Mitogenomic Comparison of the Mole Crickets Gryllotalpidae with the Phylogenetic Implications (Orthoptera: Ensifera)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Sequence Analyses
2.3. Phylogenetic Analyses
| Superfamily | Family | Species | Locality | Size (bp) | Accession No. | Resource |
|---|---|---|---|---|---|---|
| Gryllotalpoidea | Gryllotalpidae | Gryllotalpa henana Cai & Niu, 1998 | China | 15,504 | ON243749 | This study |
| G. orientalis Burmeister, 1838 | China | 15,497 | ON210982 | This study | ||
| G. orientalis Burmeister, 1838 | Korea | 15,521 | AY660929 | [12] | ||
| G. pluvialis (Mjöberg, 1913) | Australia | 15,525 | EU938371 | [11] | ||
| Gryllotalpa sp. | China | 15,506 | MK903562 | [10] | ||
| G. unispina Saussure, 1874 | China | 15,513 | KC894752 | [13] | ||
| Myrmecophilidae | Myrmecophilus kubotai Maruyama, 2004 | Japan | 15,345 | MZ440658 | [18] | |
| M. manni Schimmer, 1911 | USA | 15,323 | EU938370 | [11] | ||
| Myrmecophilus sp. | Japan | 15,341 | MZ440659 | [18] | ||
| Grylloidea | Phalangopsidae | Meloimorpha japonica (Haan, 1844) | China | 15,880 | MH580273 | [39] |
| Cacoplistes rogenhoferi Saussure, 1877 | China | 16,018 | MH580272 | [39] | ||
| Mogoplistidae | Ornebius bimaculatus (Shiraki, 1930) | China | 16,136 | MH580274 | [39] | |
| O. fuscicerci (Shiraki, 1930) | China | 16,368 | MH580275 | [39] | ||
| O. kanetataki (Matsumura, 1904) | China | 16,589 | MH580276 | [39] | ||
| Trigonidiidae | Dianemobius fascipes (Walker, 1869) | China | 15,363 | MK303550 | [40] | |
| D. furumagiensis (Ohmachi & Furukawa, 1929) | China | 15,350 | MK303551 | [40] | ||
| Homoeoxipha nigripes Xia & Liu, 1993 | China | 15,679 | MK303553 | [40] | ||
| Natula pravdini (Gorochov, 1985) | China | 15,817 | MG701239 | [40] | ||
| Svistella anhuiensis He, Li & Liu, 2009 | China | 16,494 | MG701238 | [40] | ||
| Polionemobius taprobanensis (Walker, 1869) | China | 16,641 | MK303552 | [40] | ||
| Gryllidae | Gryllodes sigillatus (Walker, 1869) | China | 16,369 | MW365703 | [41] | |
| Gryllus bimaculatus De Geer, 1773 | Korea | 16,075 | MT993975 | [42] | ||
| Loxoblemmus doenitzi Stein, 1881 | - | 15,620 | KX673202 | [43] | ||
| Oecanthus sinensis Walker, 1869 | China | 16,142 | KY783908 | [44] | ||
| Truljalia hibinonis (Matsumura, 1917) | China | 15,120 | KY783909 | [44] | ||
| Turanogryllus eous Bey-Bienko, 1956 | China | 16,045 | MK656322 | [45] | ||
| Velarifictorus hemelytrus (Saussure, 1877) | China | 16,123 | KU562918 | [46] | ||
| Acheta domesticus (Linnaeus, 1758) | Japan | 16,071 | MZ440654 | [18] | ||
| Outgroup | Tettigoniidae | Alloxiphidiopsis emarginata (Tinkham, 1944) | China | 16,207 | MN562488 | [47] |
| Tettigonia chinensis Willemse, 1933 | - | 16,244 | KX057727 | [48] | ||
| Gryllacrididae | Camptonotus carolinensis (Gerstaecker, 1860) | - | 15,211 | KM657333 | [49] | |
| Schizodactylidae | Comicus campestris Irish, 1986 | - | 15,691 | KM657337 | [49] | |
| Prophalangopsidae | Tarragoilus diuturnus Gorochov, 2001 | China | 16,144 | JQ999995 | [50] |
3. Results and Discussion
3.1. Genome Structure and Base Composition
3.2. Protein-Coding Genes and Codon Usage
3.3. Transfer and Ribosomal RNA Genes
3.4. Intergenic Spacers and Gene Overlaps
3.5. Control Region
3.6. Genetic Diversity and Selective Constraints
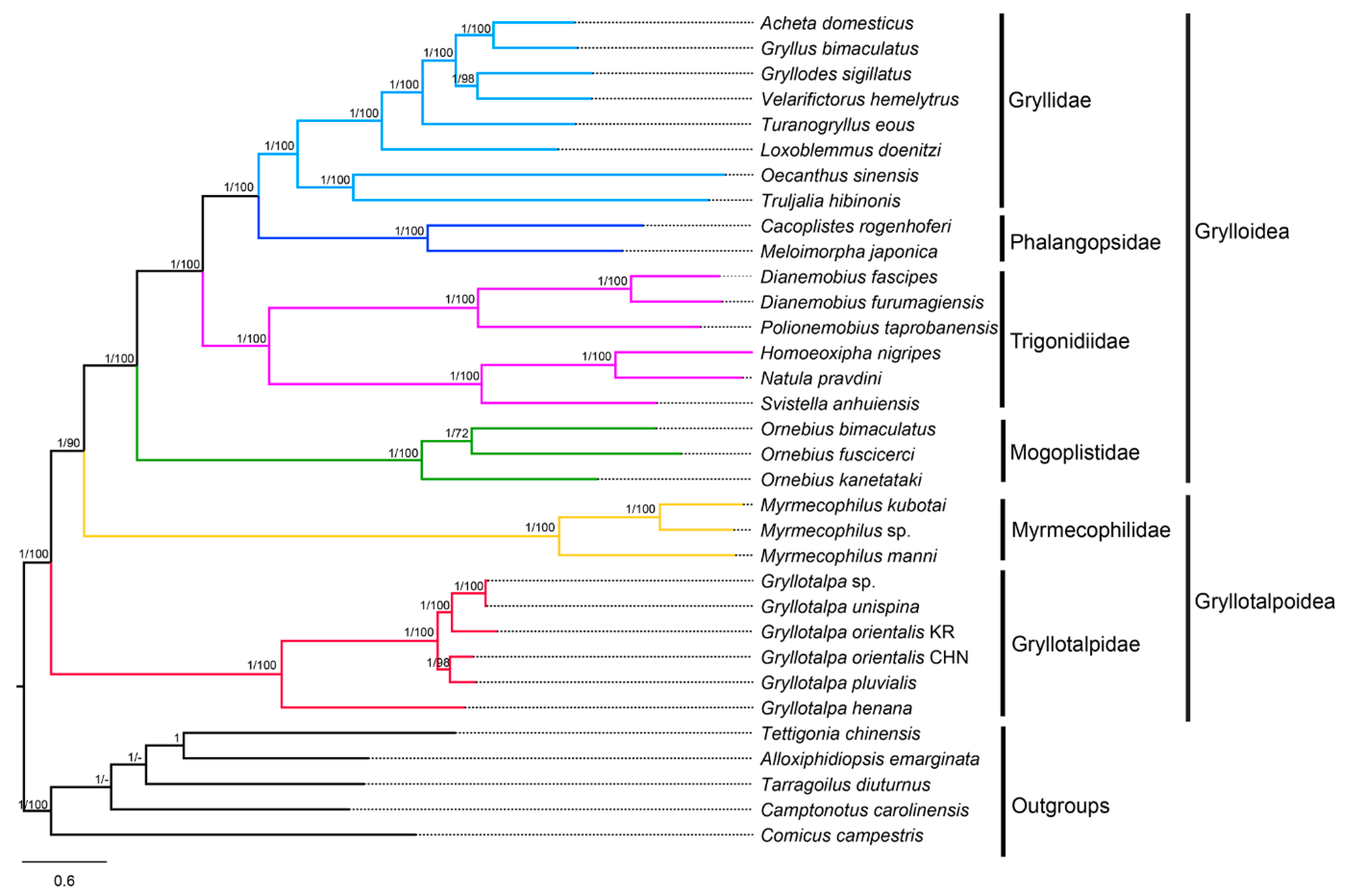
3.7. Phylogenetic Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [Green Version]
- Drosopoulou, E.; Syllas, A.; Goutakoli, P.; Zisiadis, G.A.; Konstantinou, T.; Pangea, D.; Sentis, G.; van Sauers-Muller, A.; Wee, S.L.; Augustinos, A.A.; et al. Τhe complete mitochondrial genome of Bactrocera carambolae (Diptera: Tephritidae): Genome description and phylogenetic implications. Insects 2019, 10, 429. [Google Scholar] [CrossRef] [Green Version]
- Du, H.C.; Liu, M.; Zhang, S.F.; Liu, F.; Zhang, Z.; Kong, X.B. Lineage divergence of Dendrolimus punctatus in southern China based on mitochondrial genome. Front. Genet. 2020, 11, 65. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Q.; Li, H.; Song, F.; Zhao, Y.S.; Wilson, J.J.; Cai, W.Z. Higher-level phylogeny and evolutionary history of Pentatomomorpha (Hemiptera: Heteroptera) inferred from mitochondrial genome sequences. Syst. Entomol. 2019, 44, 810–819. [Google Scholar] [CrossRef]
- Zheng, B.Y.; Han, Y.Y.; Yuan, R.Z.; Liu, J.X.; van Achterberg, C.; Tang, P.; Chen, X.X. Comparative mitochondrial genomics of 104 Darwin wasps (Hymenoptera: Ichneumonidae) and its implication for phylogeny. Insects 2022, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hu, G.L.; Hua, B.Z. Complete mitochondrial genomes of Bittacus strigosus and Panorpa debilis and genomic comparisons of Mecoptera. Int. J. Biol. Macromol. 2019, 140, 672–681. [Google Scholar] [CrossRef]
- Nie, R.; Vogler, A.P.; Yang, X.K.; Lin, M. Higher-level phylogeny of longhorn beetles (Coleoptera: Chrysomeloidea) inferred from mitochondrial genomes. Syst. Entomol. 2021, 46, 56–70. [Google Scholar] [CrossRef]
- Song, H.; Bethoux, O.; Shin, S.; Donath, A.; Letsch, H.; Liu, S.; McKenna, D.D.; Meng, G.; Misof, B.; Podsiadlowski, L.; et al. Phylogenomic analysis sheds light on the evolutionary pathways towards acoustic communication in Orthoptera. Nat. Commun. 2020, 11, 4939. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Zhu, R.Q.; Xue, H.J.; Li, Y.F.; Bu, W.J. Mitogenomics of chinch bugs from China and implications for its coevolutionary relationship with grasses. Insects 2022, 13, 643. [Google Scholar] [CrossRef]
- Chang, H.H.; Qiu, Z.Y.; Yuan, H.; Wang, X.Y.; Li, X.J.; Sun, H.M.; Guo, X.Q.; Lu, Y.C.; Feng, X.L.; Majid, M.; et al. Evolutionary rates of and selective constraints on the mitochondrial genomes of Orthoptera insects with different wing types. Mol. Phylogenet. Evol. 2020, 145, 106734. [Google Scholar] [CrossRef]
- Fenn, J.D.; Song, H.; Cameron, S.L.; Whiting, M.F. A preliminary mitochondrial genome phylogeny of Orthoptera (Insecta) and approaches to maximizing phylogenetic signal found within mitochondrial genome data. Mol. Phylogenet. Evol. 2008, 49, 59–68. [Google Scholar] [CrossRef]
- Kim, I.; Cha, S.Y.; Yoon, M.H.; Hwang, J.S.; Lee, S.M.; Sohn, H.D.; Jin, B.R. The complete nucleotide sequence and gene organization of the mitochondrial genome of the oriental mole cricket, Gryllotalpa orientalis (Orthoptera: Gryllotalpidae). Gene 2005, 353, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Shao, D.D.; Cai, M.; Yin, H.; Zhang, D.C. The complete mitochondrial genome of Gryllotalpa unispina Saussure, 1874 (Orthoptera: Gryllotalpoidea: Gryllotalpidae). Mitochondrial DNA A 2014, 27, 159–160. [Google Scholar] [CrossRef]
- Cadena-Castañeda, O.J. The phylogeny of mole crickets (Orthoptera: Gryllotalpoidea: Gryllotalpidae). Zootaxa 2015, 3985, 451–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. Available online: http://Orthoptera.SpeciesFile.org (accessed on 24 August 2022).
- Cadena-Castañeda, O.J. Two new species of mole crickets (Orthoptera: Gryllotalpidae: Scapteriscinae) from the Colombian Amazon and Orinoquia rainforests. Zootaxa 2011, 3126, 62–68. [Google Scholar] [CrossRef]
- Chintauan-Marquier, I.C.; Legendre, F.; Hugel, S.; Robillard, T.; Grandcolas, P.; Nel, A.; Zuccon, D.; Desutter-Grandcolas, L. Laying the foundations of evolutionary and systematic studies in crickets (Insecta, Orthoptera): A multilocus phylogenetic analysis. Cladistics 2016, 32, 54–81. [Google Scholar] [CrossRef] [PubMed]
- Sanno, R.; Kataoka, K.; Hayakawa, S.; Ide, K.; Nguyen, C.N.; Nguyen, T.P.; Le, B.T.N.; Kim, O.T.P.; Mineta, K.; Takeyama, H.; et al. Comparative analysis of mitochondrial genomes in Gryllidea (Insecta: Orthoptera): Implications for adaptive evolution in ant-loving crickets. Genome Biol. Evol. 2021, 13, evab222. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.K. Annotated checklist and key to species of Gryllotalpa (Orthoptera: Gryllotalpidae) from the Oriental region. Zootaxa 2016, 4132, 77–86. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Q. A checklist of Chinese crickets (Orthoptera: Gryllidea). Zootaxa 2018, 4369, 515–535. [Google Scholar] [CrossRef]
- Kang, L. The species Gryllotalpa africana Palisot from China should be a synonym of Gryllotalpa orientalis Burmeister. Entomol. Knowl. 1993, 30, 124–127. [Google Scholar]
- Townsend, B.C. A revision of the Afrotropical mole-crickets (Orthoptera: Gryllotalpidae). Bull. Br. Mus. Nat. Hist. 1983, 46, 175–203. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernt, M.; Donath, A.; Juhling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Putz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved De Novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Drechsel, O.; Kahlau, S.; Bock, R. OrganellarGenomeDraw–a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013, 41, W575–W581. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xu, H.Y.; Gu, J.X.; Mao, B.Y.; Chen, Z.L.; Huang, Y.; Huang, J.H. Phylogenetic position of the genera Caryandoides, Paratoacris, Fer and Longchuanacris (Orthoptera:Acrididae) revealed by complete mitogenome sequences. Invertebr. Syst. 2021, 35, 725–741. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.C.; Tang, P.; Zheng, B.Y.; Wu, Q.; Wei, S.J.; Chen, X.X. The first two mitochondrial genomes of the family Aphelinidae with novel gene orders and phylogenetic implications. Int. J. Biol. Macromol. 2018, 118, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.L.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, J.R.; Hiatt, K.D.; Whiting, M.F.; Song, H. Searching for the optimal data partitioning strategy in mitochondrial phylogenomics: A phylogeny of Acridoidea (Insecta: Orthoptera: Caelifera) as a case study. Mol. Phylogenet. Evol. 2013, 67, 494–508. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Li, J.K. Comparative analysis of mitochondrial genomes of the superfamily Grylloidea (Insecta, Orthoptera) reveals phylogenetic distribution of gene rearrangements. Int. J. Biol. Macromol. 2018, 120, 1048–1054. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Y.Y.; Zhang, L.C.; Li, J.K. Mitochondrial genome characterization of the family Trigonidiidae (Orthoptera) reveals novel structural features and nad1 transcript ends. Sci. Rep. 2019, 9, 19092. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.K.; Dong, H.L.; He, M.Q.; Gao, J.G. Mitochondrial Genome Characterization of Gryllodes sigillatus (Orthoptera: Gryllidae) and its phylogenetic implications. Mitochondrial DNA B 2021, 6, 1056–1058. [Google Scholar] [CrossRef]
- Park, B.; Choi, E.H.; Kim, G.; Shin, C.R.; Hwang, J.; Baek, S.Y.; Hwang, U.W. The complete mitochondrial genome of the two-spotted cricket Gryllus bimaculatus (Orthoptera: Gryllidae) from South Korea. Mitochondrial DNA B 2021, 6, 1144–1146. [Google Scholar] [CrossRef]
- Song, N.; Li, H.; Song, F.; Cai, W.Z. Molecular phylogeny of Polyneoptera (Insecta) inferred from expanded mitogenomic data. Sci. Rep. 2016, 6, 36175. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Chen, Q.; Wen, M.; Wang, J.T.; Wang, Y.L.; Ren, B.Z. Phylogeny and acoustic signal evolution of a pure tone song katydid Pseudophyllus titan (Orthoptera: Tettigoniidae) based on the complete mitogenome. Mitochondrial DNA A 2019, 30, 385–396. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, L.C.; Li, J.K. The complete mitochondrial genome of a field cricket Turanogryllus eous (Insecta: Orthoptera). Mitochondrial DNA B 2019, 4, 3852–3853. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Ren, Q.L.; Zhang, Q.; Huang, Y. Complete mitochondrial genomes of three crickets (Orthoptera: Gryllidae) and comparative analyses within Ensifera mitogenomes. Zootaxa 2016, 4092, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.L.; Lu, Y.; Xun, L.L.; Zhou, Y.F. Characterization of the mitochondrial genome of Alloxiphidiopsis emarginata (Orthoptera, Tettigoniidae, Meconematinae). Mitochondrial DNA B 2019, 4, 4192–4193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.J.; Zhao, L.; Liu, N.; Guo, H.F.; Guan, B.; Di, J.X.; Shi, F. Towards a higher-level Ensifera phylogeny inferred from mitogenome sequences. Mol. Phylogenet. Evol. 2017, 108, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Amédégnato, C.; Cigliano, M.M.; Desutter-Grandcolas, L.; Heads, S.W.; Huang, Y.; Otte, D.; Whiting, M.F. 300 million years of diversification: Elucidating the patterns of orthopteran evolution based on comprehensive taxon and gene sampling. Cladistics 2015, 31, 621–651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.J.; Shi, F.M.; Zhao, L. The first mitochondrial genome for the superfamily Hagloidea and implications for its systematic status in Ensifera. PLoS ONE 2014, 9, e86027. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y.; Shi, F. The mitochondrial genome of Ruspolia dubia (Orthoptera: Conocephalidae) contains a short A+T-rich region of 70 bp in length. Genome 2007, 50, 855–866. [Google Scholar] [CrossRef] [Green Version]
- Bernt, M.; Braband, A.; Schierwater, B.; Stadler, P.F. Genetic aspects of mitochondrial genome evolution. Mol. Phylogenet. Evol. 2013, 69, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.M.; Zhang, L.H.; Lin, Y.J.; Zheng, Y.M.; Jin, W.T.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. The genetic diversity in Thereuonema tuberculata (Wood, 1862) (Scutigeromorpha: Scutigeridae) and the phylogenetic relationship of Scutigeromorpha using the mitochondrial genome. Insects 2022, 13, 620. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ye, F.; Huang, Y. Mitochondrial genomes of four katydids (Orthoptera: Phaneropteridae): New gene rearrangements and their phylogenetic implications. Gene 2016, 575, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.Q.; Shu, X.H.; Meng, L.; Li, B.P. Complete mitochondrial genomes of three Oxya grasshoppers (Orthoptera) and their implications for phylogenetic reconstruction. Genomics 2020, 112, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, D.R. Animal mitochondrial DNA: Structure and evolution. Int. Rev. Cytol. 1992, 141, 173–216. [Google Scholar] [CrossRef]
- Li, R.; Shu, X.H.; Li, X.D.; Meng, L.; Li, B.P. Comparative mitogenome analysis of three species and monophyletic inference of Catantopinae (Orthoptera: Acridoidea). Genomics 2019, 111, 1728–1735. [Google Scholar] [CrossRef]
- Li, R.; Ying, X.; Deng, W.; Rong, W.; Li, X. Mitochondrial genomes of eight Scelimeninae species (Orthoptera) and their phylogenetic implications within Tetrigoidea. PeerJ 2021, 9, e10523. [Google Scholar] [CrossRef]
- Qiu, Z.; Chang, H.H.; Yuan, H.; Huang, Y.; Lu, H.M.; Li, X.; Gou, X.C. Comparative mitochondrial genomes of four species of Sinopodisma and phylogenetic implications (Orthoptera, Melanoplinae). Zookeys 2020, 969, 23–42. [Google Scholar] [CrossRef]
- Yin, H.; Zhi, Y.C.; Jiang, H.D.; Wang, P.X.; Yin, X.C.; Zhang, D.C. The complete mitochondrial genome of Gomphocerus tibetanus Uvarov, 1935 (Orthoptera: Acrididae: Gomphocerinae). Gene 2012, 494, 214–218. [Google Scholar] [CrossRef]
- Yuan, H.; Huang, Y.; Mao, Y.; Zhang, N.; Nie, Y.M.; Zhang, X.; Zhou, Y.F.; Mao, S.L. The evolutionary patterns of genome size in Ensifera (Insecta: Orthoptera). Front. Genet. 2021, 12, 693541. [Google Scholar] [CrossRef]
- Zhang, D.C.; Zhi, Y.C.; Yin, H.; Li, X.J.; Yin, X.C. The complete mitochondrial genome of Thrinchus schrenkii (Orthoptera: Caelifera, Acridoidea, Pamphagidae). Mol. Biol. Rep. 2011, 38, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.J.; Shi, F.M.; Huang, Y. The complete mitogenome of the Chinese bush cricket, Gampsocleis gratiosa (Orthoptera: Tettigonioidea). J. Genet. Genom. 2008, 35, 341–348. [Google Scholar] [CrossRef]
- Voronova, N.V.; Levykina, S.; Warner, D.; Shulinski, R.; Bandarenka, Y.; Zhorov, D. Characteristic and variability of five complete aphid mitochondrial genomes: Aphis fabae mordvilkoi, Aphis craccivora, Myzus persicae, Therioaphis tenera and Appendiseta robiniae (Hemiptera; Sternorrhyncha; Aphididae). Int. J. Biol. Macromol. 2020, 149, 187–206. [Google Scholar] [CrossRef]
- Wang, W.Q.; Huang, Y.X.; Bartlett, C.R.; Zhou, F.M.; Meng, R.; Qin, D.Z. Characterization of the complete mitochondrial genomes of two species of the genus Aphaena Guérin-Méneville (Hemiptera: Fulgoridae) and its phylogenetic implications. Int. J. Biol. Macromol. 2019, 141, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, Z.M.; Yin, J.; Zhang, T.T.; Zhang, X.Y.; Yuan, D.W.; Li, T.; Zhong, Y.; Ma, B.E.; Ren, Z.M. Complete mitochondrial genome of two Thitarodes species (Lepidoptera, Hepialidae), the host moths of Ophiocordyceps sinensis and phylogenetic implications. Int. J. Biol. Macromol. 2019, 140, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Huang, Y. Complete mitochondrial genome sequence of Acrida cinerea (Acrididae: Orthoptera) and comparative analysis of mitochondrial genomes in Orthoptera. Comp. Funct. Genom. 2010, 2010, 319486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Tamura, K.; Aotsuka, T. Replication origin of mitochondrial DNA in insects. Genetics 2005, 171, 1695–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
- Yang, Z.H.; Bielawski, J.P. Statistical methods for detecting molecular adaptation. Trends Ecol. Evol. 2000, 15, 496–503. [Google Scholar] [CrossRef]
- Popadin, K.Y.; Nikolaev, S.I.; Junier, T.; Baranova, M.; Antonarakis, S.E. Purifying selection in mammalian mitochondrial protein-coding genes is highly effective and congruent with evolution of nuclear genes. Mol. Biol. Evol. 2012, 30, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.L.; Gao, K.; Wang, J.S.; Hebert, P.D.; Hua, B.Z. Molecular phylogeny and species delimitation of the genus Dicerapanorpa (Mecoptera: Panorpidae). Zool. J. Linn. Soc. 2019, 187, 1173–1195. [Google Scholar] [CrossRef]
- Ricarte, A.; Nedeljkovic, Z.; Aguado-Aranda, P.; Marcos-Garcia, M.A. Assessing the diversity and systematics of Brachyopini hoverflies (Diptera: Syrphidae) in the Iberian Peninsula, including the descriptions of two new species†. Insects 2022, 13, 648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.D.; Wang, H.J.; Zhou, Z.J.; Shi, F.M. Phylogeny and integrative taxonomy of the genera Gymnaetoides and Pseudotachycines (Orthoptera: Rhaphidophoridae). Insects 2022, 13, 628. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Xie, H.C.; Liu, Y.J.; Li, K.; He, Z.Q. The complete mitochondrial genome of cricket Sclerogryllus punctatus (Orthoptera: Gryllidae) and phylogenetic analysis. J. Asia-Pac. Entomol. 2022, 25, 101933. [Google Scholar] [CrossRef]
- Li, X.D.; Jiang, G.F.; Yan, L.Y.; Li, R.; Mu, Y.; Deng, W.A. Positive selection drove the adaptation of mitochondrial genes to the demands of flight and high-altitude environments in grasshoppers. Front. Genet. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.L.; Zhang, L.; Zhao, T.X.; Wang, J.; Zhu, Q.H.; Chen, J.Y.; Yuan, M.L. Gene sequence variations and expression patterns of mitochondrial genes are associated with the adaptive evolution of two Gynaephora species (Lepidoptera: Lymantriinae) living in different high-elevation environments. Gene 2017, 610, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Bollazzi, M.; Forti, L.C.; Roces, F. Ventilation of the giant nests of Atta leaf-cutting ants: Does underground circulating air enter the fungus chambers? Insect. Soc. 2012, 59, 487–498. [Google Scholar] [CrossRef]
- Hsu, P.W.; Hugel, S.; Wetterer, J.K.; Tseng, S.P.; Ooi, C.S.M.; Lee, C.Y.; Yang, C.C.S. Ant crickets (Orthoptera: Myrmecophilidae) associated with the invasive yellow crazy ant Anoplolepis gracilipes (Hymenoptera: Formicidae): Evidence for cryptic species and potential co-introduction with hosts. Myrmecol. News 2020, 30, 103–129. [Google Scholar] [CrossRef]
- Li, T.C.; Shao, M.A.; Jia, Y.H.; Jia, X.X.; Huang, L.M. Small-scale observation on the effects of the burrowing activities of mole crickets on soil erosion and hydrologic processes. Agr. Ecosyst. Environ. 2018, 261, 136–143. [Google Scholar] [CrossRef]
- Misof, B.; Liu, S.; Meusemann, K.; Peters, R.S.; Donath, A.; Mayer, C.; Frandsen, P.B.; Ware, J.; Flouri, T.; Beutel, R.G.; et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 2014, 346, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.S.; DeBry, R.W.; Douady, C.; Amrine, H.M.; Madsen, O.; de Jong, W.W.; Stanhope, M.J. Mitochondrial versus nuclear gene sequences in deep-level mammalian phylogeny reconstruction. Mol. Biol. Evol. 2001, 18, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Hawlitschek, O.; Ortiz, E.M.; Noori, S.; Webster, K.C.; Husemann, M.; Pereira, R.J. Transcriptomic data reveals nuclear-mitochondrial discordance in Gomphocerinae grasshoppers (Insecta: Orthoptera: Acrididae). Mol. Phylogenet. Evol. 2022, 170, 107439. [Google Scholar] [CrossRef] [PubMed]
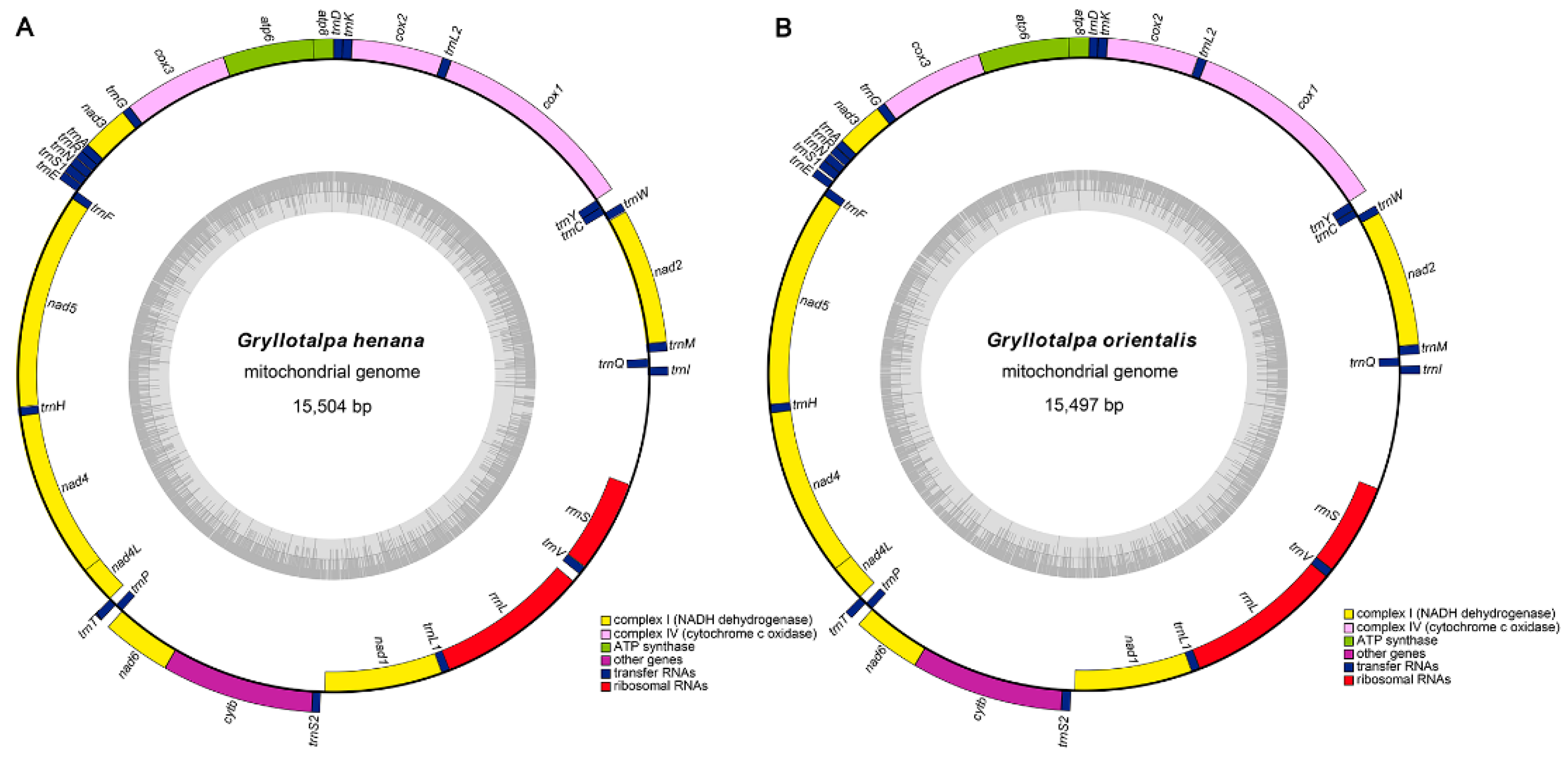
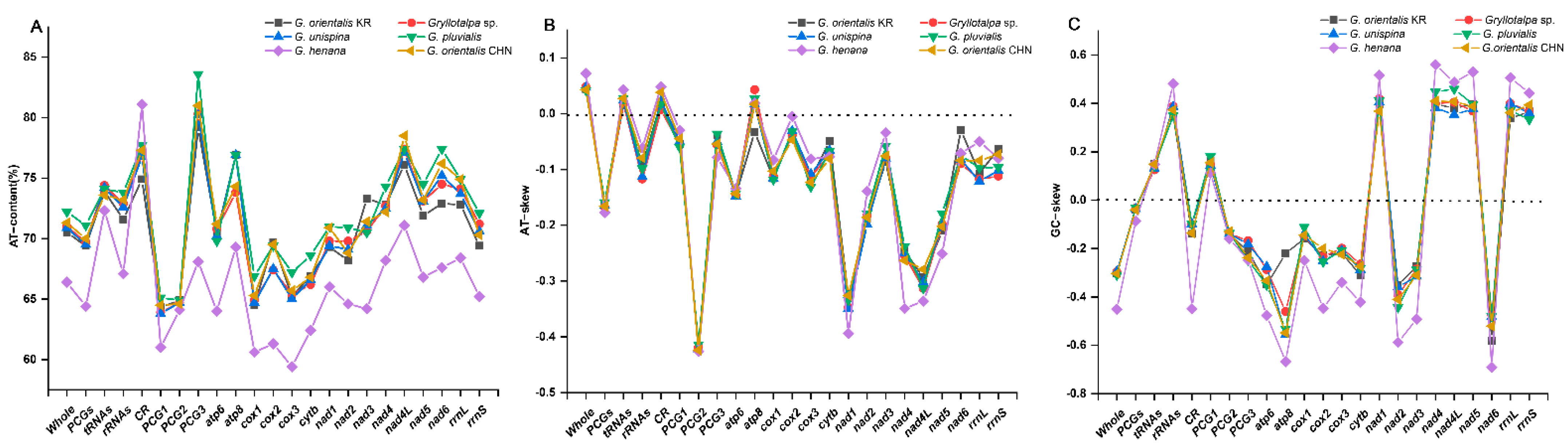
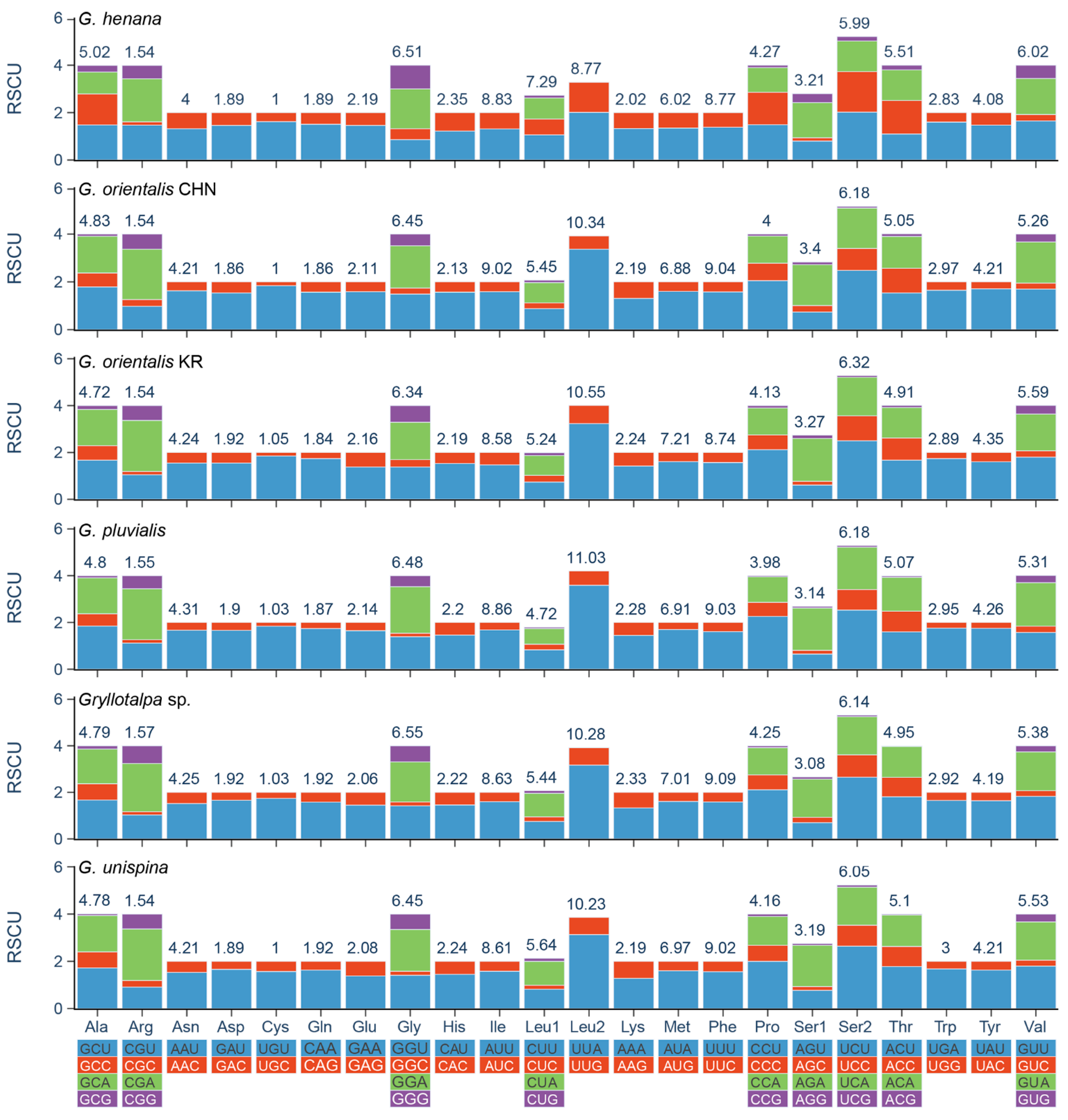
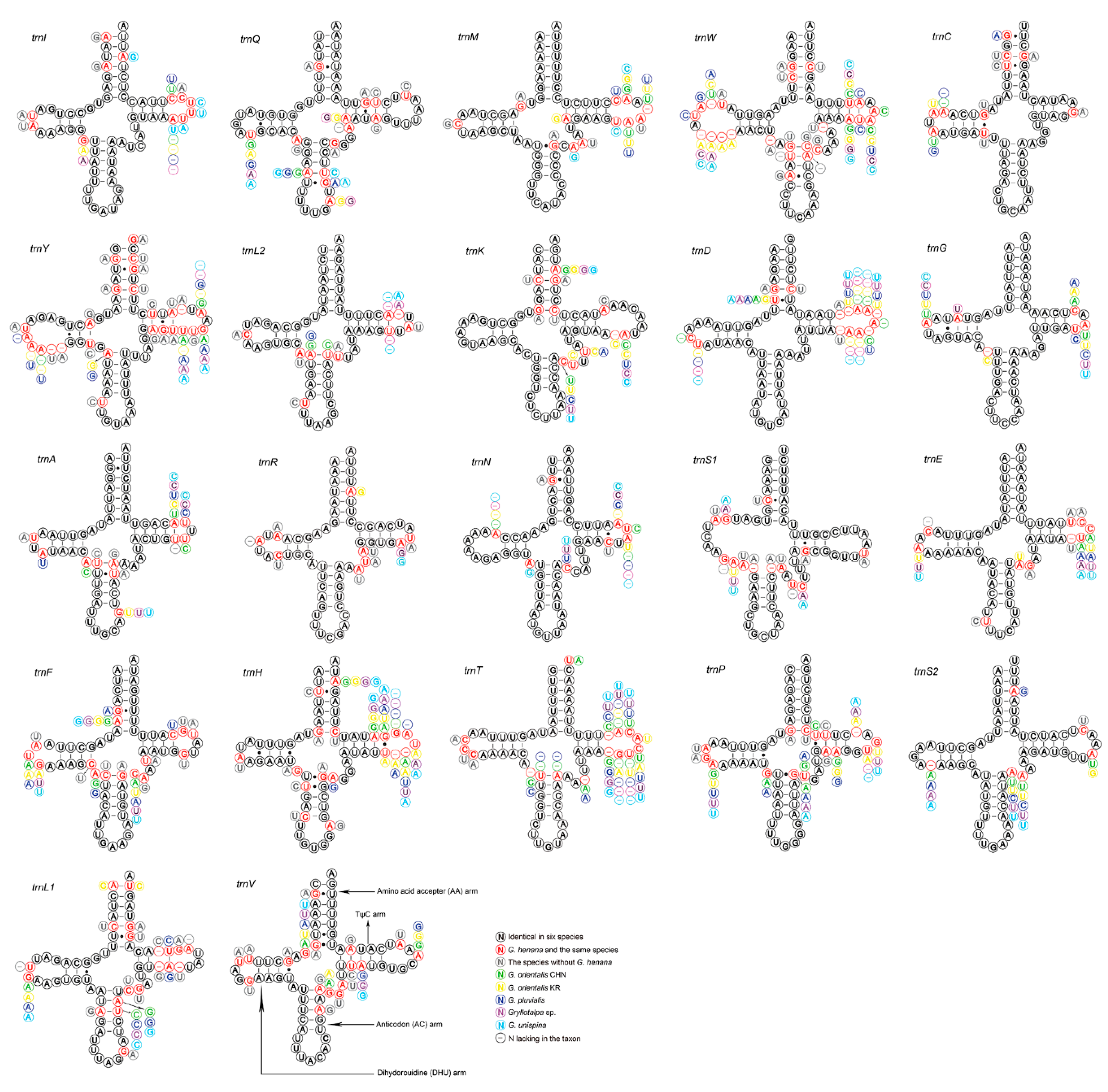

| Feature | Size (bp) | A% | G% | AT% | AT-Skew | GC-Skew |
|---|---|---|---|---|---|---|
| Whole genome | 15,504/15,497/15,521/ 15,525/15,506/15,513 | 35.6/37.2/36.8/ 37.6/37.2/37.1 | 9.2/10.0/10.3/ 9.6/10.2/10.3 | 66.4/71.3/70.5/ 72.2/71.0/70.9 | 0.072/0.043/0.044/ 0.042/0.048/0.047 | −0.451/−0.303/−0.302/ −0.309/−0.297/−0.295 |
| PCGs | 11,097/11,109/11,091/ 11,064/11,064/11,109 | 26.5/29.2/29.1/ 29.9/29.0/29.0 | 16.2/14.4/14.7/ 14.0/14.7/14.7 | 64.4/70.0/69.4/ 71.1/69.7/69.5 | −0.177/−0.166/−0.161/ −0.159/−0.168/−0.165 | −0.087/−0.040/−0.039/ −0.031/−0.033/−0.036 |
| 1st codon position | 3699/3703/3697/ 3688/3688/3703 | 29.6/30.8/30.4/ 30.6/30.2/30.3 | 21.7/20.5/20.7/ 20.6/20.7/20.7 | 61.0/64.5/64.3/ 65.1/63.9/63.8 | −0.030/−0.045/−0.054/ −0.060/−0.055/−0.050 | 0.113/0.155/0.163/ 0.181/0.147/0.144 |
| 2st codon position | 3699/3703/3697/ 3688/3688/3703 | 18.4/18.6/18.9/ 19.0/18.8/18.7 | 15.1/15.4/15.1/ 15.2/15.2/15.2 | 64.1/64.6/64.9/ 64.9/64.7/64.7 | −0.426/−0.424/−0.418/ −0.414/−0.419/−0.422 | −0.159/−0.130/−0.140/ −0.136/−0.139/−0.139 |
| 3st codon position | 3699/3703/3697/ 3688/3688/3703 | 31.4/38.3/38.1/ 40.3/37.9/38.1 | 12.0/7.2/8.2/ 6.2/8.2/8.1 | 68.1/81.0/79.2/ 83.6/80.3/80.2 | −0.078/−0.054/−0.038/ −0.036/−0.056/−0.050 | −0.248/−0.238/−0.212/ −0.248/−0.168/−0.182 |
| tRNAs | 1443/1440/1447/ 1447/1443/1443 | 37.7/37.8/37.5/ 38.1/37.9/38.0 | 15.9/15.1/15.0/ 14.7/14.4/14.6 | 72.3/73.6/73.9/ 74.1/74.4/74.2 | 0.043/0.027/0.015/ 0.028/0.019/0.024 | 0.148/0.148/0.149/ 0.140/0.125/0.132 |
| rRNAs | 1947/1966/1966/ 2019/2013/1970 | 31.5/33.7/32.5/ 33.3/32.2/32.2 | 24.3/18.4/19.2/ 17.7/18.8/19.0 | 67.1/73.2/71.6/ 73.8/72.9/72.6 | −0.061/−0.079/−0.092/ −0.098/−0.117/−0.113 | 0.482/0.373/0.347/ 0.351/0.387/0.387 |
| CR | 863/913/920/ 867/868/917 | 42.5/40.1/38.2/ 39.5/38.7/39.3 | 5.2/9.8/10.8/ 10.0/10.4/10.5 | 81.1/77.3/74.9/ 77.7/76.9/76.8 | 0.048/0.038/0.020/ 0.017/0.007/0.023 | −0.450/−0.137/−0.139/ −0.099/−0.100/−0.099 |
| Gene | Position | Size | IGN | Codon | Direction | ||
|---|---|---|---|---|---|---|---|
| From | To | Start | Stop | ||||
| trnI | 1/1/1/1/1/1/1 | 66/65/65/65/65/66 | 66/65/65/ 65/65/66 | J/J/J/J/J/J | |||
| trnQ | 64/63/63/63/63/64 | 132/130/130/ 130/130/131 | 69/68/68/ 68/68/68 | −3/−3/−3/ −3/−3/−3 | N/N/N/N/N/N | ||
| trnM | 180/148/153/ 150/153/159 | 247/216/221/ 218/221/226 | 68/69/69/ 69/69/68 | 47/17/22/ 19/22/27 | J/J/J/J/J/J | ||
| nad2 | 249/218/223/ 232/230/228 | 1256/1231/1237/ 1237/1240/1239 | 1008/1014/1015/ 1006/1011/1012 | −1/1/1/13/8/1 | GTG/GTG/GTG/ ATT/ATT/GTG | TAA/TAA/T/ T/TAA/T | J/J/J/J/J/J |
| trnW | 1261/1230/1238/ 1238/1239/1240 | 1325/1293/1305/ 1303/1303/1304 | 65/64/68/ 66/65/65 | 4/−2/-/-/−2/- | J/J/J/J/J/J | ||
| trnC | 1318/1286/1298/ 1296/1296/1297 | 1379/1346/1359/ 1356/1357/1358 | 62/61/62/ 61/62/62 | −8/−8/−8/ −8/−8/−8 | N/N/N/N/N/N | ||
| trnY | 1379/1347/1360/ 1357/1358/1359 | 1445/1415/1425/ 1425/1426/1427 | 67/69/66/ 69/69/69 | −1/-/-/-/-/- | N/N/N/N/N/N | ||
| cox1 | 1447/1419/1427/ 1427/1428/1429 | 2980/2952/2960/ 2960/2961/2962 | 1534/1534/1534/ 1534/1534/1534 | 1/3/1/1/1/1 | ATG/ATG/ATG/ ATG/ATG/ATG | T/T/T/T/T/T | J/J/J/J/J/J |
| trnL2 | 2981/2953/2961/ 2961/2962/2963 | 3045/3017/3025/ 3025/3025/3026 | 65/65/65/ 65/64/64 | -/-/-/-/-/- | J/J/J/J/J/J | ||
| cox2 | 3046/3019/3027/ 3027/3028/3029 | 3727/3700/3708/ 3708/3709/3710 | 682/682/682/ 682/682/682 | -/1/1/1/2/2 | ATG/ATG/ATG/ ATG/ATG/ATG | T/T/T/T/T/T | J/J/J/J/J/J |
| trnK | 3728/3701/3709/ 3709/3710/3711 | 3797/3770/3779/ 3778/3779/3780 | 70/70/71/ 70/70/70 | -/-/-/-/-/- | J/J/J/J/J/J | ||
| trnD | 3797/3770/3779/ 3778/3779/3780 | 3862/3834/3843/ 3847/3843/3844 | 66/65/65/ 70/65/65 | −1/−1/−1/ −1/−1/−1 | J/J/J/J/J/J | ||
| atp8 | 3863/3835/3884/ 3857/3844/3845 | 4018/3990/3999/ 4003/3999/4000 | 156/156/156/ 147/156/156 | -/-/-/9/-/- | ATT/ATT/ATT/ ATA/ATT/ATT | TAA/TAA/TAA/ TAA/TAA/TAA | J/J/J/J/J/J |
| atp6 | 4012/3984/3993/ 4000/3996/3994 | 4689/4661/4669/ 4674/4670/4671 | 678/678/677/ 675/675/678 | −7/−7/−7/ −4/−4/−7 | ATG/ATG/ATG/ATA/ATA/ATG | TAA/TAA/TA/ TAA/TAA/TAA | J/J/J/J/J/J |
| cox3 | 4689/4661/4670/ 4674/4670/4671 | 5472/5444/5453/ 5457/5453/5454 | 784/784/784/ 784/784/784 | −1/−1/-/ −1/−1/−1 | ATG/ATG/ATG/ATG/ATG/ATG | T/T/T/T/T/T | J/J/J/J/J/J |
| trnG | 5473/5445/5454/ 5458/5454/5455 | 5535/5507/5517/ 5520/5516/5517 | 63/63/64/ 63/63/63 | -/-/-/-/-/- | J/J/J/J/J/J | ||
| nad3 | 5536/5508/5518/ 5530/5517/5518 | 5887/5859/5869/ 5872/5870/5869 | 352/352/352/ 343/354/352 | -/-/-/9/-/- | ATT/ATT/ATT/ATA/ATT/ATT | T/T/T/T/TAG/T | J/J/J/J/J/J |
| trnA | 5888/5860/5870/ 5873/5869/5870 | 5951/5921/5932/ 5935/5931/5932 | 64/62/63/ 63/63/63 | -/-/-/-/−2/- | J/J/J/J/J/J | ||
| trnR | 5951/5922/5932/ 5935/5931/5932 | 6013/5983/5993/ 5996/5992/5993 | 63/62/62/ 62/62/62 | −1/-/−1/ −1/−1/−1 | J/J/J/J/J/J | ||
| trnN | 6015/5989/5999/ 6002/5994/5995 | 6080/6052/6062/ 6066/6057/6058 | 66/64/64/ 65/64/64 | 1/5/5/5/1/1 | J/J/J/J/J/J | ||
| trnS1 | 6081/6053/6063/ 6068/6058/6059 | 6147/6119/6129/ 6132/6124/6125 | 67/67/67/ 65/67/67 | -/-/-/1/-/- | J/J/J/J/J/J | ||
| trnE | 6149/6135/6141/ 6149/6135/6136 | 6213/6199/6205/ 6213/6199/6200 | 65/65/65/ 65/65/65 | 1/15/11/ 16/10/10 | J/J/J/J/J/J | ||
| trnF | 6212/6198/6204/ 6212/6198/6199 | 6276/6262/6268/ 6276/6262/6263 | 65/65/65/ 65/65/65 | −2/−2/−2/ −2/−2/−2 | N/N/N/N/N/N | ||
| nad5 | 6277/6263/6269/ 6277/6263/6264 | 7999/7985/7991/ 7999/7985/7986 | 1723/1723/1723/ 1723/1723/1723 | -/-/-/-/-/- | ATG/ATG/ATG/ATG/ATG/ATG | T/T/T/T/T/T | N/N/N/N/N/N |
| trnH | 8003/7987/7993/ 8001/7987/7988 | 8066/8050/8058/ 8064/8050/8051 | 64/64/66/ 64/64/64 | 3/1/1/1/1/1 | N/N/N/N/N/N | ||
| nad4 | 8067/8051/8059/ 8065/8051/8052 | 9402/9386/9394/ 9400/9386/9387 | 1336/1336/1336/ 1336/1336/1336 | -/-/-/-/-/- | ATG/ATG/ATG/ATG/ATG/ATG | T/T/T/T/T/T | N/N/N/N/N/N |
| nad4L | 9396/9380/9388/ 9394/9380/9381 | 9692/9676/9684/ 9690/9676/9677 | 297/297/297/ 297/297/297 | −7/−7/−7/ −7/−7/−7 | ATG/ATG/ATG/ATG/ATG/ATG | TAA/TAA/TAA/ TAA/TAA/TAA | N/N/N/N/N/N |
| trnT | 9695/9679/9687/ 9693/9679/9680 | 9759/9742/9750/ 9756/9743/9744 | 65/64/64/ 64/65/65 | 2/2/2/2/2/2 | J/J/J/J/J/J | ||
| trnP | 9760/9743/9751/ 9757/9744/9745 | 9824/9807/9816/ 9821/9808/9809 | 65/65/66/ 65/65/65 | -/-/-/-/-/- | N/N/N/N/N/N | ||
| nad6 | 9827/9810/9819/ 9842/9829/9812 | 10,345/10,322/10,330/ 10,336/10,323/10,324 | 519/513/512/ 495/495/513 | 2/2/2/20/20/2 | ATC/ATT/ATT/ATA/ATA/ATT | TAA/TAA/TA/ TAA/TAA/TAA | J/J/J/J/J/J |
| cytb | 10,345/10,322/10,331/ 10,336/10,323/10,324 | 11,478/11,455/11,462/ 11,467/11,456/11,455 | 1134/1134/1132/ 1132/1134/1132 | −1/−1/-/ −1/−1/−1 | ATG/ATG/ATG/ATG/ATG/ATG | TAA/TAA/T/T/TAA/T | J/J/J/J/J/J |
| trnS2 | 11,477/11,454/11,463/ 11,468/11,455/11,456 | 11,540/11,522/11,530/ 11,536/11,523/11,524 | 64/69/68/ 69/69/69 | −2/−2/-/-/−2/- | J/J/J/J/J/J | ||
| nad1 | 11,569/11,548/11,565/ 11,569/11,555/11,556 | 12,504/12,483/12,500/ 12,504/12,490/12,491 | 936/936/936/ 936/936/936 | 28/25/34/ 32/31/31 | ATG/ATG/ATG/ATG/ATG/ATG | TAG/TAA/TAA/ TAG/TAA/TAA | N/N/N/N/N/N |
| trnL1 | 12,506/12,485/12,502/ 12,506/12,492/12,493 | 12,570/12,549/12,566/ 12,570/12,556/12,557 | 65/65/65/ 65/65/65 | 1/1/1/1/1/1 | N/N/N/N/N/N | ||
| rrnL | 12,554/12,550/12,567/ 12,571/12,557/12,558 | 13,767/13,785/13,813/ 13,806/13,793/13,802 | 1214/1236/1247/ 1236/1237/1245 | −17/-/-/-/-/- | N/N/N/N/N/N | ||
| trnV | 13,839/13,786/13,814/ 13,807/13,794/13,803 | 13,907/13,854/13,882/ 13,875/13,862/13,871 | 69/69/69/ 69/69/69 | 71/-/-/-/-/- | N/N/N/N/N/N | ||
| rrnS | 13,909/13,855/13,883/ 13,876/13,863/13,872 | 14,641/14,584/14,601/ 14,658/14,638/14,596 | 733/730/719/ 783/776/725 | 1/-/-/-/-/- | N/N/N/N/N/N | ||
| CR | 14,642/14,585/14,602/ 14,659/14,639/14,597 | 15,504/15,497/15,521/ 15,525/15,506/15,513 | 863/913/920/ 867/868/917 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Miao, Y. Mitogenomic Comparison of the Mole Crickets Gryllotalpidae with the Phylogenetic Implications (Orthoptera: Ensifera). Insects 2022, 13, 919. https://doi.org/10.3390/insects13100919
Ma Y, Miao Y. Mitogenomic Comparison of the Mole Crickets Gryllotalpidae with the Phylogenetic Implications (Orthoptera: Ensifera). Insects. 2022; 13(10):919. https://doi.org/10.3390/insects13100919
Chicago/Turabian StyleMa, Yan, and Ying Miao. 2022. "Mitogenomic Comparison of the Mole Crickets Gryllotalpidae with the Phylogenetic Implications (Orthoptera: Ensifera)" Insects 13, no. 10: 919. https://doi.org/10.3390/insects13100919
APA StyleMa, Y., & Miao, Y. (2022). Mitogenomic Comparison of the Mole Crickets Gryllotalpidae with the Phylogenetic Implications (Orthoptera: Ensifera). Insects, 13(10), 919. https://doi.org/10.3390/insects13100919






