Phenotypic and Differential Gene Expression Analyses of Phase Transition in Oedaleus Asiaticus under High-Density Population Stress
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects Materials and Sampling
2.2. RNA Extraction and Construction of cDNA Library
2.3. Sequence Assembly and Acquisition of Unigenes
2.4. Differentially Expressed Gene Enrichment Analysis
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
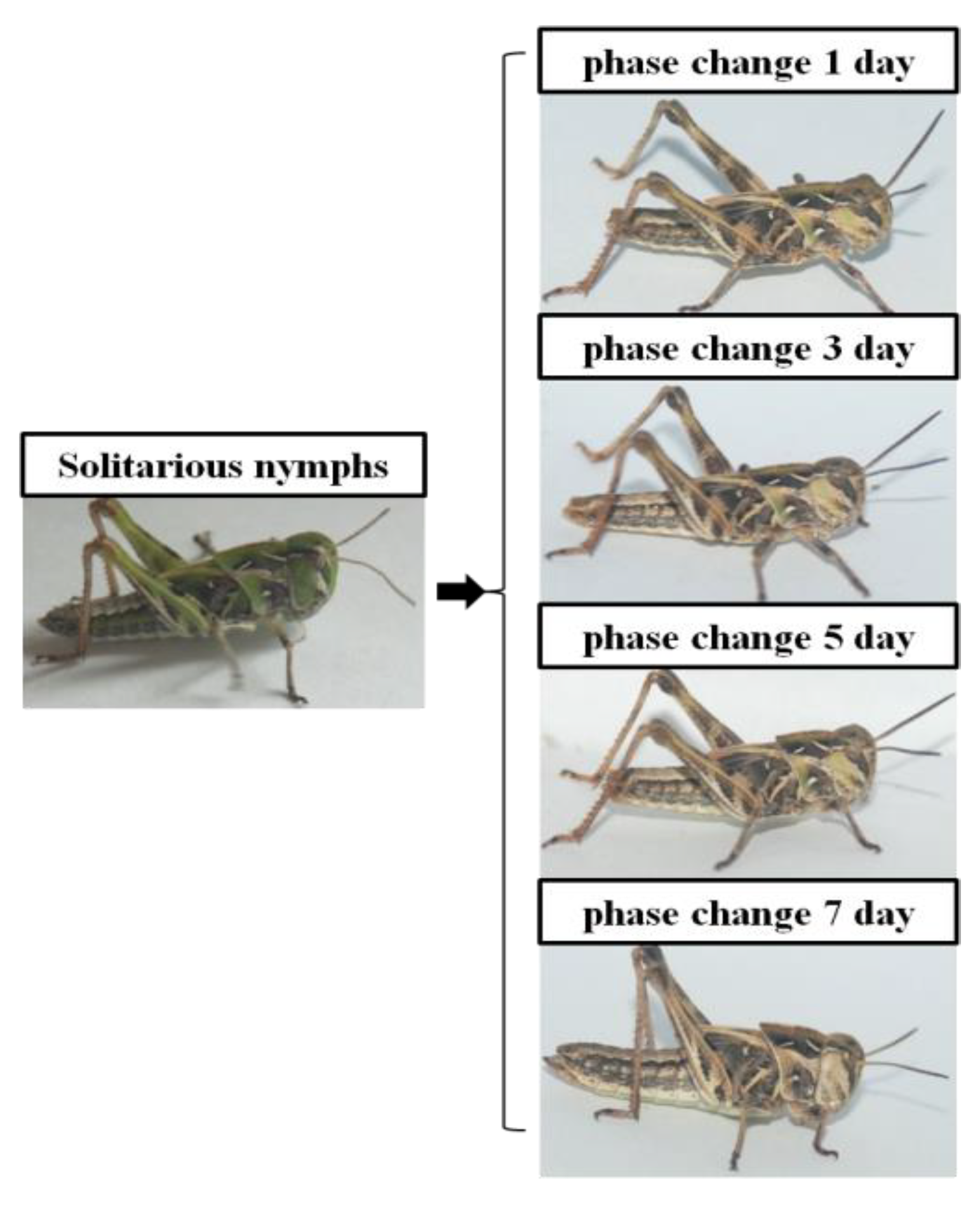
3.1. High-Density Stress Changes the Body Color of O. Asiaticus
3.2. Transcriptome Changes in Response to High-Density Treatment
3.2.1. Annotation of Unigenes
3.2.2. 698 DEGs Were Common to all Four Stages of the Phase Change
3.3. GO and KEGG Enrichment Analyses of DEGs
3.4. qRT-PCR Validation of RNA-seq Data
3.5. Expression Patterns of DEGs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fuller, T. Phenotypic plasticity: Beyond nature and nurture. Biol. Philos. 2003, 18, 381–389. [Google Scholar] [CrossRef]
- Pener, M.P.; Simpson, S.J. Locust phase polyphenism: An update. Adv. Insect Physiol. 2009, 36, 1–286. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Guo, W.; Guo, X.J.; Wang, X.H.; Kang, L. Modulation of behavioral phase changes of the migratory locust by the catecholamine metabolic pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 3882–3887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pener, M.P.; Yerushalmi, Y. The physiology of locust phase polymorphism: An update. J. Insect Physiol. 1998, 44, 365–377. [Google Scholar] [CrossRef]
- Garland, T.; Kelly, S.A. Phenotypic plasticity and experimental evolution. J. Exp. Biol. 2006, 209, 2344–2361. [Google Scholar] [CrossRef] [Green Version]
- Pfennig, D.W.; Ledon-Rettig, C. Ecological Developmental Biology Integrating Epigenetics, Medicine, and Evolution. Science 2009, 325, 268–269. [Google Scholar] [CrossRef]
- Wang, X.H.; Kang, L. Molecular Mechanisms of Phase Change in Locusts. Annu. Rev. Entomol. 2014, 59, 225–244. [Google Scholar] [CrossRef] [Green Version]
- Ernst, U.R.; Van Hiel, M.B.; Depuydt, G.; Boerjan, B.; De Loof, A.; Schoofs, L. Epigenetics and locust life phase transitions. J. Exp. Biol. 2015, 218, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.Z.; Meng, Z.P.; Guo, Y.H.; Shen, J.P. Occurrence and control of Oedaleus asiaticus in the farming and pasturing transitional ecotone of Wulanchabu City. Inner Mongolia Agric. Sci. Technol. 2005, 26, 384–387. [Google Scholar]
- Gao, S.J.; Wei, Y.S.; Te, M.E.; Liu, A.P.; Xu, L.B.; Wang, N. The flight ability of Oedaleus asiaticus and its relationship to population density. Pratacult. Sci. 2012, 29, 1915–1919. [Google Scholar]
- Jiang, X.; Mai, M.; Zhang, L. Nocturnal migration of grasshopper (Acrididae: Oedaleus asiaticus). Acta Agrestia Sin. 2003, 11, 75–77. [Google Scholar]
- Cease, A.J.; Hao, S.; Le, K.; Elser, J.J.; Harrison, J.F. Are color or high rearing density related to migratory polyphenism in the band-winged grasshopper, Oedaleus asiaticus? J. Insect Physiol. 2010, 56, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Cease, A.J.; Harrison, J.F.; Hao, S.G.; Niren, D.C.; Zhang, G.M.; Kang, L.; Elser, J.J. Nutritional imbalance suppresses migratory phenotypes of the Mongolian locust (Oedaleus asiaticus). R. Soc. Open Sci. 2017, 4, 161039. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; McCaffery, A.R.; Hagele, B.F. A behavioural analysis of phase change in the desert locust. Biol. Rev. 1999, 74, 461–480. [Google Scholar] [CrossRef]
- Ayali, A. The puzzle of locust density-dependent phase polyphenism. Curr. Opin Insect Sci. 2019, 35, 41–47. [Google Scholar] [CrossRef]
- Pener, M.P. Locust Phase Polymorphism and its Endocrine Relations. Adv. Insect Physiol. 1991, 23, 1–79. [Google Scholar]
- Simões, P.; Ott, S.R.; Niven, J.E. Environmental Adaptation, Phenotypic Plasticity, and Associative Learning in Insects: The Desert Locust as a Case Study. Integr. Comp. Biol. 2016, 56, 914–924. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Shao, W.; Wang, X.; Ge, J.; Chen, X. Composition and emission dynamics of migratory locust volatiles in response to changes in developmental stages and population density. Insect Sci. 2016, 24, 60–72. [Google Scholar] [CrossRef]
- Lucas, C.; Kornfein, R.; Chakaborty-Chatterjee, M.; Schonfeld, J.; Geva, N.; Sokolowski, M.B.; Ayali, A. The locust foraging gene. Arch. Insect Biochem. Physiol. 2011, 76, 52–66. [Google Scholar] [CrossRef]
- Ott, S.R.; Verlinden, H.; Rogers, S.M.; Brighton, C.H.; Quah, P.S.; Vleugels, R.K.; Verdonck, R.; Vanden Broeck, J. Critical role for protein kinase A in the acquisition of gregarious behavior in the desert locust. Proc. Natl. Acad. Sci. USA 2012, 109, E381–E387. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Yang, P.C.; Jiang, F.; Liu, Q.; Wang, X.H.; Kang, L. The neuropeptide F/nitric oxide pathway is essential for shaping locomotor plasticity underlying locust phase transition. Elife 2017, 6, e22526. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, H.; Badisco, L.; Marchal, E.; Van Wielendaele, P.; Vanden Broeck, J. Endocrinology of reproduction and phase transition in locusts. Gen. Comp. Endocrinol. 2009, 162, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Korb, J. Chapter Four—Juvenile Hormone: A Central Regulator of Termite Caste Polyphenism. In Advances in Insect Physiology; Zayed, A., Kent, C.F., Eds.; Academic Press: Freiburg, Germany, 2015; Volume 48, pp. 131–161. [Google Scholar]
- Miura, T. Juvenile hormone as a physiological regulator mediating phenotypic plasticity in pancrustaceans. Dev. Growth Differ. 2019, 61, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.; Wu, Z.M.; Wang, X.H.; Yang, P.C.; Yu, D.; Zhao, C.X.; Xu, G.W.; Kang, L. Metabolomic analysis reveals that carnitines are key regulatory metabolites in phase transition of the locusts. Proc. Natl. Acad. Sci. USA 2012, 109, 3259–3263. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, X.; Ma, Z.; Xue, L.; Han, J.; Dan, Y.; Le, K. CSP and Takeout Genes Modulate the Switch between Attraction and Repulsion during Behavioral Phase Change in the Migratory Locust. PLoS Genet. 2011, 7, e1001291. [Google Scholar] [CrossRef]
- Anton, S.; Dufour, M.C.; Gadenne, C. Plasticity of olfactory-guided behaviour and its neurobiological basis: Lessons from moths and locusts. Entomol. Exp. Appl. 2007, 123, 1–11. [Google Scholar] [CrossRef]
- Rogers, S.M.; Matheson, T.; Sasaki, K.; Kendrick, K.; Simpson, S.J.; Burrows, M. Substantial changes in central nervous system neurotransmitters and neuromodulators accompany phase change in the locust. J. Exp. Biol. 2004, 207, 3603–3617. [Google Scholar] [CrossRef] [Green Version]
- Kim, D. Different Physiological Roles of Two Dopamine Receptors in Isolated Salivary Glands of the Blacklegged Tick, Ixodes Scapularis. In Proceedings of the 16th North Central Branch Annual Meeting, Knoxville, TN, USA, 11–14 November 2012. [Google Scholar]
- Roessingh, P.; Simpson, S.J. The time-course of behavioral phase change in nymphs of the desert locust, Schistocerca gregaria. Physiol. Entomol. 1994, 19, 191–197. [Google Scholar] [CrossRef]
- Claeys, I.; Breugelmans, B.; Simonet, G.; Soest, S.V.; Sas, F.; Loof, A.D.; Broeck, J.V. Neuroparsin transcripts as molecular markers in the process of desert locust (Schistocerca gregaria) phase transition. Biochem. Biophys. Res. Commun. 2006, 341, 599–606. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2018. [Google Scholar]
- Wilkins, O.; Capitanchik, C.; Luscombe, N.; Ule, J. Ultraplex: A Rapid, Flexible, All-in-One Fastq Demultiplexer. Wellcome OpenRes. 2021, 6, 141. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Jiang, H. rSeqDiff: Detecting differential isoform expression from RNA-Seq data using hierarchical likelihood ratio test. PLoS ONE 2013, 18, e79448. [Google Scholar]
- Wang, H.; Yu, Y.; Chen, C.; Wang, Q.; Huang, T.; Hong, F.; Zhu, L. Involvement of enhancer of zeste homolog 2 in cisplatin-resistance in ovarian cancer cells by interacting with several genes. Mol. Med. Rep. 2015, 12, 2503–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, S.; Han, Z.; Chen, X.; Qingchang, L.I.; Xiao, S.; Jiakai, L.I.; Liu, X. Transcriptome analysis of high-temperature adaptation in large yellow croaker(Larimichthys crocea). J. Fish. China 2018, 42, 1673–1683. [Google Scholar]
- Sun, X.; Yang, A.; Wu, B.; Zhou, L.; Liu, Z. KEGG (Kyoto Encyclopedia of Genes and Genomes) assignment of unigenes in the mantle transcriptome of P. yessoensis. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Ziwen, H.; Zhang, Z.; Wuxia, G.; Ying, Z.; Renchao, Z.; Suhua, S.; Zhong-Hua, C. De Novo Assembly of Coding Sequences of the Mangrove Palm (Nypa fruticans) Using RNA-Seq and Discovery of Whole-Genome Duplications in the Ancestor of Palms. PLoS ONE 2015, 10, e0145385. [Google Scholar]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lun, A.; Smyth, G.K. Differential Expression Analysis of Complex RNA-Seq Experiments Using edgeR; Springer International Publishing: Cham, Switzerland, 2014. [Google Scholar]
- Sun, L.; Yang, H.; Chen, M.; Ma, D.; Lin, C. Significantly enriched GO terms in DEGs. PLoS ONE 2015. [Google Scholar]
- Wilson, K. Density-Dependent Prophylaxis in Insects. In Phenotypic Plasticity of Insects Mechanisms & Consequences; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Whitman, D.; Ananthakrishnan, T. Phenotypic Plasticity of Insects; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Srygley, R.B. Age- and Density-Dependent Prophylaxis in the Migratory, Cannibalistic Mormon Cricket Anabrus simplex (Orthoptera: Tettigoniidae). Environ. Entomol. 2012, 41, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Despland, E.; Simpson, S.J. The role of food distribution and nutritional quality in behavioural phase change in the desert locust. Anim. Behav. 2000, 59, 643–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.J.; Yu, Q.Q.; Chen, D.F.; Wei, J.N.; Yang, P.C.; Yu, J.; Wang, X.H.; Kang, L. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 2020, 584, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Seiji, T.; Shigemi, Y. Evidence for the Involvement of a Neuropeptide in the Control of Body Color in the Desert Locust, Schistocerca gregaria. Jpn. J. Entomol 1997, 65, 447–457. [Google Scholar]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Amsalem, E.; Malka, O.; Grozinger, C.; Hefetz, A. Exploring the role of juvenile hormone and vitellogenin in reproduction and social behavior in bumble bees. BMC Evol. Biol. 2014, 14, 45. [Google Scholar]
- Yamamoto, R.; Bai, H.; Dolezal, A.G.; Amdam, G.; Tatar, M. Juvenile hormone regulation of Drosophila aging. BMC Biol. 2013, 11, 85. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.H.; Pham, V.; Jablonka, W.; Goodman, W.G.; Ribeiro, J.M.C.; Andersen, J.F. A mosquito hemolymph odorant-binding protein family member specifically binds juvenile hormone. J. Biol. Chem. 2017, 292, 15329–15339. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Song, J.; Yang, P.; Chen, X.; Wang, X. Juvenile hormone suppresses aggregation behavior through influencing antennal gene expression in locusts. PLoS Genet. 2020, 16, e1008762. [Google Scholar] [CrossRef]
- Ignell, R.; Couillaud, F.; Anton, S. Juvenile-hormone-mediated plasticity of aggregation behaviour and olfactory processing in adult desert locusts. J. Exp. Biol. 2001, 204, 249. [Google Scholar] [CrossRef]
- Zhu, J.S.; Busche, J.M.; Zhang, X. Identification of juvenile hormone target genes in the adult female mosquitoes. Insect Biochem. Mol. Biol. 2010, 40, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.A.; Cease, A.J.; Latchininsky, A.V.; Ayali, A.; Rogers, S.M. From Molecules to Management: Mechanisms and Consequences of Locust Phase Polyphenism; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Guo, X.; Ma, Z.; Kang, L. Two dopamine receptors play different roles in phase change of the migratory locust. Front. Behav. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Hou, L.; Wang, X.; Kang, L. Core transcriptional signatures of phase change in the migratory locust. Protein Cell 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, S. Dopamine Signalling in Mushroom Bodies Regulates Temperature-Preference Behaviour in Drosophila. PLoS Genet. 2011, 7, e1001346. [Google Scholar] [CrossRef]
- Ueno, K.; Suzuki, E.; Naganos, S.; Ofusa, K.; Horiuchi, J.; Saitoe, M. Coincident postsynaptic activity gates presynaptic dopamine release to induce plasticity in Drosophila mushroom bodies. eLife 2017, 6, e21076. [Google Scholar] [CrossRef]
- Verlinden, H. Dopamine signalling in locusts and other insects. Insect Biochem. Mol. Biol. 2018, 97, 40–52. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; True, J.R.; Carroll, S.B. Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigment patterns. Development 2002, 129, 1849–1858. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Williams, B.L.; Selegue, J.E.; Carroll, S.B. Drosophila pigmentation evolution: Divergent genotypes underlying convergent phenotypes. Proc. Natl. Acad. Sci. USA 2003, 100, 1808–1813. [Google Scholar] [CrossRef]






| High-Density Treatment Time (d) | Number of Insects Changed to Brown (out of 100) | Proportion (%) |
|---|---|---|
| 1 | 9 | 6.92 |
| 2 | 10 | 7.69 |
| 3 | 6 | 4.61 |
| 4 | 6 | 4.61 |
| 5 | 4 | 3.08 |
| 6 | 5 | 3.85 |
| 7 | 4 | 3.08 |
| 8 | 7 | 5.38 |
| 9 | 6 | 4.61 |
| 10 | 5 | 3.85 |
| 11 | 4 | 3.08 |
| 12 | 1 | 0.77 |
| total | 67 | 51.53 |
| Annotated Database and Unigenes | Numbers of Unigenes | Proportion (%) |
|---|---|---|
| Nr | 27,820 | 45.98 |
| KEGG | 21,529 | 35.58 |
| COG | 14,346 | 23.71 |
| SwissProt | 15,154 | 25.05 |
| Annotated genes | 27,995 | 46.27 |
| Not-annotated genes | 32,507 | 53.73 |
| Total unigenes | 60,502 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, N.; Ma, H.; Han, H.; Yan, F.; Gao, H.; Zhang, Y.; Gao, S. Phenotypic and Differential Gene Expression Analyses of Phase Transition in Oedaleus Asiaticus under High-Density Population Stress. Insects 2022, 13, 1034. https://doi.org/10.3390/insects13111034
Guo N, Ma H, Han H, Yan F, Gao H, Zhang Y, Gao S. Phenotypic and Differential Gene Expression Analyses of Phase Transition in Oedaleus Asiaticus under High-Density Population Stress. Insects. 2022; 13(11):1034. https://doi.org/10.3390/insects13111034
Chicago/Turabian StyleGuo, Na, Hongyue Ma, Haibin Han, Feng Yan, Haiyan Gao, Yuanyuan Zhang, and Shujing Gao. 2022. "Phenotypic and Differential Gene Expression Analyses of Phase Transition in Oedaleus Asiaticus under High-Density Population Stress" Insects 13, no. 11: 1034. https://doi.org/10.3390/insects13111034





