The Effect of Ice-Nucleation-Active Bacteria on Metabolic Regulation in Evergestis extimalis (Scopoli) (Lepidoptera: Pyralidae) Overwintering Larvae on the Qinghai-Tibet Plateau
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Insects and Bacterial Strains
2.2. Cold Resistance of Overwintering Larvae
2.3. Effect of Different Temperature and Exposure Duration on Cold Resistance of E. extimalis
2.4. Measurement of Trehalase Activity
2.5. Quantification of TRE Expression after Cold Acclimation
3. Results
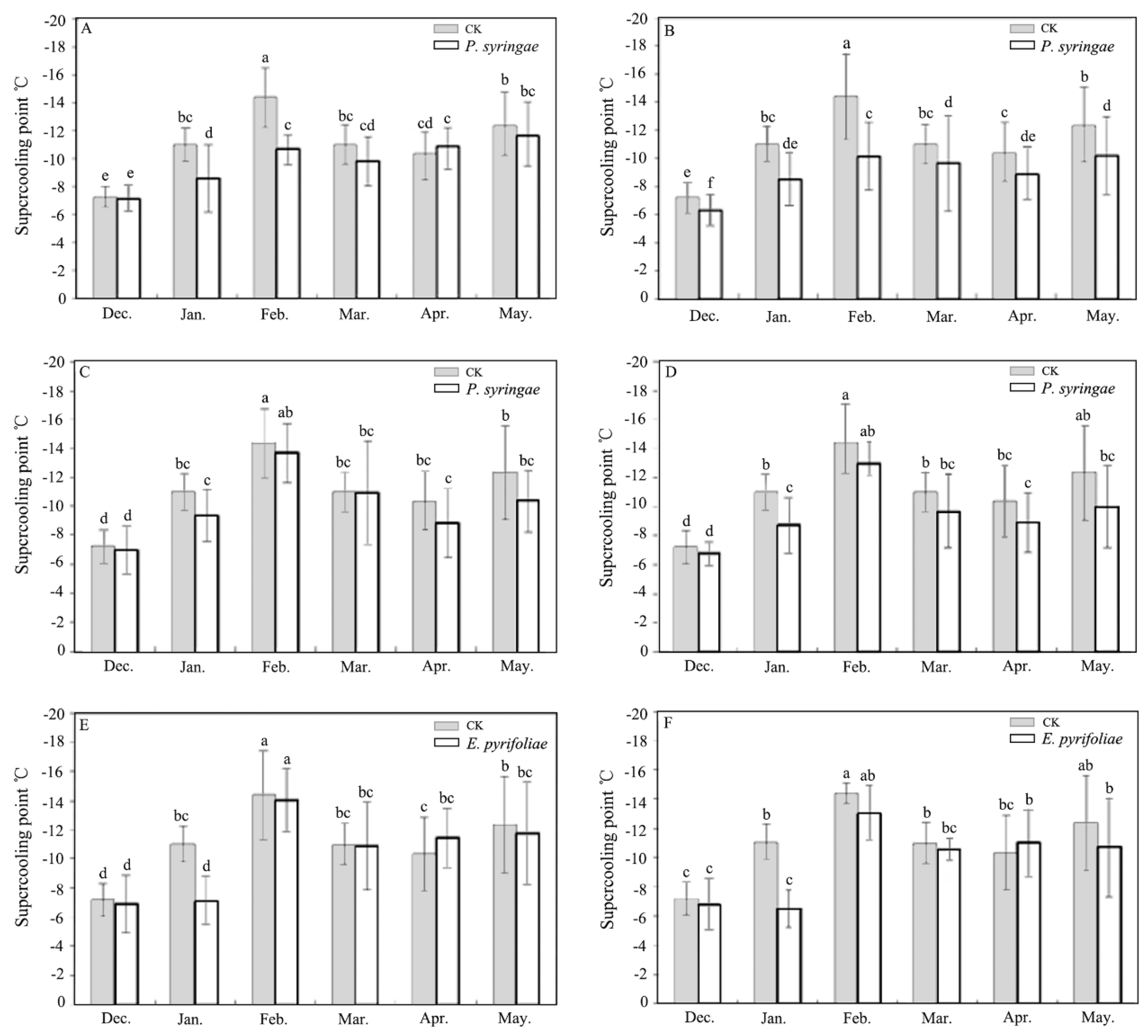
3.1. Changes inSCP under INA Bacteria Stress
3.2. Changes in SCP under Different Concentrations and Temperatures
3.3. Effect of Temperature and Exposure Duration on Trehalose Content
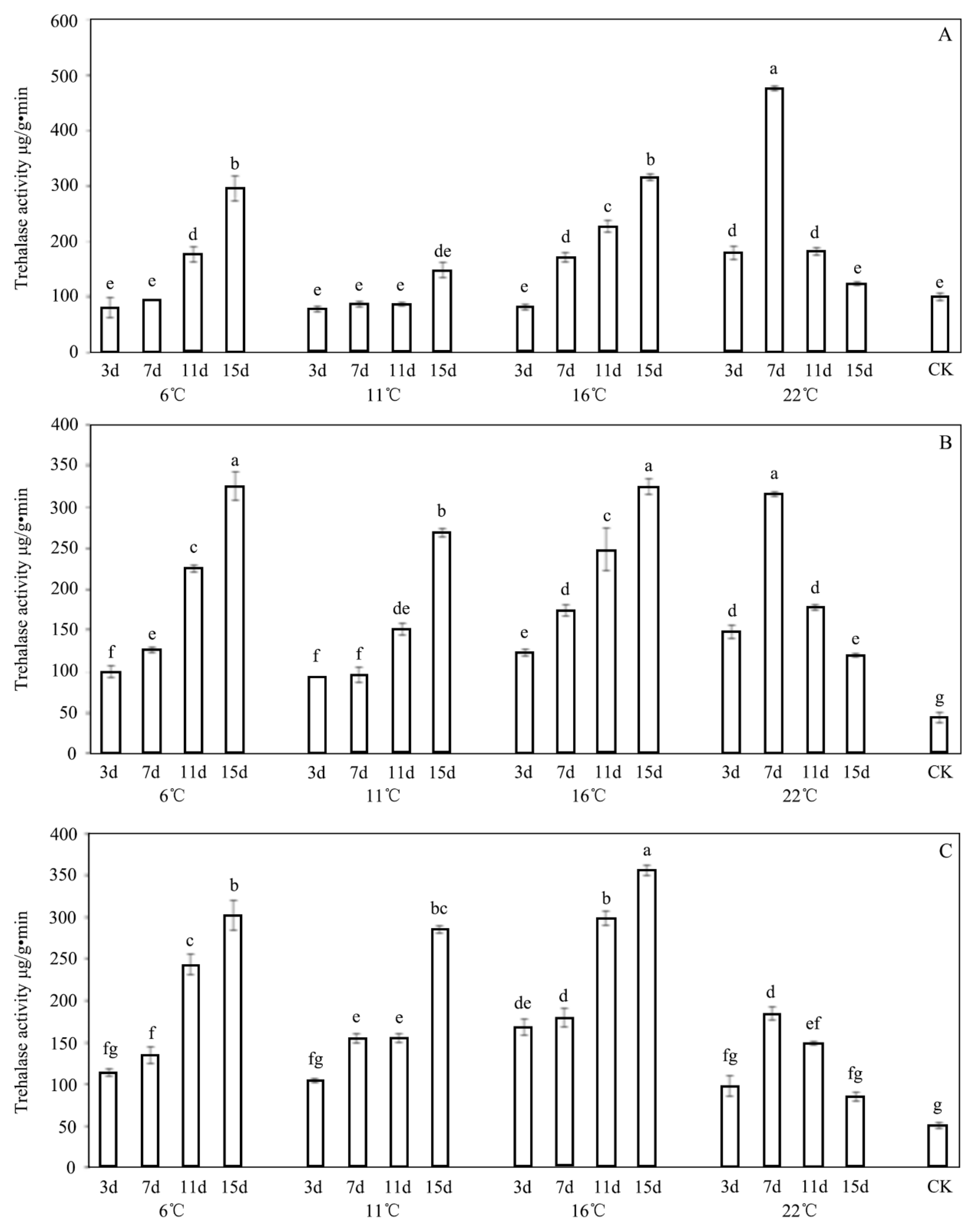
3.4. Effect of INA Bacteria on Trehalase Activity
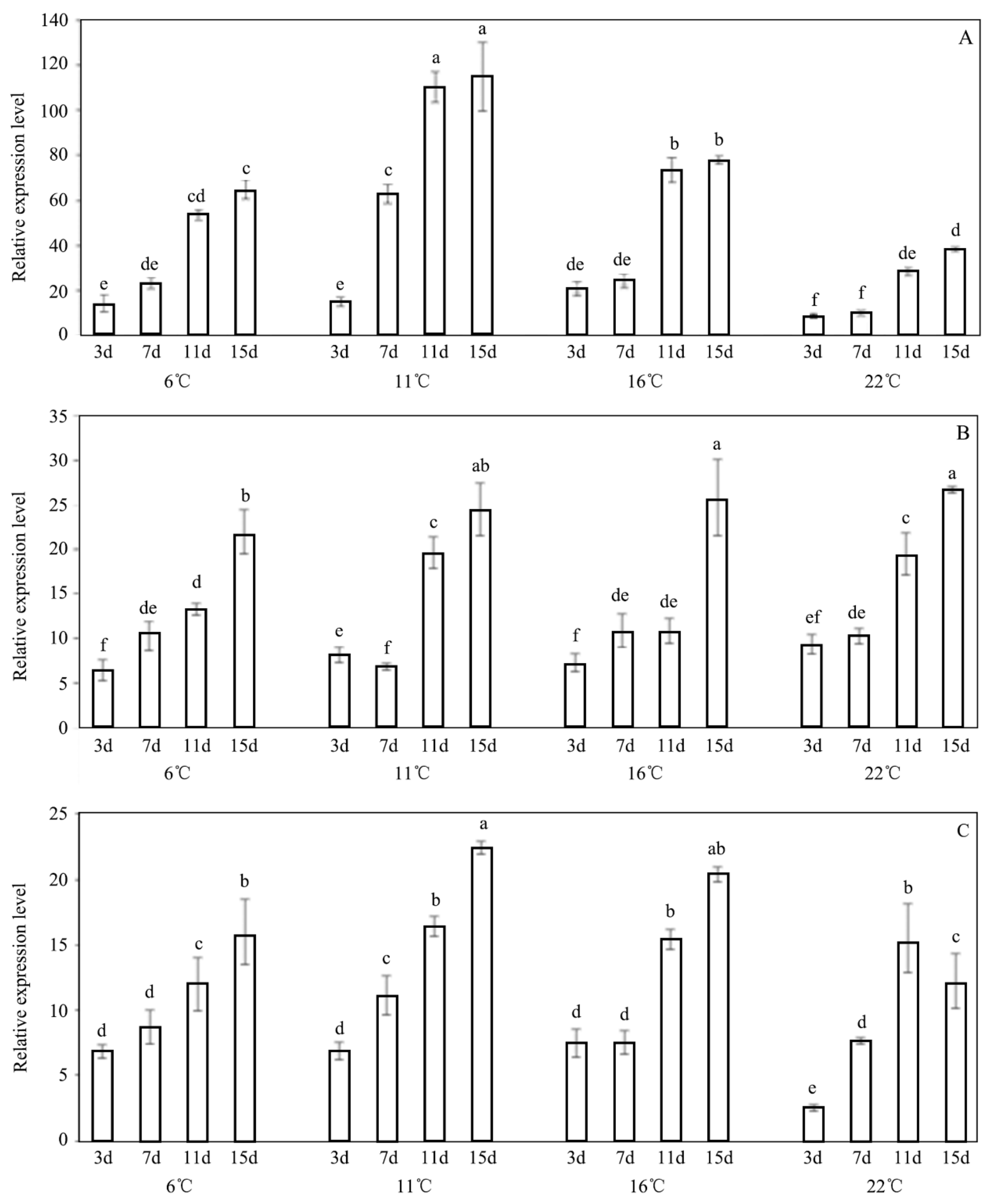
3.5. Expression Profiles of Trehalase Genes after Exposure to INA Bacteria
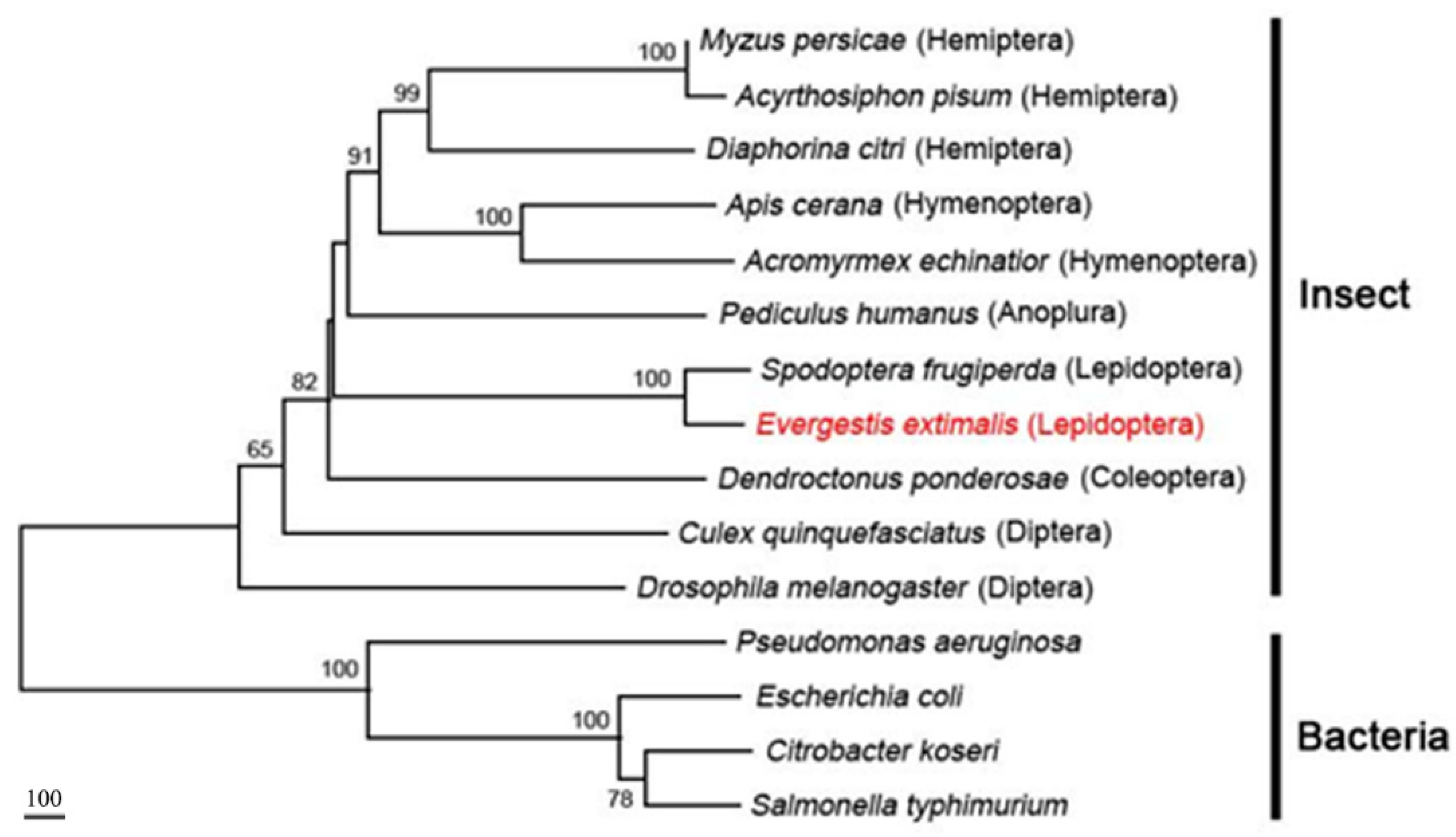
3.6. Phylogenetic Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. S. N. Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Li, S.; Zhou, Q.; Zhou, D.; He, N.; Qian, Z. Safety evaluation of microbial pesticide (HaNPV) based on PCR method. Front. Chem. Sci. Eng. 2019, 13, 377–384. [Google Scholar] [CrossRef]
- Maki, L.R.; Galyan, E.L.; Chang, C.M.M.; Caldwell, D.R. Ice nucleation induced by Pseudomonas syringae. Appl. Microbiol. 1974, 28, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Lindow, S.E.; Arny, D.C.; Upper, C.D.; Barchet, W.R. The role of bacterial ice nuclei in frost injury to sensitive plants. In Plant Cold Hardiness and Freezing Stress; Li, P.H., Sakai, A., Eds.; Academic Press Inc.: New York, NY, USA, 1978; pp. 249–263. [Google Scholar]
- Kim, H.K.; Orser, C.; Lindow, S.E.; Sands, D.C. Xanthomonas-Campestris pv. translucens strains active in ice nucleation. Plant Dis. 1987, 71, 994–997. [Google Scholar] [CrossRef]
- Lee, M.R.; Lee, R.E.; Strong-Gunderson, J.M.; Minges, S.R. Isolation of ice-nucleating active bacteria from the freeze-tolerant frog, Rana sylvatica. Cryobiology 1995, 32, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Lee Jr, R.E.; Strong-Gunderson, J.M.; Lee, M.R.; Grove, K.S.; Riga, T.J. Isolation of ice nucleating active bacteria from insects. J. Exp. Zool. 1991, 257, 124–127. [Google Scholar]
- Tang, C.; Sun, F.; Zhao, T.C. Construction of ice nucleation active Enterobacter cloacae for control of insect pests. Chin. Sci. Bull. 2003, 48, 178–180. [Google Scholar] [CrossRef]
- Costanzo, J.P.; Humphreys, T.L.; Lee, R.E.; Moor, J.B.; Lee, M.R.; Wyman, J.A. Long-term reduction of cold hardiness following ingestion of ice-nucleating bacteria in the Colorado potato beetle, Leptinotarsa decemlineata. J. Insect Physiol. 1998, 44, 1173–1180. [Google Scholar] [CrossRef]
- Zhang, D.F.; Lai, Y.P.; Xian, W.R.; Wang, X. Control efficacy of seven insecticides to Evergestis extimalis. J. Plant Protec. 2009, 6, 169–171. [Google Scholar]
- Zhang, D.F.; Xian, W.R.; Lai, Y.P.; Wang, X.; Wang, A.L.; Geng, G.G. Investigation of the occurrence dynamics of Evergestis extimalis. Chin. J. Appl. Entomol. 2010, 47, 201–203. [Google Scholar]
- Lai, Y.P.; Zhang, D.F.; Xian, W.R. The relationship between the population density of Evergestis extimalis rape yield loss and control index. Chin. J. Appl. Entomol. 2012, 49, 526–528. [Google Scholar]
- Lai, Y.P.; Zhang, D.F.; Hou, T.P.; Liu, T.X. Analysis of super-cooling point and biochemical indexes on Evergestis extimalis (Scopoli) (Lepidoptera: Pyralidae) larvae over-wintering. Chin. J. Northwest Agric. 2014, 2, 44–48. [Google Scholar]
- Lai, Y.P.; Li, Q.R.; Zhang, G. Effects of altitude and ice nucleating active bacteria on the super-cooling point of Evergestis extimalis (Scopli) (Lepidoptera: Pyralidida). J. Appl. Entomol. 2019, 56, 1347–1352. [Google Scholar]
- Fu, D.; Dai, L.; Ning, H.; Kang, X.; Sun, Y.; Chen, H. Effects of cold stress on metabolic regulation in the overwintering larvae of the Chinese white pine beetle, Dendroctonus armandi. Entomol. Exp. Appl. 2020, 168, 836–850. [Google Scholar] [CrossRef]
- Tamang, A.M.; Kalra, B.; Parkash, R. Cold and desiccation stress induced changes in the accumulation and utilization of proline and trehalose in seasonal populations of Drosophila immigrans. Comp. Biochem. Phys. A 2017, 203, 304–313. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Nawaz, A.; Lee, D.J.; Alghamdi, S.S.; Siddique, K.H.M. Seed priming improves chilling tolerance in chickpea by modulating germination metabolism, trehalose accumulation and carbon assimilation. Plant Physiol. Biochem. 2017, 111, 274–283. [Google Scholar] [CrossRef]
- Lambhod, C.; Pathak, A.; Munjal, A.K.; Parkash, R. Tropical Drosophila ananassae of wet-dry seasons show cross resistance to heat, drought and starvation. Biol. Open 2017, 6, 1698–1706. [Google Scholar] [CrossRef] [Green Version]
- Mitsumasu, K.; Azuma, M.; Niimi, T.; Yamashita, O.; Yaginuma, T. Membrane-penetrating trehalase from silkworm Bombyx mori. molecular cloning and localization in larval midgut. Insect Mol. Biol. 2005, 14, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.M.; Ding, J.H.; Jiang, D.L.; Liu, Z.X.; Li, Y.J.; Wang, J.; Wang, J.; Sheng, S.; Wu, F.A. Characterization and functional analysis of trehalase related to chitin metabolism in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Insects 2021, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.M.; Zhao, L.M.; Shen, Q.D.; Xie, G.Q.; Wang, S.G.; Tang, B. Knockdown of two trehalose-6-phosphate synthases severely affects chitin metabolism gene expression in the brown planthopper Nilaparvata lugens. Pest. Manag. Sci. 2017, 73, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Z.; Huang, Y.L.; Lu, Z.J.; Zhang, Q.; Su, H.N.; Du, Y.M.; Yi, L.; Zhong, B.L.; Chen, C.X. Inhibition of trehalase affects the trehalose and chitin metabolism pathways in Diaphorina citri (Hemiptera: Psyllidae). Insect Sci. 2021, 28, 718–734. [Google Scholar] [CrossRef]
- Lqbal, J.; Zhang, X.X.; Chang, Y.W.; Du, Y.Z. Differential response of leafminer files Liriomyza trifolli (Burgess) and Liriomyza sativae (Blanchard) to rapid cold hardening. Insects 2021, 12, 1041. [Google Scholar]
- Li, X.W.; Li, D.; Zhang, Z.J.; Huang, J.; Zhang, J.M.; Hafeez, M.; Wang, L.K.; Guo, C.; Lu, Y.B. Supercooling capacity and cold tolerance of the South American tomato pinworm, Tuta absoluta, a newly invaded pest in China. J. Pest. Sci. 2021, 94, 845–858. [Google Scholar] [CrossRef]
- Sun, F.Z.; Xing, W.; Zhang, Y.X. Preliminary study on effect of INA bacteria to kill larva of Anoplophora glabripennis through freezing-induction. For. Res. 1997, 10, 96. [Google Scholar]
- Castrillo, L.A.; Lee, R.E.; Lee, M.R.; Rutherford, S.T. Identification of ice-nucleating active Pseudomonas fluorescens strains for biological control of overwintering Colorado potato beetles (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2000, 92, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.L.; Cheng, J.E.; Luo, Y.Z. Effects of ice nucleating active bacteria (INAB) on the immunity of haemolymph of Pieris rapae. Chin. Bull. Entomol. 2005, 42, 391–394. [Google Scholar]
- Huang, Q.T.; Zhang, G.J.; Nan, J.L.; Cheng, W.N.; Salzman, K.Z. Characterization of trehalose metabolic genes and corresponding enzymatic activities during diapause of Sitodiplosis mosellana. J. Insect Physiol. 2021, 135, 104324. [Google Scholar] [CrossRef]
- Masoumi, Z.; Noghabi, S.S.; Lzadi, H. Trehalose and proline failed to enhance cold tolerance of the cowpea weevil, Callosobruchus maculatus (F.) (Col.: Bruchidae). J. Stored Prod. Res. 2021, 93, 101853. [Google Scholar] [CrossRef]
- Zhou, H.; Lei, G.; Chen, Y.; You, M.; You, S. PxTret1-like affects the temperature adaptability of cosmopolitan pest by altering trehalose tissue distribution. Int. J. Mol. Sci. 2022, 23, 9019. [Google Scholar] [CrossRef] [PubMed]
- Wharton, D.A. Cold tolerance of New Zealand alpine insects. J. Insect Physiol. 2011, 57, 1090–1095. [Google Scholar] [CrossRef]
- Kandror, O.; Deleon, A.; Goldberg, A.L.; Kandror, O.; DeLeon, A.; Goldberg, A.L. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperature. Proc. Natl. Acad. Sci. USA 2002, 99, 9727–9732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowe, L.M. Lessons from nature: The role of sugars in anhydrobiosis. Comparative Biochem. Phys. A 2002, 131, 505–513. [Google Scholar] [CrossRef]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.N. Trehalose—The insect ‘blood’ sugar. Adv. Insect Physiol. 2003, 31, 205–285. [Google Scholar]
- Ge, L.Q.; Zhao, K.F.; Huang, L.J.; Wu, J.C. The effects of triazophos on the trehalose content, trehalase activity and their gene expression in the brown planthopper Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Pestic. Biochem. Phys. 2011, 100, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Liu, X.; Xu, Q.; Qin, Z.; Wang, S.; Zhang, F.; Wang, S.; Tang, B. Two novel soluble trehalase genes cloned from Harmonia axyridis and regulation of the enzyme in a rapid changing temperature. Comp. Biochem. Physiol. B 2016, 198, 10–18. [Google Scholar] [CrossRef]
- Mu, Y.; Shi, X.; Zhang, Z.; Zhang, Z.; Wang, T.; Wang, Y.; Wei, Y.; Zhou, X.; Xiang, M.; Liu, Y.; et al. Validamycin reduces the transmission of Tomato chlorotic virus by Bemisia tabaci. J. Pest. Sci. 2021, 95, 1261–1272. [Google Scholar] [CrossRef]
- Castaldi, S.; Cimmino, A.; Masi, M.; Evidente, A. Bacterial Lipodepsipeptides and Some of Their Derivatives and Cyclic Dipeptides as Potential Agents for Biocontrol of Pathogenic Bacteria and Fungi of Agrarian Plants. J. Agric. Food Chem. 2022, 70, 4591–4598. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Atri, A.; Banyal, D.K.; Bhardwaj, N.R.; Roy, A.K. Exploring the integrated use of fungicides, bio-control agent and biopesticide for management of foliar diseases (anthracnose, grey leaf spot and zonate leaf spot) of sorghum. Int. J. Pest. Manag. 2022, 1–12. [Google Scholar] [CrossRef]
- You, J.; Chen, Z.M.; Hou, X.Y.; Guo, J.S.; Wang, C.C.; Gao, J.M. Occurrence, potential sources and risks of organophosphate esters in the high-elevation region, Tibet, China. Sci. Total Environ. 2022, 806, 1348. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, P.; Wang, C.; Ren, J.; Yao, T. A review of current knowledge and future prospects regarding persistent organic pollutants over the Tibetan Plateau. Sci. Total Environ. 2016, 573, 139–154. [Google Scholar] [CrossRef]
- Cengiz, M.F.; Basancelebi, O.; Baslar, M.; Certel, M. A novel technique for the reduction of pesticide residues by a combination of low-intensity electrical current and ultrasound applications: A study on lettuce samples. Food Chem. 2021, 354, 129360. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, H.; Liu, Y.; Liu, Y.; Lai, Y. The Effect of Ice-Nucleation-Active Bacteria on Metabolic Regulation in Evergestis extimalis (Scopoli) (Lepidoptera: Pyralidae) Overwintering Larvae on the Qinghai-Tibet Plateau. Insects 2022, 13, 909. https://doi.org/10.3390/insects13100909
Shao H, Liu Y, Liu Y, Lai Y. The Effect of Ice-Nucleation-Active Bacteria on Metabolic Regulation in Evergestis extimalis (Scopoli) (Lepidoptera: Pyralidae) Overwintering Larvae on the Qinghai-Tibet Plateau. Insects. 2022; 13(10):909. https://doi.org/10.3390/insects13100909
Chicago/Turabian StyleShao, Hainan, Yunxiang Liu, Yujiao Liu, and Youpeng Lai. 2022. "The Effect of Ice-Nucleation-Active Bacteria on Metabolic Regulation in Evergestis extimalis (Scopoli) (Lepidoptera: Pyralidae) Overwintering Larvae on the Qinghai-Tibet Plateau" Insects 13, no. 10: 909. https://doi.org/10.3390/insects13100909
APA StyleShao, H., Liu, Y., Liu, Y., & Lai, Y. (2022). The Effect of Ice-Nucleation-Active Bacteria on Metabolic Regulation in Evergestis extimalis (Scopoli) (Lepidoptera: Pyralidae) Overwintering Larvae on the Qinghai-Tibet Plateau. Insects, 13(10), 909. https://doi.org/10.3390/insects13100909



