Fall Armyworm Infestation and Development: Screening Tropical Maize Genotypes for Resistance in Zambia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Method
2.1. Description of the Study Sites
2.2. Mass Production of FAW
2.2.1. Sampling of Eggs and Larvae
2.2.2. Laboratory Procedures
2.2.3. Assessing the Survival Rate of FAW Larvae on the Natural and Artificial Diets
2.2.4. Determining the Developmental Stages of FAW
2.3. Screening of Selected Maize Genotypes for FAW Resistance
2.3.1. Genetic Materials
2.3.2. Experimental Design and Trial Establishment
2.3.3. Seedling Infestation with FAW Larvae
2.4. Data Collection
2.4.1. FAW Larvae Survival and Developmental Stages
2.4.2. Reaction of Maize Genotypes to FAW
2.5. Data Analysis
2.5.1. Survival Rates of FAW Larvae under Two Contrasting Diets
2.5.2. Developmental Stages of FAW under Laboratory Conditions
2.5.3. Analysis of Variance on Leaf Damage Scores Mean Comparison Maize Genotypes
2.5.4. The Area under the Pest Progress Curve
- represents the mean of the ith fall armyworm leaf damage (FLD) across the three replications, beginning with FLD0 to FLD5
- represents the mean of the ith FLD plus 1
- represents the ith time point at which leaf damage assessments were made, beginning with 14 days to 32 days after the first signs of FAW infestation
- represents the ith time plus 1
3. Results
3.1. Survival Rates of FAW Larvae under Two Contrasting Diets
3.2. Developmental Stages of FAW under Zambian Conditions
3.3. Selection of Maize Genotypes with FAW Resistance under Controlled Screening
3.3.1. Analysis of Variance Based on Leaf Damage Scores
3.3.2. Mean Performance of Test Genotypes
3.3.3. Nature of FAW Damage and Reaction of Test Genotypes to Artificial FAW Infestation
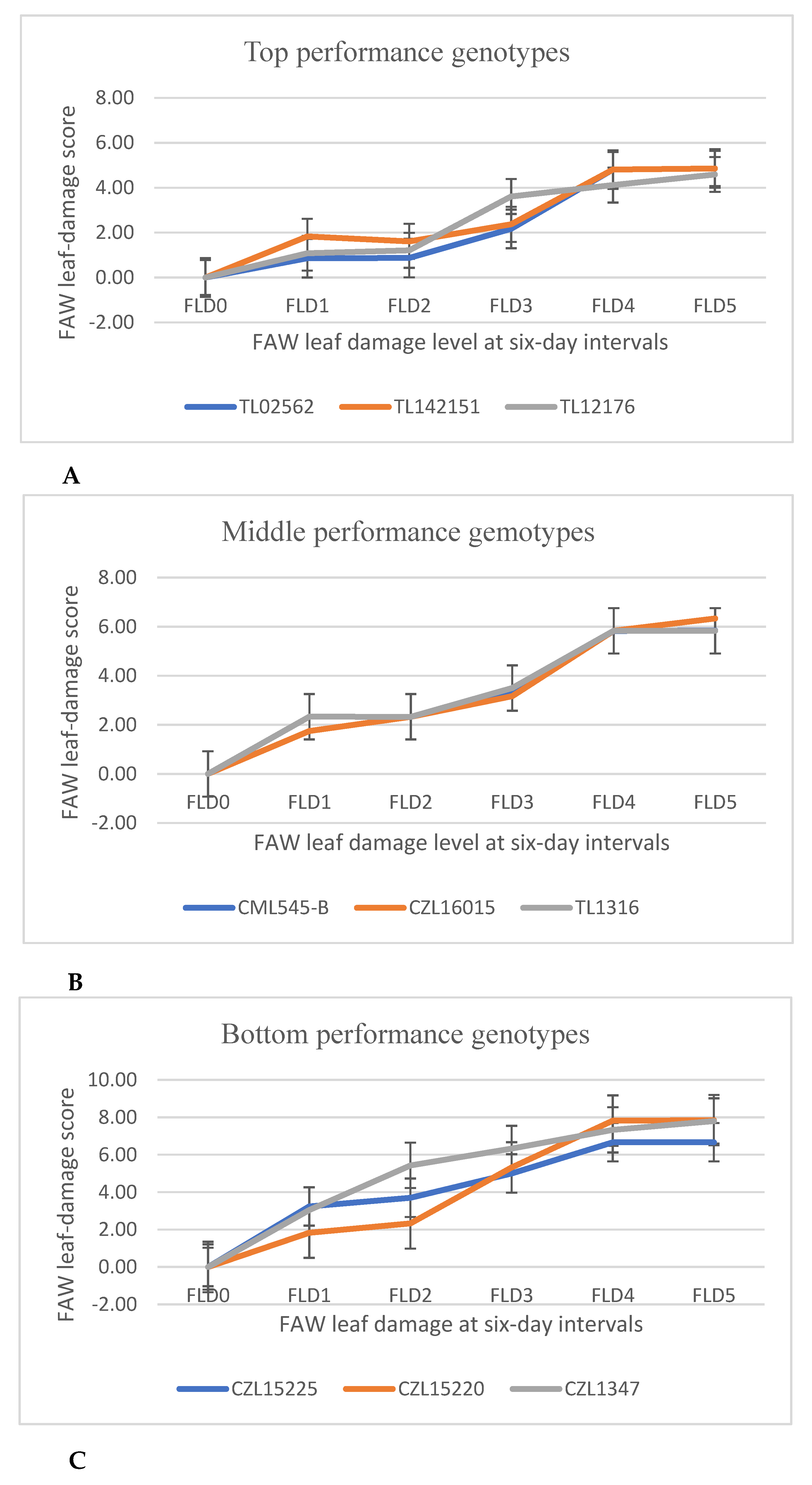
3.3.4. The Area under Pest Progress Curve (AUPPC)
4. Discussion
4.1. Rearing of FAW on the Natural vs. Artificial Diet
4.2. Developmental Stages of the FAW
4.3. Artificial Infestation of Maize Genotypes
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrahams, P.; Beale, T.; Cock, M.; Corniani, N.; Day, R.; Godwin, J.; Gomez, J.; Moreno, P.G.; Murphy, S.T.; Opon-Mensah, B.; et al. Fall Armyworm Status, Impacts and Control Options in Africa: Preliminary Evidence Note; CABI, UKAid: Wallingford, UK, 2017. [Google Scholar]
- Guimapi, R.A.; Niassy, S.; Mudereri, B.T.; Abdel-Rahman, E.M.; Tepa-Yotto, G.T.; Subramanian, S.; Mohamed, S.A.; Thunes, K.H.; Kimathi, E.; Agboka, K.M.; et al. Harnessing data science to improve integrated management of invasive pest species across Africa: An application to fall amyworm (Spodoptera frugiperda) (J.E. Smith) (Lepidoptera: Noctuidae). Glob. Ecol. Conserv. 2022, 35, e0205. [Google Scholar] [CrossRef]
- Niassy, S.; Agbodzavu, M.K.; Kimathi, E.; Mutune, B.; Abdel-Rahman, E.F.M.; Salifu, D.; Hailu, G.; Belayneh, Y.T.; Felege, E.; Tonnang, H.E.Z.; et al. Bioecology of fall armyworm Spodoptera frugiperda (J. E. Smith), its management and potential patterns of seasonal spread in Africa. PLoS ONE 2021, 16, e0249042. [Google Scholar] [CrossRef] [PubMed]
- Sokame, B.M.; Musyoka, B.; Obonyo, J.; Rebaudo, F.; Abdel-Rahman, E.M.; Subramanian, S.; Kila-lo, D.C.; Juma, G.; Calatayud, P.A. Impact of an exotic invasive pest, Spodoptera frugiperda (Lepidoptera: Noctuidae), on resident communities of pest and natural enemies in maize fields in Kenya. Agronomy 2021, 11, 1074. [Google Scholar] [CrossRef]
- Macauley, H.; Ramadjita, T. Cereal crops: Rice, maize, millet, sorghum wheat. Feed. Afr. 2015, 36. [Google Scholar]
- CABI Centre for Agriculture and Bioscience International. Spodoptera frugiperda [Original text by Rwomushana]. In Invasive Species Compedium; CABI International: Wallingford, UK, 2019; Available online: www.cabi.org.ics (accessed on 8 January 2020).
- Kasoma, C.; Shimelis, H.; Laing, M.D.; Shayanowako, A.I.T.; Mathew, I. Outbreaks of the fall armyworm Spodoptera frugiperda and maize production constraintsin Zambia with special emphaiss on coping strategies. Sustainability 2021, 13, 10771. [Google Scholar] [CrossRef]
- Prasanna, B.; Huesing, J.; Eddy, R.; Peschke, V. Fall Armyworm in Africa: A Guide for Integrated Pest ManagementCIMMYT; USAID, Mexico. 2018. Available online: https://repository.cimmyt.org/xmlui/handle/10883/19204 (accessed on 21 October 2018).
- Stokstad, E. New crop pest takes Africa at lightning speed. Science 2017, 356, 473–474. [Google Scholar] [CrossRef]
- Overton, K.; Maino, J.L.; Day, R.; Umina, P.A.; Bett, B.; Carnovale, D.; Ekesi, S.; Meagher, R.; Reynolds, O.L. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop. Prot. 2021, 145, 105641. [Google Scholar] [CrossRef]
- Abro, Z.; Kimathi, E.; De Groote, H.; Tefera, T.; Sevgan, S.; Niassy, S.; Kassie, M. Socioeconomic and health impacts of fall armyworm in Ethiopia. PLoS ONE 2021, 16, e0257736. [Google Scholar] [CrossRef]
- Agyei-Holmes, A.; Ankrah, D.A.; Boakye, A.A. Covid-1 and Ghana’s agri food system: An assessment of resilience. Afr. Geogr. Rev. 2021, 2021, 1–22. [Google Scholar] [CrossRef]
- FAO Food and Agricultural Organization Integrated Management of the Fall Armyworm on Maize. A Guide for Farmer Field Schools in Africa. Food and Agricultural Organization (FAO) of the United Nations, Rome. 2018. Available online: http://www.fao.org/3/18665EN/i8665en.pdf (accessed on 2 December 2018).
- Kasoma, C.; Shimelis, H.; Laing, M.D. Fall armyworm invasion in Africa: Implications for maize production and breeding. J. Crop. Improv. 2020, 35, 111–146. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Prasanna, B.M.; Kalleshwaraswamy, C.M.; Jaba, J.; Choudhary, B. Fall Armyworm (Spodoptera frugiperda). In Polyphagous Pests of Crops; Omkar, Ed.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Padhee, A.K.; Prasanna, B.M. The emmerging threat of fall amryworm in India. Indian Farming 2019, 69, 51–54. [Google Scholar]
- Mihn, J. Efficient Mass Rearing and Infestation Techniques to Screen for Resistance to Spodoptera frugiperda; CIMMYT: Mexico City, Mexico, 1983. [Google Scholar]
- Castro, M.H.; Pitre, T.N. Development of fall armyworm Spodoptera frugiperda from Honduras and Mississipi on sorghum or corn in the laboratory. Fla. Entomol. Soc. 1988, 71, 49–59. [Google Scholar] [CrossRef]
- Williams, W.P.; Davis, F.M. Response of corn to artificial infestation with fall armyworm and southwestern corn borer larvae. Southwest. Entomol. 1990, 15, 163–166. [Google Scholar]
- Santos, L.M.; Redaelli, L.R.; Diefenbach, L.M.G.; Efrom, C.F.S. Larval and pupal stage of fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in sweet and field corn genotypes. Braz. J. Biol. 2003, 63, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Maruthadurai, R.; Ramesh, R. Occurrence, damage pattern and biology of fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) on fodder crops and green amaranth in Goa, India. Phytoparasitica 2019, 48, 15–23. [Google Scholar] [CrossRef]
- Jin, T.; Lin, Y.; Chi, H.; Xiang, K.; Ma, G.; Peng, Z.; Yi1, K. Comparative performance of the fall armyworm (Lepidoptera: Noctuidae) reared on various cereal-based artificial diets. J. Econ. Entomol. 2020, 113, 2986–2996. [Google Scholar] [CrossRef]
- Koffi, D.; Kyerematen, R.; Eziah, V.Y.; Agboka, K.; Adom, M.; Goergen, G.; Jr, R.L.M. Natural Enemies of the Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in Ghana. Fla. Èntomol. 2020, 103, 85–90. [Google Scholar] [CrossRef]
- Laminou, S.A.; Ba, M.N.; Karimoune, L.; Doumma, A.; Muniappan, R. Parasitism of Locally Recruited Egg Parasitoids of the Fall Armyworm in Africa. Insects 2020, 11, 430. [Google Scholar] [CrossRef]
- Walaa, E.G. Fall Armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) Biological Aspects as A New Alien Invasive Pest in Egypt. Egypt. Acad. J. Biol. Sci. 2020, 13, 189–196. [Google Scholar]
- Wiseman, B.R.; Painter, R.H.; Wassom, C.E. Detecting corn seedling differences in the green house by visual classification of damage by the fall armyworm. J. Econ. Entomol. 1966, 59, 1211–1214. [Google Scholar] [CrossRef]
- Perkins, W.D. Laboratory Rearing of the Fall Armyworm. Fla. Èntomol. 1979, 62, 87. [Google Scholar] [CrossRef]
- Silva, C.S.B.; Parra, J.R.P. New method for rearing Spodoptera frugiperda in the laboratory shows that larval cannibalism is not obligatory. Rev. Bras. Entomol. 2013, 57, 347–349. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Developmental parameters of Spodoptera frugiperda (Lepidoptera: Noctuidae) immature stages under controlled and standardized conditions. J. Agric. Sci. 2019, 8, 11. [Google Scholar] [CrossRef]
- Kasoma, C.; Shimelis, H.; Laing, M.D.; Shayanowako, A.I.T.; Mathew, I. Combining ability of maize genotypes for fall armyworm (Spodoptera frugiperda J.E. Smith) resistance, yield and yield-related traits. Crop Prot. 2021, 149, 105762. [Google Scholar] [CrossRef]
- Kasoma, C.; Shimelis, H.; Laing, M.; Shayanowako, A.I.; Mathew, I. Screening of inbred lines of tropical maize for resistance to fall armyworm, and for yield and yield-related traits. Crop Prot. 2020, 136, 105218. [Google Scholar] [CrossRef]
- Mukanga, M.; Matumba, L.; Makwenda, B.; Alfred, S.; Sakala, W.; Kanenga, K.; Chancellor, T.; Mugabe, J.; Bennett, B. Participatory evaluation of groundnut planting methods for pre-harvest aflatoxin management in Eastern Province of Zambia. Cah. Agric. 2019, 28, 1. [Google Scholar] [CrossRef]
- Deole, S.; Paul, N. First report of fall army worm, Spodoptera frugiperda (J.E. Smith), their nature of damage and biology on maize crop at Raipur, Chhattisgarh. J. Entomol. Zool. Stud. 2018, 6, 219–221. [Google Scholar]
- Capinera, J. Fall armyworm, Spodoptera frugiperda (J. E. Smith) (Insecta: Lepidoptera: Noctuidae); University of Florida: Gainesville, FL, USA, 2002; pp. 2–5. [Google Scholar]
- Du Plessis, H.; Schlemmer, M.-L.; Van den Berg, J. The Effect of Temperature on the Development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef]
- Luginbill, P. The Fall Armyworm; Technical Bulletin No. 34; United States Department of Agriculture: Washington, DC, USA, 1928; pp. 1–92. [Google Scholar]
- Firake, D.; Behere, G.; Babu, S.; Prakash, N. Fall armyworm.: Diagnosis and Management. (An Extension Pocket Book); ICAR Research Complex for NEH Region: Umiam, India, 2019. [Google Scholar]
- Mesce, K.A.; Fahbrach, S.E. Intergration of endocrine signals that regulate insect ecdysis. Front. Neuroendocrinol. 2002, 23, 179–199. [Google Scholar] [CrossRef]
- Davis, F.M.; Ng, S.S.; Williams, W.P. Visual rating scales for screening whorlstage corn for resistance to fall armyworm. Mississippi Agricultural; Forestry Experiment Station. Tech. Bull. 1992, 186, 2–5. [Google Scholar]
- Payne, R. A Guide to ANOVA and Design. In Genstat®, 18th ed.; VSN International: Hemel Hempstead, UK, 2017. [Google Scholar]
- Heinrichs, E.A.; Miller, T.A. Rice Insects: Management Strategies; Springer: New York, NY, USA, 1991; pp. 39–41. [Google Scholar]
- Jeger, M.J.; Viljanen-Rollinson, S.L.H. The use of the area under the disease-progress curve (AUDPC) to assess quantitative disease resistance in crop cultivars. Theor. Appl. Genet. 2001, 102, 32–40. [Google Scholar] [CrossRef]
- Acharya, R.; Hwang, H.; Mostafiz, M.; Yu, Y.; Lee, K. Susceptibility of Various Developmental Stages of the Fall Armyworm, Spodoptera frugiperda, to Entomopathogenic Nematodes. Insects 2020, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Baur, M.E.; Yeargan, K.V. Behavioral Interactions Between the Hyperparasitoid Mesochorus discitergus (Hymenoptera: Ichneumonidae) and Four Species of Noctuid Caterpillars: Evasive Tactics and Capture Efficiency. J. Èntomol. Sci. 1994, 29, 420–427. [Google Scholar] [CrossRef]
- López, R.Y.; Ortega, A.V.; Ceteno, J.H.A.; Ruiz, J.S.; Carranza, J.A.Q. Alert system against the fall armyworm Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae). Rev. Mex. De Cienc. Agrícolas 2019, 10, 405–416. [Google Scholar] [CrossRef]
- He, L.-M.; Wu, Q.-L.; Gao, X.-W.; Wu, K.-M. Population life tables for the invasive fall armyworm, Spodoptera frugiperda fed on major oil crops planted in China. J. Integr. Agric. 2021, 20, 745–754. [Google Scholar] [CrossRef]
- Hardke, J.T.; Lorenz, G.M.; Leonard, B.R. Fall Armyworm (Lepidoptera: Noctuidae) Ecology in Southeastern Cotton. J. Integr. Pest Manag. 2015, 6, 10. [Google Scholar] [CrossRef]
- Chapman, J.W.; Williams, T.; Escribano, A.; Caballero, P.; Cave, R.D.; Goulson, D. Fitness consequences of cannibalism in the fall armyworm, Spodoptera frugiperda. Behav. Ecol. 1999, 10, 298–303. [Google Scholar] [CrossRef]
- Badji, A.; Kwemoi, D.; Machida, L.; Okii, D.; Mwila, N.; Agbahoungba, S.; Kumi, F.; Ibanda, A.; Bararyenya, A.; Solemanegy, M.; et al. Genetic Basis of Maize Resistance to Multiple Insect Pests: Integrated Genome-Wide Comparative Mapping and Candidate Gene Prioritization. Genes 2020, 11, 689. [Google Scholar] [CrossRef]
- Mueller, D.; Sisson, A. Corn Field Guide: A Reference for Production Integrated Pest Management and Identification of Diseases, Insects and Disorders of Corn, 2nd ed.; Iowa State University: Ames, IA, USA, 2013. [Google Scholar]
| Instar | Mean Head Capsule Width (mm) |
|---|---|
| FIRST | 0.35 ± 0.02 |
| Second | 0.56 ± 0.03 |
| Third | 0.87 ± 0.04 |
| Fourth | 1.27 ± 0.06 |
| Fifth | 1.85 ± 0.12 |
| Sixth | 2.72 ± 0.20 |
| Genotype | Breeding History | Source | Presumed FAW Resistance * | Genotype | Breeding History | Source | Presumed FAW Resistance * | Genotypes | Breeding History | Source | Presumed FAW Resistance * |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CKDHL0323 | Inbred line | CIMMYT | MR | CZL15225 | Inbred line | CIMMYT | MR | Teost | OPV | NPGRC | - |
| CML441-B | Inbred line | CIMMYT | MR | CZL15123 | Inbred line | CIMMYT | MR | TL101711 | Inbred line | CIMMYT | - |
| CML488 | Inbred line | CIMMYT | MR | CZL15231 | Inbred line | CIMMYT | S | TL102562 | Inbred line | CIMMYT | - |
| CML491 | Inbred line | CIMMYT | S | CZL15234 | Inbred line | CIMMYT | MR | TL116163 | Inbred line | CIMMYT | MR |
| CML538 | Inbred line | CIMMYT | MR | CZL16015 | Inbred line | CIMMYT | S | TL118367 | Inbred line | CIMMYT | - |
| CML539 | Inbred line | CIMMYT | S | CZL16016 | Inbred line | CIMMYT | MR | TL12176 | Inbred line | CIMMYT | MR |
| CML545-B | Inbred line | CIMMYT | MR | CZL16080 | Inbred line | CIMMYT | S | TL13159 | Inbred line | CIMMYT | MR |
| CML546-B | Inbred line | CIMMYT | S | CZL16084 | Inbred line | CIMMYT | MR | TL1316 | Inbred line | CIMMYT | MR |
| CML547-B | Inbred line | CIMMYT | S | CZL16091 | Inbred line | CIMMYT | MR | TL139113 | Inbred line | CIMMYT | - |
| CML548-B | Inbred line | CIMMYT | MR | CZL16093 | Inbred line | CIMMYT | S | TL139180 | Inbred line | CIMMYT | MR |
| CML572 | Inbred line | CIMMYT | MR | CZL16095 | Inbred line | CIMMYT | MR | TL142017 | Inbred line | CIMMYT | R |
| CZL03011 | Inbred line | CIMMYT | S | CZL16098 | Inbred line | CIMMYT | MR | TL142139 | Inbred line | CIMMYT | MR |
| CZL052 | Inbred line | CIMMYT | MR | CZL16137 | Inbred line | CIMMYT | S | TL142151 | Inbred line | CIMMYT | MR |
| CZL1310c | Inbred line | CIMMYT | MR | CZL16141 | Inbred line | CIMMYT | MR | TL14266 | Inbred line | CIMMYT | - |
| CZL1347 | Inbred line | CIMMYT | S | EBL1611480 | Inbred line | CIMMYT | - | TL145748 | Inbred line | CIMMYT | - |
| CZL1369 | Inbred line | CIMMYT | MR | EBL169550 | Inbred line | CIMMYT | R | TL1512847 | Inbred line | CIMMYT | S |
| CZL1466 | Inbred line | CIMMYT | MR | EBL173782 | Inbred line | CIMMYT | - | TL1512845 | Inbred line | CIMMYT | MR |
| CZL15033 | Inbred line | CIMMYT | MR | EBL1738809 | Inbred line | CIMMYT | - | TL173 | Inbred line | CIMMYT | MR |
| CZL15142 | Inbred line | CIMMYT | S | MM501 | Hybrid | ZAMSEED | S | VL05120 | Inbred line | CIMMYT | - |
| CZL15209 | Inbred line | CIMMYT | MR | MM502 | Hybrid | ZAMSEED | MR | ZM4236 | OPV | NPGRC | MR |
| CZL15220 | Inbred line | CIMMYT | MR | Pool 16 | OPV | ZAMSEED | S | ZM7114 | OPV | NPGRC | MR |
| Symptom Description | Score |
|---|---|
| No visible damage | 1 |
| 2–4 windowpane-damaged portions | 2 |
| 2–4 windowpane-damaged portions and 2–4 pin/shot holes | 3 |
| 5–10 windowpane-damaged portions and shot holes | 4 |
| 10–15 windowpane-damaged portions and shreds only | 5 |
| 10–15 windowpane-damaged portions shot holes and shreds | 6 |
| 10–15 windowpane-damaged portions, shot holes, shreds and traces of whorl damage | 7 |
| ≥15 windowpane-damaged portions, shot holes, shreds and moderately damaged whorl | 8 |
| ≥15 windowpane-damaged portions, shot holes, shreds and completely damaged whorl | 9 |
| Set | Diet | |
|---|---|---|
| Natural | Artificial | |
| 1 | 11 (73.3%) | 4 (26.7%) |
| 2 | 13 (86.7%) | 8 (53.3%) |
| 3 | 9 (60.0%) | 7 (46.7%) |
| 4 | 15 (100.0%) | 5 (33.3%) |
| 5 | 12 (80.0%) | 6 (40.0% |
| 6 | 13 (86.7%) | 6 (40.0%) |
| 7 | 11 (73.3%) | 10 (66.67%) |
| 8 | 12 (80.00%) | 2 (13.3%) |
| t-statistics, (df = 7) | 5.15 | |
| Significance level | 0.000662 | |
| Standard deviation (SD) | 1.77 | 2.45 |
| Mean | 12 (80.00%) | 6 (40.00%) |
| Minimum | 9 | 2 |
| Maximum | 15 | 10 |
| Egg Batch ID | Life Cycle Stages | Number of FAW for Observation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egg | L1 | L2 | L3 | L4 | L5 | L6 | Pre-Pupa | Pupa | Adult | Initial Number at Neonate Stage | Number at Pupal Stage | |
| A | 2.0 | 2.0 | 3.6 | 3.6 | 1.4 | 2.3 | 12.2 | 4.7 | 17.7 | 7.0 | 15.0 | 5.0 |
| B | 3.0 | 3.0 | 2.9 | 3.2 | 2.7 | 2.7 | 13.4 | 3.6 | 19.7 | 23.0 | 15.0 | 8.0 |
| C | - | 2.8 | 2.7 | 2.7 | 2.7 | 3.4 | 11.3 | 4.3 | 19.8 | 5.0 | 15.0 | 13.0 |
| D | 2.3 | - | 2.9 | 2.9 | 2.9 | 2.2 | 9.8 | 3.0 | 22.0 | 7.0 | 15.0 | 13.0 |
| E | 2.2 | 2.8 | 2.8 | 2.8 | 2.8 | - | 10.8 | 2.8 | 25.6 | 2.0 | 15.0 | 15.0 |
| F | 2.0 | 2.9 | 2.6 | 3.0 | 2.7 | 2.6 | 11.0 | 3.4 | 18.2 | 17.3 | 15.0 | 9.0 |
| G | 2.7 | 2.0 | 2.0 | 3.0 | 3.4 | 2.6 | 5.4 | 2.8 | 15.0 | 20.3 | 15.0 | 4.0 |
| Mean | 2.4 | 2.6 | 2.8 | 3.0 | 2.7 | 2.6 | 10.6 | 3.5 | 19.7 | 11.7 | 15.0 | 9.6 |
| Standard deviation | 0.9 | 1.0 | 1.0 | 1.1 | 1.0 | 1.0 | 2.8 | 1.3 | 4.3 | 21.6 | 5.3 | 5.0 |
| Minimum | 2.0 | 2.0 | 2.0 | 2.7 | 1.4 | 2.2 | 5.4 | 2.8 | 15.0 | 2.0 | 15.0 | 4.0 |
| Maximum | 3.0 | 3.0 | 3.6 | 3.6 | 3.4 | 3.4 | 13.4 | 4.7 | 25.6 | 23.0 | 15.0 | 15.0 |
| F-statistic | 130.85 *** | 1.42 ns | 13.63 *** | 4.09 ** | 17.23 *** | 2.04 ns | 3.20 ** | 2.21 * | 2.63 * | - | - | - |
| SE | 0.13 | 0.45 | 0.39 | 0.40 | 0.40 | 0.79 | 3.21 | 1.42 | 5.76 | - | - | - |
| CV (%) | 5.6 | 15.9 | 13.8 | 13.9 | 15.1 | 29.7 | 30.0 | 40 | 31.5 | - | - | - |
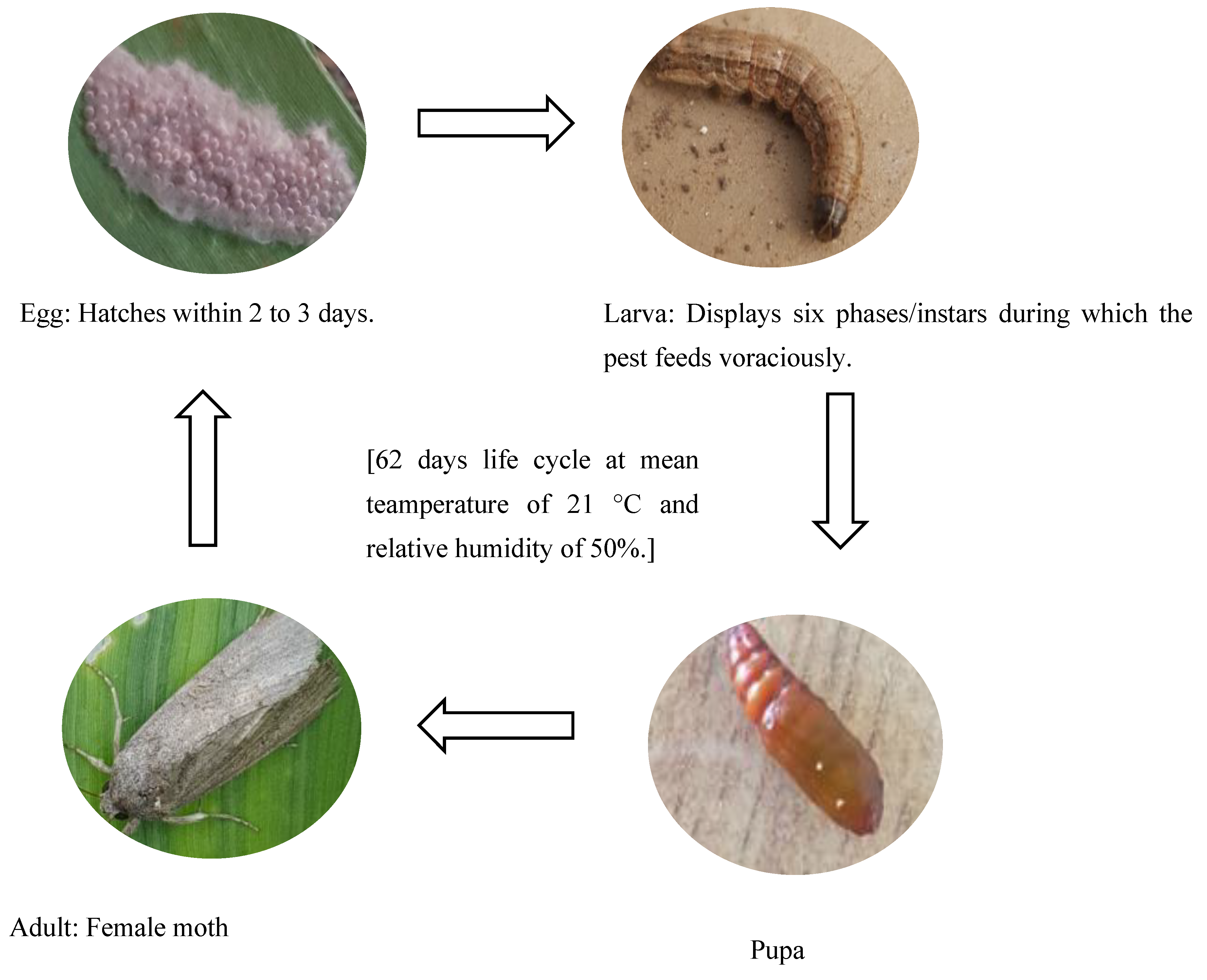
| Stage | Features | Sub-Stage | Descriptions | Duration (Days) | Appearance |
|---|---|---|---|---|---|
| Egg | With three sub-stages distinguishable by colour changes from green to cream white to black. | I | Eggs are covered by scales from the female moth. They appear green to grey for 12 h and begin to darken. | <1 |  |
| II | Cream-white to pink, transitioning into brown. | 1 |  | ||
| III | Dark egg mass approaching hatching. Egg mass appears grey to black before hatching. | 1 |  | ||
| Egg–larva | Immobile, circular, and shiny black protrusions in fur-like mass | Eclosion | Black shiny heads are visible as they emerge from the eggshells. | <1 |  |
| Larva | Feeding stage: with six substages distinguishable by body colour changes and feeding patterns | Blackhead | Newly hatched larvae on a tender maize stalk remain dormant for about 5 to 6 h. | <1 |  |
| Instar I | First instar larvae on a maize leaf. Small bodies and shiny blackheads. | 2–3 |  | ||
| Instar II | Second instar larva on a maize leaf with a cream to pale white body and blackhead. | 2–4 |  | ||
| Instar III | Third instar larva: light brown, begins to turn green after feeding on leaves. | 3–4 |  | ||
| Instar IV | Fourth instar larva: dark green or dark brown body. | 1.5–3 |  | ||
| Instar V | Fifth instar larva: well defined brown body with an inverted Y marking on the head. | 2–3 |  | ||
| Instar VI | Sixth instar larva surrounded by frass. Grey or dark brown body with fully defined segments and head markings. | 5–13 |  | ||
| Progressive shedding of the exoskeleton with a pale colour prior to the darkening of the body | Ecdysis/moulting | Larva sheds its outer cuticular skeleton between instars, leaving a colourless patch in the neck area. Ecdysis lasts approximately 12 to 28 h. | >1 |  | |
| Dark skeleton | Ecdysis/moulting | The skeleton appears black. | >1 |  | |
| Larva–Pupa | Inactive and compacted body | Pre-pupa | It stops feeding, and the body becomes short, preparing for pupation. Body segments with defined ridges and markings. | 3–5 |  |
| Pupa | Stiff pupal casing with localized circular movements in the head area of the insect | Consists of the early, mid, and late pupal stages | Forms an oval-shaped cocoon using leaf particles. The cocoon gradually changes from green to pink to orange-brown. | 15–26 |  |
| Adult | Dull-coloured moth | No distinguishable sub-stages | Female is comparatively plain in appearance with no prominent marks on the wings. | 12 |  |
| Male moth has conspicuous markings on wings. |  |
| Source | df | FLD1 | FLD2 | FLD3 | FLD4 | FLD5 |
|---|---|---|---|---|---|---|
| Replications | 2 | 0.2979 | 1.604 | 5.748 | 8.8314 | 5.0628 |
| Genotypes | 62 | 0.9604 | 6.334 * | 3.398 ** | 1.505 ** | 1.3976 ** |
| Residual | 100 | 0.8583 | 4.39 | 2.191 | 0.9676 | 0.8671 |
| Genotype | FAW Leaf-Damage Rates | |||||||
|---|---|---|---|---|---|---|---|---|
| FLD0 | FLD1 | FLD2 | FLD3 | FLD4 | FLD5 | Mean FLD | AUPPC | |
| Top five performing genotypes | ||||||||
| TL02562 | 0.00 | 0.86 | 0.87 | 2.17 | 4.81 | 4.86 | 2.26 | 61.63 |
| TL142151 | 0.00 | 1.83 | 1.61 | 2.37 | 4.81 | 4.86 | 2.58 | 67.29 |
| TL12176 | 0.00 | 1.08 | 1.21 | 3.61 | 4.12 | 4.59 | 2.44 | 67.39 |
| TL13159 | 0.00 | 2.33 | 1.25 | 1.83 | 5.83 | 5.83 | 2.85 | 70.98 |
| Teost | 0.00 | 1.33 | 1.75 | 2.00 | 5.50 | 5.67 | 2.71 | 72.50 |
| Middle five performing genotypes | ||||||||
| EBL169550 | 0.00 | 1.50 | 1.50 | 3.17 | 6.17 | 6.83 | 3.20 | 85.52 |
| Pool 16 | 0.00 | 1.33 | 2.09 | 4.29 | 4.60 | 6.82 | 3.19 | 86.33 |
| CML545-B | 0.00 | 2.33 | 2.33 | 3.32 | 5.82 | 5.85 | 3.28 | 86.39 |
| CZL16015 | 0.00 | 1.75 | 2.33 | 3.17 | 5.83 | 6.33 | 3.24 | 87.00 |
| TL1316 | 0.00 | 2.33 | 2.33 | 3.50 | 5.83 | 5.83 | 3.30 | 87.48 |
| Bottom five performing genotypes | ||||||||
| CML547-B | 0.00 | 2.91 | 2.84 | 4.00 | 7.00 | 7.33 | 4.01 | 105.04 |
| CZL16141 | 0.00 | 1.92 | 1.72 | 5.78 | 6.76 | 6.82 | 3.83 | 106.04 |
| CZL15225 | 0.00 | 3.24 | 3.70 | 5.00 | 6.67 | 6.67 | 4.21 | 112.20 |
| CZL15220 | 0.00 | 1.83 | 2.33 | 5.32 | 7.82 | 7.85 | 4.19 | 116.39 |
| CZL1347 | 0.00 | 3.05 | 5.43 | 6.33 | 7.33 | 7.80 | 4.99 | 137.94 |
| Statistics | ||||||||
| Grand mean | 0.00 | 1.85 | 2.05 | 3.26 | 6.05 | 6.33 | 3.35 | 87.33 |
| CV (%) | - | 49.60 | 92.60 | 45.60 | 16.30 | 14.80 | - | - |
| LSD (0.05) | - | 1.50 | 3.40 | 2.40 | 1.60 | 1.51 | - | - |
| SED | - | 0.93 | 2.10 | 1.48 | 0.98 | 0.93 | - | - |
| Damage Type | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | None | Whorl Only | Leaf Only | Stalk | Leaf/Whorl and Fresh Frass | Leaf and Whorl | Number of Plants at the Final Assessment |
| CML545-B | 0 | 0 | 5 | 0 | 0 | 1 | 6 |
| CZL1466 | 0 | 2 | 0 | 0 | 1 | 1 | 4 |
| CML491 | 0 | 0 | 3 | 0 | 0 | 0 | 3 |
| CZL0310c | 0 | 0 | 2 | 0 | 0 | 3 | 5 |
| CML539 | 0 | 0 | 5 | 1 | 0 | 0 | 6 |
| CZL15142 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| VL050120 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| CZL16095 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| ZM4236 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| EBL1611480 | 0 | 1 | 4 | 0 | 0 | 0 | 5 |
| EBL169550 | 0 | 0 | 0 | 0 | 1 | 5 | 6 |
| EBL173782 | 1 | 0 | 1 | 0 | 1 | 0 | 3 |
| MM501 | 0 | 0 | 3 | 0 | 1 | 1 | 5 |
| Pool 16 | 1 | 0 | 1 | 0 | 0 | 1 | 3 |
| TL142151 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Total number of plants showing the damage * | 3 | 7 | 101 | 1 | 14 | 80 | 206 |
| FAW Assessment Level | FAW Leaf Damage Score |
|---|---|
| FLD1 | 1.87 |
| FLD2 | 2.26 |
| FLD3 | 3.24 |
| FLD4 | 6.03 |
| FLD5 | 6.28 |
| Genotype | Mean FAW Damage Score | FAW Reaction |
|---|---|---|
| TL13159 | 3.41 | Resistance |
| TL02562 | 2.71 | Resistance |
| VL050120 | 3.49 | Resistance |
| CML548-B | 3.13 | Resistance |
| CML545-B | 3.92 | Moderate resistance |
| CZL1310c | 3.93 | Moderate resistance |
| CZL16095 | 3.97 | Moderate resistance |
| EBL169550 | 3.83 | Moderate resistance |
| ZM4236 | 4.18 | Moderate resistance |
| Pool 16 | 3.82 | Moderate resistance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasoma, C.; Shimelis, H.; Laing, M.D.; Mekonnen, B. Fall Armyworm Infestation and Development: Screening Tropical Maize Genotypes for Resistance in Zambia. Insects 2022, 13, 1020. https://doi.org/10.3390/insects13111020
Kasoma C, Shimelis H, Laing MD, Mekonnen B. Fall Armyworm Infestation and Development: Screening Tropical Maize Genotypes for Resistance in Zambia. Insects. 2022; 13(11):1020. https://doi.org/10.3390/insects13111020
Chicago/Turabian StyleKasoma, Chapwa, Hussein Shimelis, Mark D. Laing, and Bethelihem Mekonnen. 2022. "Fall Armyworm Infestation and Development: Screening Tropical Maize Genotypes for Resistance in Zambia" Insects 13, no. 11: 1020. https://doi.org/10.3390/insects13111020
APA StyleKasoma, C., Shimelis, H., Laing, M. D., & Mekonnen, B. (2022). Fall Armyworm Infestation and Development: Screening Tropical Maize Genotypes for Resistance in Zambia. Insects, 13(11), 1020. https://doi.org/10.3390/insects13111020







