 | 0.24 | 0.628 | 0.364 | 0.0020 | 0.11 | 0.743 | |
| 4 | 42 | 37.8 ± 2.24 | 7.308 | 0.2110 | 10.69 | 0.002 | 2.273 | 0.0238 | 0.98 | 0.329 | 1.017 | 0.0107 | 0.43 | 0.515 | |
| 5 | 21 | 31.2 ± 2.69 | 3.072 | 0.0479 | 0.96 | 0.341 | 1.049 | 0.0062 | 0.12 | 0.735 | 0.531 | 0.0031 | 0.06 | 0.810 | |
| 1 + 2 | 57 | 98.9 ± 2.70 | 17.266 | 0.6220 | 90.43 | <0.001 | −0.048 | 0.0000 | 0.00 | 0.980 | −0.022 | 0.0000 | 0.00 | 0.987 | |
| 1 + 2 + 3 | 57 | 145.7 ± 3.90 | 23.788 | 0.5650 | 71.54 | <0.001 | 0.751 | 0.0012 | 0.06 | 0.801 | 0.343 | 0.0005 | 0.03 | 0.865 | |
| 1 + 2 + 3 + 4 | 42 | 184.9 ± 5.37 | 30.557 | 0.5770 | 54.56 | <0.001 | 3.730 | 0.0187 | 0.76 | 0.387 | 1.668 | 0.0084 | 0.34 | 0.564 | |
| 1 + 2 + 3 + 4 + 5 | 21 | 217.5 ± 8.96 | 32.143 | 0.4740 | 17.11 | <0.001 | 7.027 | 0.0454 | 0.90 | 0.354 | 3.555 | 0.0230 | 0.45 | 0.512 | |
| 2022 | |||||||||||||||
| 1 | 45 | 44.1 ± 1.30 | 4.269 | 0.1800 | 10.32 | 0.002 | 1.761 | 0.0339 | 1.65 | 0.205 | 0.648 | 0.0125 | 0.60 | 0.444 | |
| 2 | 44 | 48.8 ± 1.73 | 5.005 | 0.1420 | 6.98 | 0.012 | 1.963 | 0.0255 | 1.10 | 0.301 | 1.126 | 0.6960 | 0.38 | 0.540 | |
| 3 | 43 | 52.3 ± 1.60 | 2.683 | 0.0489 | 2.11 | 0.154 | 3.381 | 0.0830 | 3.71 | 0.061 | 1.222 | 0.0300 | 1.27 | 0.267 | |
| 4 | 40 | 54.7 ± 1.50 | 4.916 | 0.2050 | 9.79 | 0.003 | −1.848 | 0.0340 | 1.34 | 0.255 | −0.662 | 0.0122 | 0.47 | 0.498 | |
| 5 | 30 | 51.5 ± 2.18 | 6.098 | 0.1680 | 6.88 | 0.013 | −0.730 | 0.0025 | 0.09 | 0.771 | −0.229 | 0.0008 | 0.03 | 0.871 | |
| 1 + 2 | 44 | 93.6 ± 2.62 | 9.189 | 0.2090 | 11.11 | 0.002 | 4.195 | 0.0549 | 2.44 | 0.126 | 1.490 | 0.0195 | 0.84 | 0.366 | |
| 1 + 2 + 3 | 43 | 147.1 ± 3.65 | 10.56 | 0.1450 | 6.96 | 0.012 | 7.830 | 0.0949 | 4.30 | 0.044 | 2.829 | 0.0343 | 1.46 | 0.234 | |
| 1 + 2 + 3 + 4 | 40 | 204.0 ± 4.57 | 15.836 | 0.2060 | 9.59 | 0.004 | 3.900 | 0.0145 | 0.55 | 0.465 | 1.280 | 0.0048 | 0.18 | 0.676 | |
| 1 + 2 + 3 + 4 + 5 | 30 | 251.6 ± 6.24 | 21.15 | 0.2580 | 11.46 | 0.002 | 2.090 | 0.0028 | 0.09 | 0.762 | 0.644 | 0.0009 | 0.03 | 0.866 | |
| Regression | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clutch | Egg Mass | (a) Female Size | (b) Male Size (abs) | (c) Male Size (rel) | ||||||||||
| N | mean ± SE | a | Rsqr | F | P | a | Rsqr | F | P | a | Rsqr | F | P | |
| 1 | 45 | 3.80 ± 0.070 | 0.296 | 0.3180 | 20.02 | <0.001 | 0.037 | 0.0068 | 0.30 | 0.590 | 0.014 | 0.0026 | 0.11 | 0.738 |
| 2 | 44 | 3.61 ± 0.059 | 0.074 | 0.0269 | 1.13 | 0.293 | −0.112 | 0.0615 | 2.69 | 0.109 | −0.040 | 0.0218 | 0.91 | 0.345 |
| 3 | 43 | 3.61 ± 0.052 | 0.053 | 0.0193 | 0.71 | 0.405 | −0.070 | 0.0383 | 1.44 | 0.239 | −0.025 | 0.0129 | 0.49 | 0.491 |
| 4 | 40 | 3.55 ± 0.048 | 0.081 | 0.0659 | 2.40 | 0.131 | 0.037 | 0.0120 | 0.41 | 0.525 | 0.012 | 0.0039 | 0.13 | 0.718 |
| 5 | 30 | 3.38 ± 0.082 | 0.021 | 0.0017 | 0.05 | 0.825 | 0.093 | 0.0251 | 0.75 | 0.395 | 0.028 | 0.0075 | 0.22 | 0.644 |
| Regression | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clutch | To (Days) | (a) Female Size | (b) Male Size (abs) | (c) Male Size (rel) | ||||||||||
| N | mean ± SE | a | Rsqr | F | P | a | Rsqr | F | P | a | Rsqr | F | P | |
| 1 | 45 | 7.4 ± 0.15 | 0.188 | 0.0273 | 1.29 | 0.262 | 0.053 | 0.0021 | 0.10 | 0.758 | 0.020 | 0.0008 | 0.04 | 0.850 |
| 2 | 44 | 12.2 ± 0.23 | 0.406 | 0.0542 | 2.35 | 0.133 | 0.088 | 0.0027 | 0.11 | 0.739 | 0.032 | 0.0010 | 0.04 | 0.841 |
| 3 | 43 | 18.2 ± 0.41 | 0.445 | 0.0205 | 0.86 | 0.360 | 0.295 | 0.0094 | 0.39 | 0.537 | 0.107 | 0.0034 | 0.14 | 0.711 |
| 4 | 40 | 23.2 ± 0.59 | 0.893 | 0.0423 | 1.72 | 0.197 | 0.735 | 0.0287 | 1.15 | 0.290 | 0.265 | 0.0103 | 0.41 | 0.527 |
| 5 | 30 | 28.0 ± 0.77 | 0.257 | 0.0025 | 0.08 | 0.776 | 1.113 | 0.0390 | 1.34 | 0.256 | 0.343 | 0.0120 | 0.40 | 0.531 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honek, A.; Martinkova, Z. Effect of Male Body Size on Female Reproduction in Pyrrhocoris apterus (L.) (Heteroptera, Pyrrhocoridae). Insects 2022, 13, 902. https://doi.org/10.3390/insects13100902
Honek A, Martinkova Z. Effect of Male Body Size on Female Reproduction in Pyrrhocoris apterus (L.) (Heteroptera, Pyrrhocoridae). Insects. 2022; 13(10):902. https://doi.org/10.3390/insects13100902
Chicago/Turabian StyleHonek, Alois, and Zdenka Martinkova. 2022. "Effect of Male Body Size on Female Reproduction in Pyrrhocoris apterus (L.) (Heteroptera, Pyrrhocoridae)" Insects 13, no. 10: 902. https://doi.org/10.3390/insects13100902






