RNA Interference of Phenoloxidases of the Fall Armyworm, Spodoptera frugiperda, Enhance Susceptibility to Bacillus thuringiensis Protein Vip3Aa19
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Bt Toxin and Bioassays
2.3. Sample Preparation
2.4. RNA Extraction and cDNA Synthesis
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. RNA Interference (RNAi)
2.7. Midgut, Hemolymph and Hemocyte Sample Preparation and PO Activity Assay
2.8. Statistical Analysis
3. Results
3.1. Differential PO Genes Screening and qRT-PCR Verification
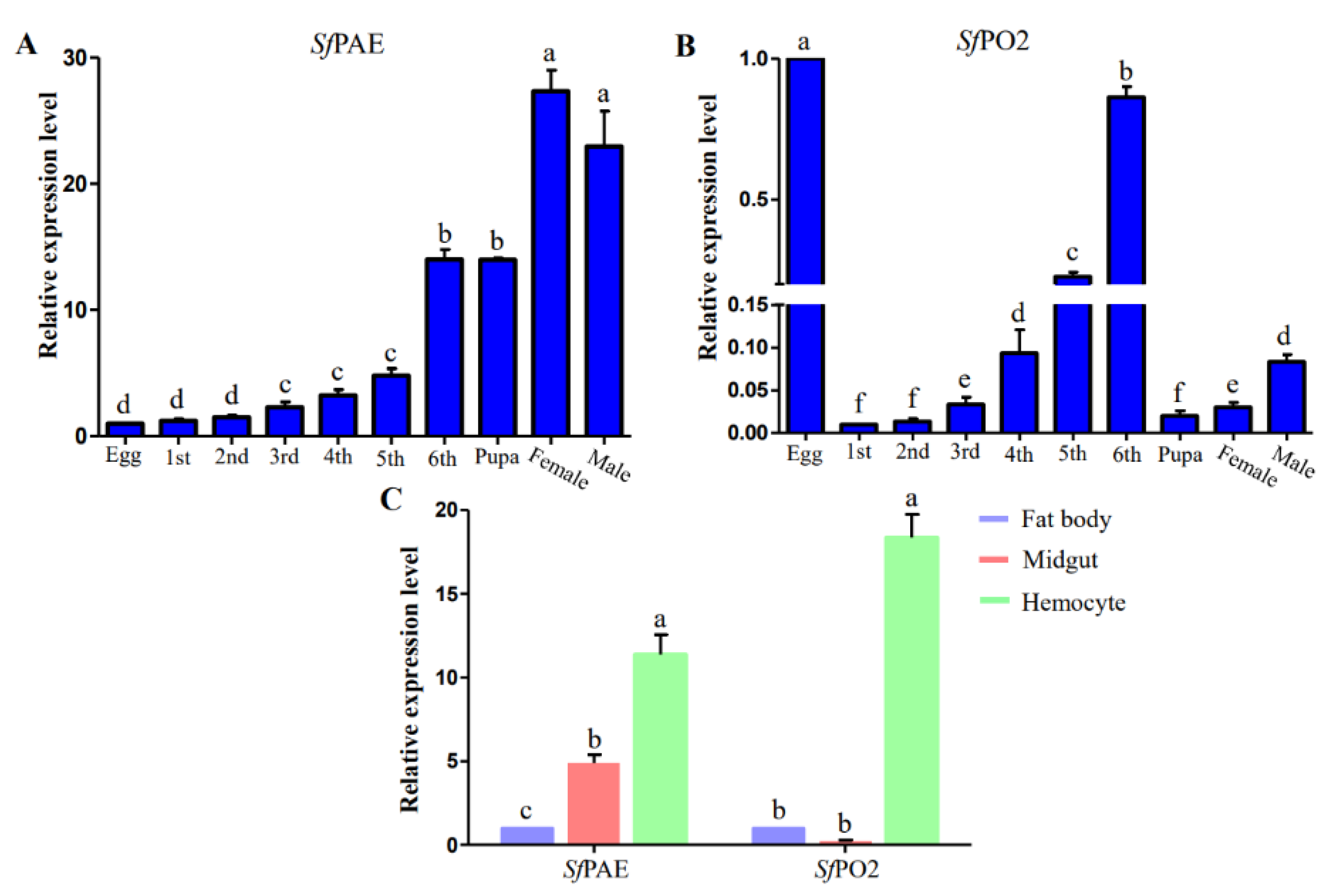
3.2. S. frugiperda POs Expression Profiles
3.3. RNAi-Mediated Downregulation of dsPAE and dsPO2 Genes in S. frugiperda via qRT-PCR
3.4. PO Activity in the Midgut, Hemolymph and Hemocyte Lysate Supernatant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemaitre, B.; Hoffmann, J. The Host Defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [Green Version]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Rosales, C. Phagocytosis, a cellular immune response in insects. Invert. Surv. J. 2011, 8, 109–131. [Google Scholar]
- Kim, M.S.; Baek, M.J.; Lee, M.H.; Park, J.W.; Lee, S.Y.; Söderhäll, K.; Lee, B.L. A New Easter-type Serine Protease Cleaves a Masquerade-like Protein during Prophenoloxidase Activation in Holotrichia diomphalia Larvae. J. Biol. Chem. 2002, 277, 39999–40004. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.; Zhang, Q.; Zhang, J.; Yang, B.; Wu, K.; Xie, W.; Luan, Y.-X.; Ling, E. Insect prophenoloxidase: The view beyond immunity. Front. Physiol. 2014, 5, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Söderhäll, K.; Cerenius, L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998, 10, 23–28. [Google Scholar] [CrossRef]
- Andersen, S.O.; Peter, M.G.; Roepstorff, P. Cuticlar sclerotization in insects. Comp. Biochem. Physiol. Physiol. 1996, 113, 689–705. [Google Scholar] [CrossRef]
- Jing, D.P.; Guo, J.F.; Jiang, Y.Y.; Zhao, J.Z.; Sethi, A.; He, K.L.; Wang, Z.Y. Initial detections and spread of invasive Spodoptera frugiperda in China and comparisons with other noctuid larvae in corn fields using molecular techniques. Insect Sci. 2020, 27, 780–790. [Google Scholar] [CrossRef]
- Yang, X.; Wyckhuys, K.A.; Jia, X.; Nie, F.; Wu, K. Fall armyworm invasion heightens pesticide expenditure among Chinese smallholder farmers. J. Environ. Manag. 2021, 282, 111949. [Google Scholar] [CrossRef]
- Gui, F.; Lan, T.; Zhao, Y.; Guo, W.; Dong, Y.; Fang, D.; Liu, H.; Li, H.; Wang, H.; Hao, R.; et al. Genomic and transcriptomic analysis unveils population evolution and development of pesticide resistance in fall armyworm Spodoptera frugiperda. Protein Cell 2020, 13, 513–531. [Google Scholar] [CrossRef]
- Shelton, A.M.; Zhao, J.Z.; Roush, R.T. Economic, Ecological, Food Safety, and Social Consequences of the Deployment of Bt Transgenic Plants. Annu. Rev. Èntomol. 2002, 47, 845–881. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Sisterson, M.S.; Ellsworth, P.C.; Dennehy, T.J.; Antilla, L.; Liesner, L.; Whitlow, M.; Staten, R.T.; Fabrick, J.A.; Unnithan, G.C.; et al. Suppressing resistance to Bt cotton with sterile insect releases. Nat. Biotechnol. 2010, 28, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Chandrasena, D.I.; Signorini, A.M.; Abratti, G.; Storer, N.P.; Olaciregui, M.L.; Alves, A.P.; Pilcher, C.D. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F δ-endotoxin in Spodoptera frugiperda populations from Argentina. Pest Manag. Sci. 2018, 74, 746–754. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef]
- Rahman, M.; Roberts, H.; Sarjan, M.; Asgari, S. Induction and transmission of Bacillus thuringiensis tolerance in the flour moth, Ephestia kuehniella. Proc. Natl. Acad. Sci. USA 2004, 101, 2696–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.; Kim, Y. Immunosuppressive action of pyriproxyfen, a juvenile hormone analog, enhances pathogenicity of Bacillus thuringiensis subsp. kurstaki against diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Biol. Control 2007, 42, 72–76. [Google Scholar] [CrossRef]
- Kwon, B.; Kim, Y. Benzylideneacetone, an immunosuppressant, enhances virulence of Bacillus thuringiensis against beet armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 2008, 101, 36–41. [Google Scholar] [CrossRef]
- Wang, J.; Yang, K.; Wang, S.; Li, X.; Liu, J.; Yu, Y.; Liu, X. Infection of the entomopathogenic fungus Metarhizium rileyi suppresses cellular immunity and activates humoral antibacterial immunity of the host Spodoptera frugiperda. Pest Manag. Sci. 2022, 78, 2828–2837. [Google Scholar] [CrossRef]
- Yang, L.; Xing, B.; Wang, L.; Yuan, L.; Manzoor, M.; Li, F.; Wu, S. Identification of serine protease, serine protease homolog and prophenoloxidase genes in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Asia-Pac. Èntomol. 2021, 24, 1144–1152. [Google Scholar] [CrossRef]
- Prabu, S.; Jing, D.; Shabbir, M.Z.; Yuan, W.; Wang, Z.; He, K. Contribution of phenoloxidase activation mechanism to Bt insecticidal protein resistance in Asian corn borer. Int. J. Biol. Macromol. 2020, 153, 88–99. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Zhang, J.-D.; Luo, W.-C. Inhibitory kinetics of quercetin on phenoloxidase from loopworm. Insect Sci. 2005, 12, 435–441. [Google Scholar] [CrossRef]
- Tang, F.F.; Yang, W.K.; Zhu, F.; Shao, Y.L.; Zhang, Y.H.; Bai, X.R. BmNPV affecting the activity and gene expression of phenoloxidase in Bombyx mori. Chin. Agricul. Sci. Bull. 2016, 32, 25–28. [Google Scholar]
- Liu, A.; Huang, X.; Gong, L.; Guo, Z.; Zhang, Y.; Yang, Z. Characterization of immune-related PGRP gene expression and phenoloxidase activity in Cry1Ac-susceptible and -resistant Plutella xylostella (L.). Pestic. Biochem. Physiol. 2019, 160, 79–86. [Google Scholar] [CrossRef]
- Estruch, J.J.; Warren, G.W.; Mullins, M.A.; Nye, G.J.; Craig, J.A.; Koziel, M.G. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc. Natl. Acad. Sci. USA 1996, 93, 5389–5394. [Google Scholar] [CrossRef] [Green Version]
- Fatoretto, J.C.; Michel, A.P.; Silva-Filho, M.; Silva, N. Adaptive Potential of Fall Armyworm (Lepidoptera: Noctuidae) Limits Bt Trait Durability in Brazil. J. Integr. Pest Manag. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.-S.; Yu, H.-K.; Dai, J.-H.; Xie, Z.-G.; Qian, W.-Q.; Lin, J.-T. Stability evaluation of reference genes for real-time quantitative PCR normalization in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 2471–2482. [Google Scholar] [CrossRef]
- Cerenius, L.; Söderhäll, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.; Christensen, B. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005, 35, 443–459. [Google Scholar] [CrossRef]
- Sanahuja, G.; Banakar, R.; Twyman, R.M.; Capell, T.; Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol. J. 2011, 9, 283–300. [Google Scholar] [CrossRef] [Green Version]
- He, K.L.; Wang, Z.Y. Resistance evolution to Bt maize in the fall armyworm and consideration on IRM strategy in China. Plant Protec. 2020, 46, 1–15. [Google Scholar]
- Chen, X.; Head, G.P.; Price, P.; Kerns, D.L.; Rice, M.E.; Huang, F.; Gilreath, R.T.; Yang, F. Fitness costs of Vip3A resistance in Spodoptera frugiperda on different hosts. Pest Manag. Sci. 2019, 75, 1074–1080. [Google Scholar] [CrossRef]
- Wang, N.-M.; Li, J.-J.; Shang, Z.-Y.; Yu, Q.-T.; Xue, C.-B. Increased Responses of Phenoloxidase in Chlorantraniliprole Resistance of Plutella xylostella (Lepidoptera: Plutellidae). J. Insect Sci. 2020, 20, 2. [Google Scholar] [CrossRef]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef]
- Marinotti, O.; Ngo, T.; Kojin, B.B.; Chou, S.P.; Nguyen, B.; Juhn, J.; Carballar-Lejarazú, R.; Marinotti, P.N.; Jiang, X.; Walter, M.F.; et al. Integrated proteomic and transcriptomic analysis of the Aedes aegypti egg shell. BMC Dev. Biol. 2014, 14, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eychenne, M.; Girard, P.-A.; Frayssinet, M.; Lan, L.; Pagès, S.; Duvic, B.; Nègre, N. Mutagenesis of both prophenoloxidases in the fall armyworm induces major defects in metamorphosis. J. Insect Physiol. 2022, 139, 104399. [Google Scholar] [CrossRef]
- Binggeli, O.; Neyen, C.; Poidevin, M.; Lemaitre, B. Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS Pathog. 2014, 10, e1004067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, C.; Korayem, A.M.; Scherfer, C.; Loseva, O.; Dushay, M.S.; Theopold, U. Proteomic analysis of the Drosophila larval hemolymph clot. J. Biol. Chem. 2004, 279, 52033–52041.39. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.C.; Chen, C.C.; Hou, R.F. Immunolocalization of prophenoloxidase in the process of wound healing in the Mosquito Armigeres subalbatus (Diptera: Culicidae). J. Med. Entomol. 2002, 39, 266–274. [Google Scholar] [CrossRef]
- Snežana, M.O.; Tatjana, V.Č.; Jelena, S.P.; Elvira, L.V.; Danijela, K.K. Acute toxicity of sublethal concentrations of thiacloprid and clothianidin to immune response and oxidative status of honey bees. Apidologie 2022, 53, 50. [Google Scholar]
- Giglio, A.; Cavaliere, F.; Giulianini, P.G.; Kurtz, J.; Vommaro, M.L.; Brandmayr, P. Continuous Agrochemical Treatments in Agroecosystems Can Modify the Effects of Pendimethalin-Based Herbicide Exposure on Immunocompetence of a Beneficial Ground Beetle. Diversity 2019, 11, 241. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.; Kim, Y. RNA interference of an antimicrobial peptide, gloverin, of the beet armyworm, Spodoptera exigua, enhances susceptibility to Bacillus thuringiensis. J. Invertebr. Pathol. 2011, 108, 194–200. [Google Scholar] [CrossRef]
- Schmidt, S.L.; Christensen, B.M. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 2003, 313, 117–127. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Schmidt, S.L.; Christensen, B.M. The antibacterial innate immune response by the mosquito Aedes aegypti is mediated by hemocytes and independent of Gram type and pathogenicity. Microbes Infect. 2004, 6, 448–459. [Google Scholar] [CrossRef]
- Feng, F.; Wang, P.; Zhang, Y.; An, Q.; Lin, Y.; Tong, W.; Chu, P.K.; Liang, M. Natural Nanominerals Show Enzyme-Like Activities. J. Nanomater. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Luo, M.-J.; Zhang, Q.-W.; Cai, P.-M.; Idrees, A.; Ji, Q.-E.; Yang, J.-Q.; Chen, J.-H. Molecular characterization of prophenoloxidase-1 (PPO1) and the inhibitory effect of kojic acid on phenoloxidase (PO) activity and on the development of Zeugodacus tau (Walker) (Diptera: Tephritidae). Bull. Èntomol. Res. 2018, 109, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, J.; Wright, D.J. Bottom-up cascading effects in a tritrophic system: Interactions between plant quality and host-parasitoid immune responses. Ecol. Èntomol. 2008, 33, 45–52. [Google Scholar] [CrossRef]

| Gene Name | Genes | Primer | Sequences (5′3′) | Amplified Fragment Length (bp) |
|---|---|---|---|---|
| SfPAE | LOC118279360 | qPAE-F | CGGCCCATCTGTTTACCAAC | 168 bp |
| qPAE-R | GTACGCTGGTTCGCATTGAT | |||
| dsPAE | dsPAE-F | GGATCCTAATACGACTCACTATAGGATTAGGCGTTGGACTGGTG | 433 bp | |
| dsPAE-R | GGATCCTAATACGACTCACTATAGGCTCATCGCGCGTTCTTGAAT | |||
| SfPO2 | LOC118275234 | qPO2-F | CATACACAACAACGGGCACA | 170 bp |
| qPO2-R | ACTGAGACTCCTTGTGCCTC | |||
| dsPO2 | dsPO2-F | GGATCCTAATACGACTCACTATAGGTGGTCCCAATTCTCGTGGAAC | 452 bp | |
| dsPO2-R | GGATCCTAATACGACTCACTATAGGCTTGAAGTTGACCTTGATGCC | |||
| GADPH | AF400219.1 | GADPH-F | CGGTGTCTTCACAACCACAG | 209 bp |
| GADPH-R | TTGACACCAACGACGAACAT | |||
| dsGFP | AB062168.1 | dsGFP-F | GGATCCTAATACGACTCACTATAGGGTGGTCCCAATTCTCGTGGAAC | 468 bp |
| dsGFP-F | GGATCCTAATACGACTCACTATAGGGCTTGAAGTTGACCTTGATGCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Jing, D.; Prabu, S.; Zhang, T.; Wang, Z. RNA Interference of Phenoloxidases of the Fall Armyworm, Spodoptera frugiperda, Enhance Susceptibility to Bacillus thuringiensis Protein Vip3Aa19. Insects 2022, 13, 1041. https://doi.org/10.3390/insects13111041
Huang X, Jing D, Prabu S, Zhang T, Wang Z. RNA Interference of Phenoloxidases of the Fall Armyworm, Spodoptera frugiperda, Enhance Susceptibility to Bacillus thuringiensis Protein Vip3Aa19. Insects. 2022; 13(11):1041. https://doi.org/10.3390/insects13111041
Chicago/Turabian StyleHuang, Xiaodan, Dapeng Jing, Sivaprasath Prabu, Tiantao Zhang, and Zhenying Wang. 2022. "RNA Interference of Phenoloxidases of the Fall Armyworm, Spodoptera frugiperda, Enhance Susceptibility to Bacillus thuringiensis Protein Vip3Aa19" Insects 13, no. 11: 1041. https://doi.org/10.3390/insects13111041
APA StyleHuang, X., Jing, D., Prabu, S., Zhang, T., & Wang, Z. (2022). RNA Interference of Phenoloxidases of the Fall Armyworm, Spodoptera frugiperda, Enhance Susceptibility to Bacillus thuringiensis Protein Vip3Aa19. Insects, 13(11), 1041. https://doi.org/10.3390/insects13111041







