Simple Summary
Acanthoscelides obtectus is an insect pest that attacks wild and cultivated common beans (Phaseolus vulgaris L). Four Trichoderma strains, T. arundinaceum IBT 40837, Ta37-17.139 (=Δtri17), and Ta37-23.74 (=Δtri23), and T. brevicompactum IBT 40841 were assessed to establish their direct effect on insect attacks and their indirect effect on the plants grown from the beans treated with those fungal strains and exposed to insect attacks. Treatments of bean seeds with different Trichoderma strains led to different survival rates in the insects. Insect cadavers (in contact with Δtri23) showed growth of this strain. The emergence of insects was reduced in the beans treated with the Ta37, Tb41, and Δtri17 strains. The undamaged beans (treated with Ta37 and Δtri23) provided a high capacity of germination, whereas the Δtri17 and Tb41 treatments increased the capacity of germination in the damaged beans. The undamaged beans treated with Δtri23 obtained the greatest dry weights of the aerial part and root system in the plants. More studies on the mechanisms of insect control, plant growth promotion, and volatile compound production by Δtri23 and Tb41 should be explored in order to commercialize these fungal species on a massive scale.
Abstract
Acanthoscelides obtectus is an insect pest that attacks wild and cultivated common beans (Phaseolus vulgaris L). Four Trichoderma strains, the T. arundinaceum IBT 40837 wild-type strain (=Ta37), a producer of trichothecene harzianum A (HA), two transformants of T. arundinaceum strain, Ta37-17.139 (=Δtri17) and Ta37-23.74 (=Δtri23), and the T. brevicompactum IBT 40841 wild-type strain (=Tb41), which produces the trichothecene trichodermin, were assessed to establish their direct effect on insect attacks and their indirect effect on the plants grown from the beans treated with those fungal strains and exposed to insect attacks. Treatments of bean seeds with different Trichoderma strains led to different survival rates in the insects, and the Tb41 strain caused the lowest survival rate of all. An 86.10% of the insect cadavers (in contact with Δtri23) showed growth of this strain. This was the treatment that attracted the greatest number of insects. The daily emergence was reduced in beans treated with the Ta37, Tb41, and Δtri17 strains. The undamaged beans treated with Ta37 and Δtri23 showed a high capacity of germination (80.00% and 75.00%, respectively), whereas the Δtri17 and Tb41 treatments increased the capacity of germination in the damaged beans (66.67%). The undamaged beans treated with Δtri23 had the greatest dry weights for the aerial part (4.22 g) and root system in the plants (0.62 g). More studies on the mechanisms of insect control, plant growth promotion, and trichodermol and trichodermin production by Δtri23 and Tb41, respectively, should be explored in order to commercialize these fungal species on a large scale.
1. Introduction
Beauveria bassiana (Hypocreales, Cordycipitaceae) and Metarhizium anisopliae (Hypocreales, Clavicipitaceae) are two fungi capable of managing postharvest insects [1]. The most significant insect pests that cause damage to stored products are Sithophilus zeamais [2], A. obtectus [3], and Callosobruchus maculatus (F.) [4], which can be controlled with B. bassiana; and Rhyzopertha dominica [5] and Sitophilus oryzae [6], which can be controlled with M. anisopliae.
Trichoderma (Hypocreales, Hypocreaceae) is a well-characterized fungal genus that currently comprises more than 200 molecularly defined species [7]. Trichoderma species are cosmopolitan and prevalent components of different ecosystems in a wide range of climatic zones [8]. Several Trichoderma spp. are non-pathogenic soil-borne fungi that are recognized as opportunistic, avirulent, and plant symbionts capable of colonizing the root system of many plants [9]. This genus is known to provide significant advantages in agriculture for its capacity to protect crops against diseases and to generally improve crop yield [10]. Biocontrol using Trichoderma strains can be performed by mycoparasitism, antibiosis, or competition, but the most effective biocontrol strains use more than one of these strategies [11].
Trichoderma strains have an enormous potential to synthesize an extensive variety of secondary metabolites [12,13], such as pyrones [6-pentyl-2H-pyran-2-one (6-PP) derivatives] [14,15], butenolides [16], peptaibols [17,18], terpenes (e.g., trichothecenes, triterpenes) [19,20,21,22], and gliotoxin, viridin, harzianopyridone, and harziandione [13,23].
Terpenes include a plethora of compounds with a great variety of roles in mediating antagonistic and beneficial interactions between organisms [24]. However, there is a certain lack of information regarding the effect of terpene mycotoxins, e.g., volatile mycotoxins or intermediates, produced by saprophytic-beneficial fungi, on their interaction with plants and the herbivores that eat them [25].
Trichothecenes are a group of non-volatile sesquiterpene mycotoxins with a central core of fused cyclohexene/tetrahydropyran rings [26,27]. The majority of these compounds are phytotoxic, have significant antibiotic activity, and are highly toxic for humans and animals, irritating the skin or the intestinal mucous membrane, and affecting the immune and nervous systems [28]. Information about the plant–trichothecene interactions remains largely unknown, and it is thought that despite their phytotoxic activity, they also suppress the defense response in host plants [29]. Nevertheless, assays in plants showed that trichothecene harzianum A (HA), produced by T. arundinaceum, not only lacks phytotoxic activity but also induces the expression of the plant-defense-related genes belonging to the salicylic acid (SA) and jasmonic acid (JA) signaling pathways [20].
Bean weevil, Acanthoscelides obtectus (Coleoptera: Chrysomelidae: Bruchidae), is an insect pest that threatens wild and cultivated common beans (Phaseolus vulgaris L.) [30,31,32], both in the field and in storage, where the pest causes the highest number of losses [33]. Thus, if the initial population of A. obtectus insects is not controlled, it can grow exponentially and spoil the entire stored product [34]. Traditionally, several strategies such as the application of synthetic insecticides (phosphine, pyrethroids, and organophosphates) [35], or the use of physical barriers, e.g., hermetic packaging [36], have been used to prevent this pest from causing damage to stored beans.
However, issues about pest resistance and risk to human health or the environment have arisen in relation to the continued application of synthetic insecticides [37]. There is, therefore, a growing demand to look for more sustainable alternatives regarding pest control, including the use of essential oils [38,39], volatile compounds that intervene in the natural defense of the plant against insects [40,41], or the implementation of biological control agents such as filamentous fungi or bacteria [42,43,44,45,46,47]. Over the last few years, the utilization of fungi to control diverse pests and plant diseases has markedly increased, resulting in a great number of commercial products available [42,48], all of which are able to minimize the side effects not only on the indigenous-beneficial organisms but also on the environment.
The main objectives of this study were to determine how A. obtectus insect adults are affected by the terpene compounds produced by different wild-type strains of T. arundinaceum and T. brevicompactum, and two transformant strains of T. arundinaceum; how terpene production affects the A. obtectus insect adults; and also, the effect of these strains on the germination capacity and agronomic parameters of the plants obtained either from healthy beans or from beans previously damaged by A. obtectus larvae.
2. Materials and Methods
2.1. Fungal Strains
Two wild Trichoderma strains, Trichoderma arundinaceum IBT 40837 (=Ta37) (IBT = strains collected from the “Institut for Biotecknologi”), which produces trichothecene harzianum A (HA), T. brevicompactum IBT 40841 (=Tb41), producer of trichodermin [49], and two transformants of Ta37, Ta37-17.139 (Δtri17) and Ta37-23.74 (Δtri23), in which the genes tri17 and tri23 (related to the harzianum A biosynthetic pathway) were deleted, [22,49] were used in this experiment. The Δtri17 and Δtri23 mutants do not produce HA, but they accumulate trichodermol, an intermediate in the synthesis of HA [22,50]. The fungal strains were kept in the mycological collection of the “Grupo de Investigación en Ingeniería y Agricultura Sostenible (GUIIAS)”, located in the “Plant Pests and Diseases Laboratory”, Escuela de Ingeniería Agraria y Forestal (EIAF)—Universidad de Leon (ULE). Spores of all the fungal strains were conserved in a 50% glycerol suspension at −80 °C for long-term storage.
The Trichoderma strains were grown on a PPG solid medium (2% mashed potatoes (Nestlé, Vevey, Switzerland), 2% glucose (Panreac Applichem, Chicago IL, USA), 2% agar (Oxoid, Ltd., Hampshire, UK)) (Sigma-Aldrich Chemie GmbH, Steinheim, Germany)) and incubated in controlled conditions (described in Section 2.2.) for 7–10 days. To collect the spores, 20 mL of autoclaved sterile water was poured on the grown Trichoderma strains, and the surface was scrubbed with a brush. It was then placed under a microscope (200× magnification and a suspension of 1 × 107 spores/mL), using a Neubauer chamber. The spore suspension was then put in Eppendorf tubes (1.5 mL) (EppendorfAG, Hamburg, Germany), which were stored at 20 ± 1 °C for a few hours until they were used in the experiments [43,47].
2.2. Insect Collection and Rearing
The original population of A. obtectus was collected from 2017 to 2019 in storage rooms inside the Protected Geographical Indication (PGI) “Alubia de La Bañeza-León”. The common bean (P. vulgaris L.) “Canela” variety was used to feed the different A. obtectus stages, which were put into glass jars (150 mm diameter, 250 mm high) and covered with a cloth to allow air to enter. Every 3 days, A. obtectus adults were removed from the glass jar with the beans in order to maintain a group of young adults (1–3 days old) to use in the experiments. Insects, before and after the treatments, were kept in a chamber with controlled temperature (24 ± 1 °C), humidity (60 ± 5%), and placed under a photoperiod of 16 h of light (luminous intensity of 1000 lux) and 8 h of darkness [43,47].
2.3. Design of Experiments
2.3.1. Effects of Beans Sprayed with Spores of the Trichoderma Strains on Insects
One milliliter of the spore suspension (1 × 107 spores/mL) of each Trichoderma strain was directly applied to 40 P. vulgaris beans placed in Petri dishes (90 mm diameter) (Sigma-Aldrich, Steinheim, Germany). Eight treatments (four Trichoderma strains and their respective four controls) with four replicates for each treatment were applied. Sterile water was used as the control treatment and as the carrier (support in which the spores of the fungi were diluted) in all the treatments with Trichoderma strains. The application of the treatments, Trichoderma strains, and their respective controls was carried out with a manual loading Potter Tower (Burkard Scientific Limited, P.O. Box 55 Uxbridge, Middx UB8 2RT, UK), according to the methodology of [51]. Once the treatments were applied to the beans, the beans were subsequently transferred to a structure made of 5 circular plastic containers according to the methodology described by Mazzonetto and Vendramim [52] and Fouad et al. [53]. There were four containers (A, B, C, and D) (40 mm diameter, 70 mm high) with a central container (E) (120 mm diameter, 60 mm high) connected to the other 4 containers by plastic cylinders (70 mm long, 7 mm diameter). In the structure, containers B and D were arranged diagonally and filled with 40 beans treated with the same Trichoderma strains, and containers A and C (also arranged diagonally) were filled with 40 beans treated with the respective control of the Trichoderma strain, which had previously been filled in containers B and D. In total, 20 insects (10 males and 10 females, 1 to 3 days old) were released into the central container (E). After 24 h, once the insects decided their location in containers A, B, C, or D, the beans (treated with Trichoderma strains or their controls) and insects were transferred back to the Petri dishes, where the treatments had been applied using the Potter Tower, as previously described. The mortality of the insects in contact with the beans (the insects were considered dead if there was no reaction when prodded) was recorded daily over a 30-day period. During the entire process of Experiment 1, the Petri dishes were kept in a chamber with the same controlled conditions as described in Section 2.2. Once all the dead insects were collected for each of the treatments, the insect cadavers were placed in Petri dishes with a Rose-Bengal Chloramphenicol Agar medium (4 insect cadavers/dish), to verify the presence of fungal strains in their bodies.
2.3.2. Effect of the Fungal Strains on the Biological Development of the Insects
The insects that emerged from the beans in the Petri dishes (sprayed in Experiment 1) were used to estimate the effect of Trichoderma strains on the biological development of insects. The progeny of the insects that emerged from the beans (2nd generation) and the damaged beans (by the insect emergence holes) in each Petri dish were recorded daily over an 18-day period (day 1 of this period being the equivalent to day 31 after the treatments were applied) according to the methodology described by [43]. The insects that emerged were removed from the Petri dishes, and the daily insect emergence of the 2nd generation was recorded. The number of treatments, replicates, and controlled conditions were similar to those described previously in Section 2.3.1.
2.3.3. Determination of Germination Capacity of Beans Attacked by Insects
This study was performed in a climatic chamber to test the germination capacity of the beans previously sprayed with Trichoderma strains or with sterile water (the control) and that were damaged or not damaged after being in contact with the insects, as was described in Section 2.3.1. Polypropylene pots (1-L capacity) with peat (TYPical, Brill, Georgsdorf, Germany) were used to make this bioassay. Each pot was filled with 250 mL of water prior to sowing. Five undamaged beans (one bean without any holes from each Petri dish, as described in Section 2.3.1.) were randomly selected and sown in five pots (one bean/pot). Five damaged beans (beans with at least one hole per bean caused by insect emergence) were selected and sown in five pots (one bean/pot). The culture was maintained for 45 days under controlled conditions with a photoperiod of 16/8 h (light/darkness), a temperature of 25 °C/16 °C (day/night), 60 ± 5% RH, and brightness of 3500 lux. They were watered every 4 days with 250 mL of tap water per pot according to the methodology described by Mayo et al. [54,55]. A nutrient solution was added during the 2nd–4th week according to the methodology described by [56]. Plant emergence was evaluated on the 11th and 17th day after sowing. Plants were then removed after 45 days, and the agronomic parameter “dry weight” (72 h in an oven, at 82 °C) of both the aerial part and the root system was evaluated.
2.4. Statistical Analysis
All the data analyzed were normally distributed and presented homoscedasticity and were subjected (in each of the experiments) to the statistical analyses described below.
The survival data of the insects were submitted to the Kaplan–Meier estimator, and the functions obtained from each treatment were compared using the log-rank test (Mantel-Cox) (p < 0.05).
A randomly completed experiment using the generalized linear model (GLM) procedure, with four treatments and four replicates was subjected to an ANOVA (IBM SPSS Statistics, Version 26.0.). Differences (p < 0.05) in all the evaluated parameters were examined by mean comparisons using the Fisher least significant difference (LSD) tests.
A randomly completed experiment following a generalized linear model (GLM) procedure, with eight treatments and twenty replicates (undamaged or damaged beans) was subjected to an ANOVA (IBM SPSS Statistics, Version 26.0.). Differences (p < 0.05) in all the evaluated parameters were examined by mean comparisons using the Fisher least significant difference (LSD) test.
3. Results
3.1. Effect of Beans Sprayed with Spores of Trichoderma Strains on the Survival Rate of Insects
The type of treatment influenced the survival rate of the insects (log-rank χ2 = 25.019; df = 7141; p < 0.001).
The number and survival rate of the insects exposed to the beans sprayed with different Trichoderma strains were 15 insects with a mean of 18.00 ± 1.00 days for the Ta37 strain, 22 insects with a mean of 21.00 ± 3.00 days for the Δtri23 strain, 16 insects with a mean of 19.50 ± 1.50 days for the Δtri17 strain, and 14 insects with a mean of 21.00 ± 3.00 days for the Tb41 strain.
The number and survival rate of the insects exposed to the beans sprayed with sterile water (controls) were 20 insects with a mean of 19.00 ± 1.00 days for Ta37 (control), 17 insects with a mean of 20.80 ± 0.37 days for Δtri23 (control), 23 insects with a mean of 19.00 ± 0.45 days for Δtri17 (control), and 22 insects with a mean of 18.75 ± 0.31 days for Tb41 (control).
Only the 14 insects subjected to the Tb41 strain had a significantly (F = 8.000; df = 1.34; p < 0.005) lower life expectancy (17.00 days) than those obtained with Tb41 (control), 18.75 days. In addition, the Tb41 strain and Tb41 (control) treatments (log-rank χ2 = 25.019; df = 7141; p < 0.001) lowered life expectancy significantly more than those obtained with the Δtri23 strain and Δtri23 (control), 21.00 and 20.80 days, respectively.
3.2. Effect of Trichoderma Strains on the Biological Development of Insects
3.2.1. Daily Emergence Curves of Insects Exposed to Different Trichoderma Strains
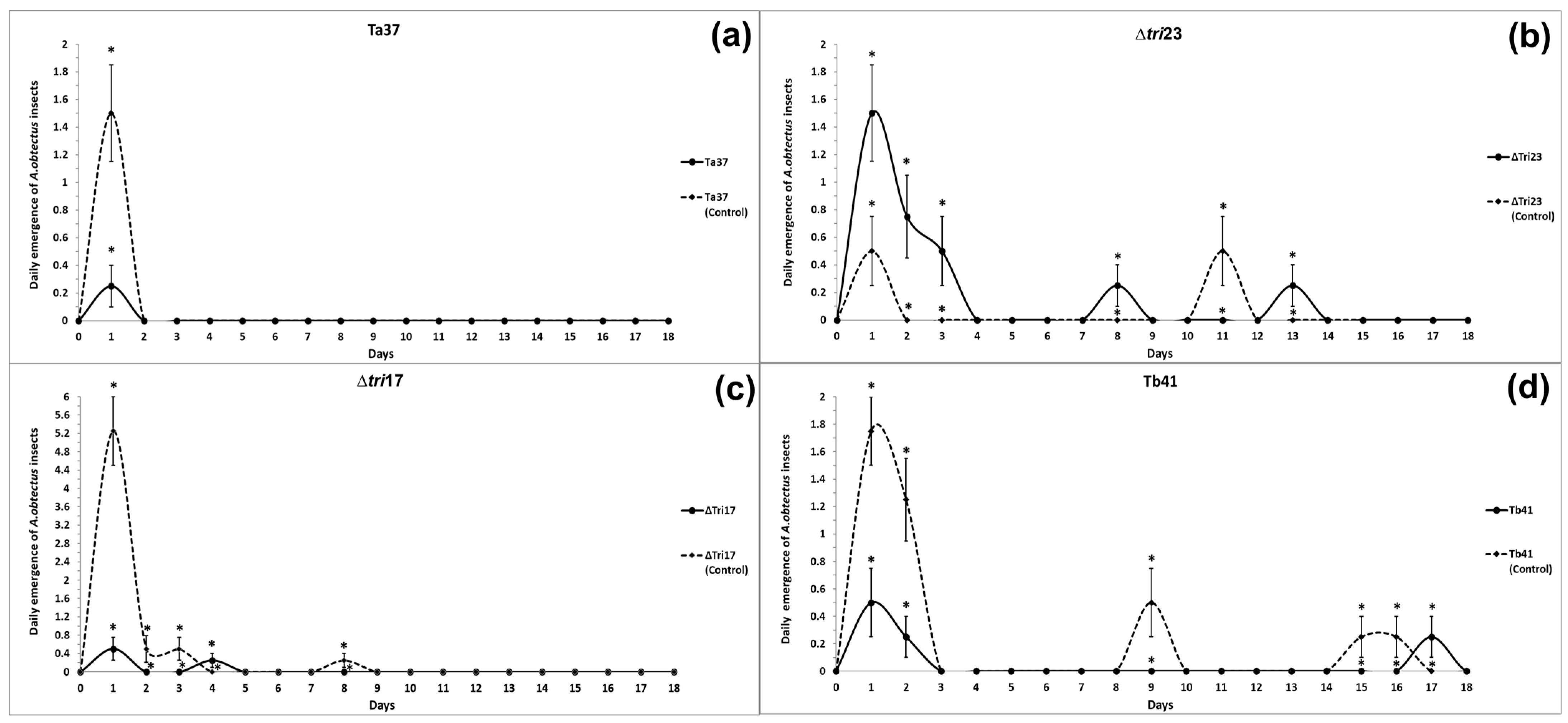
The beans treated with Ta37 and Δtri17 saw a reduction in insect emergence on the 1st day of the evaluation (means of 0.25 and 0.50 insects, respectively) compared with those obtained for their respective controls on the same day (Figure 1a,c). Similarly, the Tb41 treatment provided a significant reduction in insect emergence in comparison to its control, but its effect lasted longer than the rest of the treatments, so differences were significant on days 1, 2, 9, 15, and 16 (Figure 1d).
Figure 1.
Daily emergence of A. obtectus adults from beans treated with different Trichoderma strains: (a) Ta37, (b) Δtri23, (c) Δtri17, and (d) Tb41. The values represent the mean of four replicates for each fungal isolate. The vertical bars represent the standard error (SE). Asterisks indicate significant differences for a specific date.
By contrast, insect emergence from the beans treated with Δtri23 on days 1, 2, 3, 8, and 13 was significantly greater in comparison to the Δtri23 control (Figure 1b).
3.2.2. Fungal Growth on Insect Cadavers
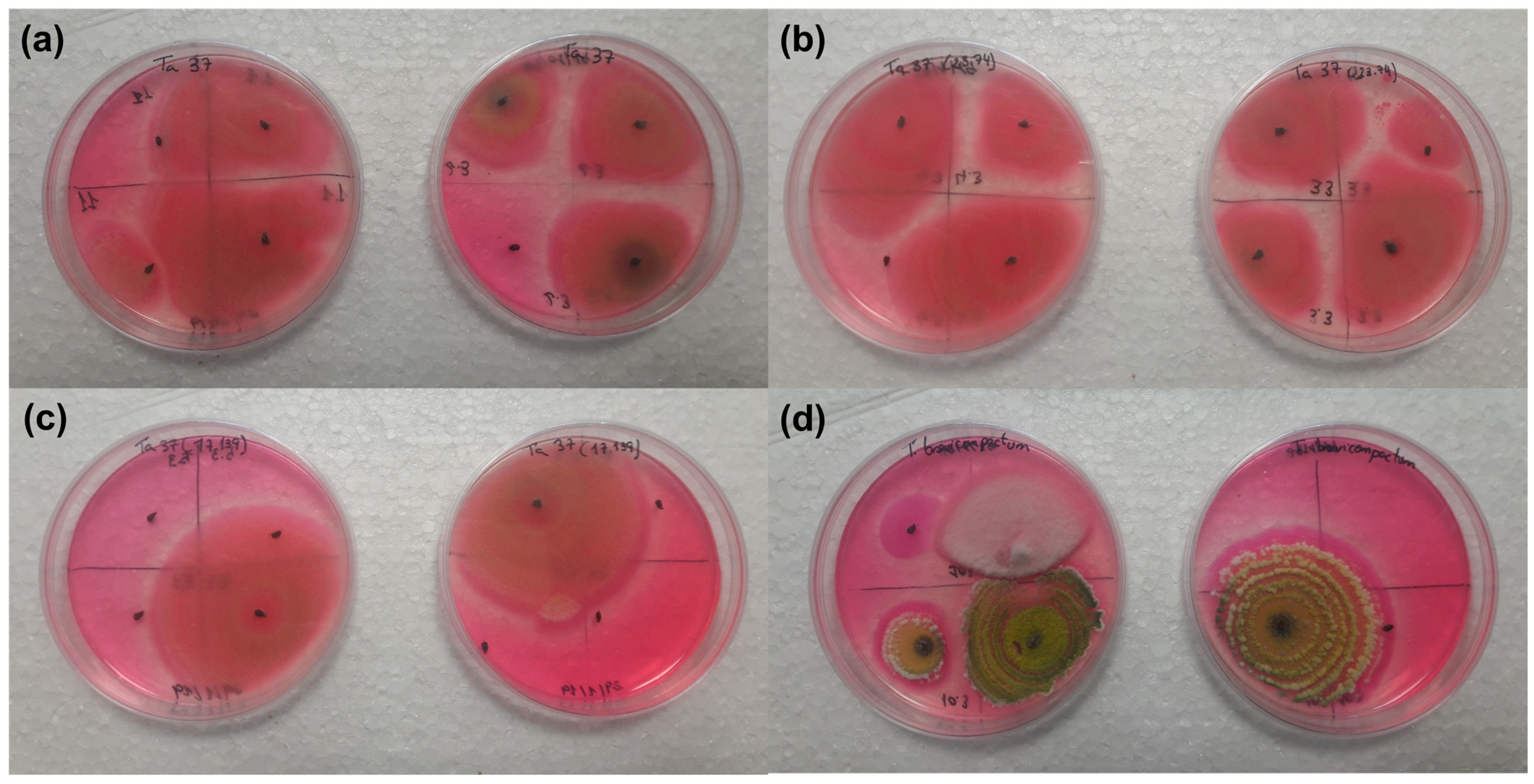
The insect cadavers showed the growth of Trichoderma strains when placed on the Rose-Bengal Chloramphenicol Agar medium after being in contact with the beans sprayed with Trichoderma strains and their controls.
The number of insect cadavers and the growth percentage of the Trichoderma strains on them were 15 cadavers with a mean of 64.60 ± 22.10% for the Ta37 strain (Figure 2a), 22 cadavers with a mean of 86.10 ± 8.30% for the Δtri23 strain (Figure 2b), 16 cadavers with a mean of 37.50 ± 14.20% for the Δtri17 strain (Figure 2c), and 14 cadavers with a mean of 17.50 ± 11.80% for the Tb41 strain (Figure 2d).
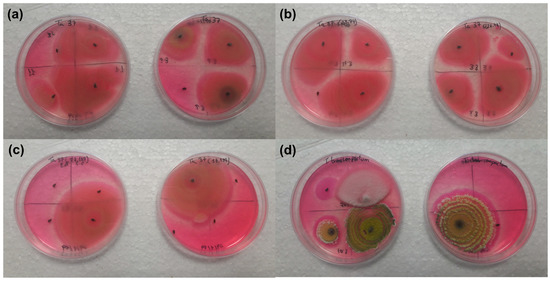
Figure 2.
Growth of different Trichoderma strains on insect cadavers placed on the Rose-Bengal Chloramphenicol Agar medium: (a) Ta37, (b) Δtri23, (c) Δtri17, and (d) Tb41.
The number of insect cadavers and the growth percentage of the Trichoderma strains on them were 20 cadavers with a mean of 0.00 ± 0.00% in the Ta37 (control), 17 cadavers with a mean of 0.00 ± 0.00% for the Δtri23 (control), 23 cadavers with a mean of 0.00 ± 0.00% for the Δtri17 (control), and 22 cadavers with a mean of 0.00 ± 0.00% for the Tb41 (control).
The percentages of the Trichoderma strains grown in the cadavers that were previously in contact with the beans treated with fungal isolates were significantly higher than those obtained for their respective controls (F = 8.504; df = 1.6; p = 0.027 in the Ta37 strain; F = 106.780; df = 1.6; p ≤ 0.001 in the Δtri23 strain; F = 6.943; df = 1.6; p = 0.039 in the Δtri17 strain; and F = 4.194; df = 1.6; p = 0.048 in the Tb41 strain). Only the percentages of Trichoderma growth in the cadavers that were previously in contact with the beans treated with the Δtri23 strain were significantly higher (F = 4.022; df = 3.12; p = 0.034) than those obtained for the other strains.
3.3. Effect of Insects on the Germination of Beans
The germination rates (% ± SE) of the P. vulgaris-undamaged beans sprayed with Trichoderma strains were 80.00 ± 9.17% for the Ta37 strain, 75.00 ± 9.93% for the Δtri23 strain, 70.00 ± 10.51% for the Δtri17 strain, and 45.00 ± 11.41% for the Tb41 strain. The germination rates of the damaged beans were with no data for the Ta37 strain, 62.50 ± 18.29% for the Δtri23 strain, 66.67 ± 33.33% for the Δtri17 strain, and 66.67 ± 33.33% for the Tb41 strain.
The germination rates (% ± SE) of the P. vulgaris-undamaged beans sprayed with the controls were 70.00 ± 10.51% for Ta37 (control), 65.00 ± 10.94% for Δtri23 (control), 60.00 ± 11.23% for Δtri17 (control), and 65.00 ± 10.94% for Tb41 (control). The germination rates of the damaged beans were 0.00 ± 0.00% with no data for Ta37 (control), 100.00 ± 0.00% for Δtri23 (control), 87.50 ± 12.50% for Δtri17 (control), and 55.56 ± 17.57% for Tb41 (control).
The undamaged beans sprayed with Ta37 and Δtri23 reached higher germination rates (80.00 ± 9.17% and 75.00 ± 9.93%, respectively) than the beans treated with Tb41 (45.00 ± 11.41%). No significant differences were found between the undamaged beans sprayed with the fungal strains and their respective controls in relation to their germination capacity.
The damaged beans sprayed with Ta37 (control) reached the lowest germination rate of all the treatments, significantly lower than the germination rates for the rest of the control treatments, Δtri23 (control) and Δtri17 (control). Significant differences between the damaged beans sprayed with the fungal strains and their respective controls were only found for the beans sprayed with the Δtri23 strain, whose germination rate was significantly (F = 3.246; df = 3.18; p = 0.046) lower (62.50 ± 18.29%) than that for Δtri23 (control).
3.4. Other Parameters Evaluated in the Plants after 45 Days
3.4.1. The Effect of Fungal Strains on the Agronomic Parameters of the Plants Grown from Treated Beans
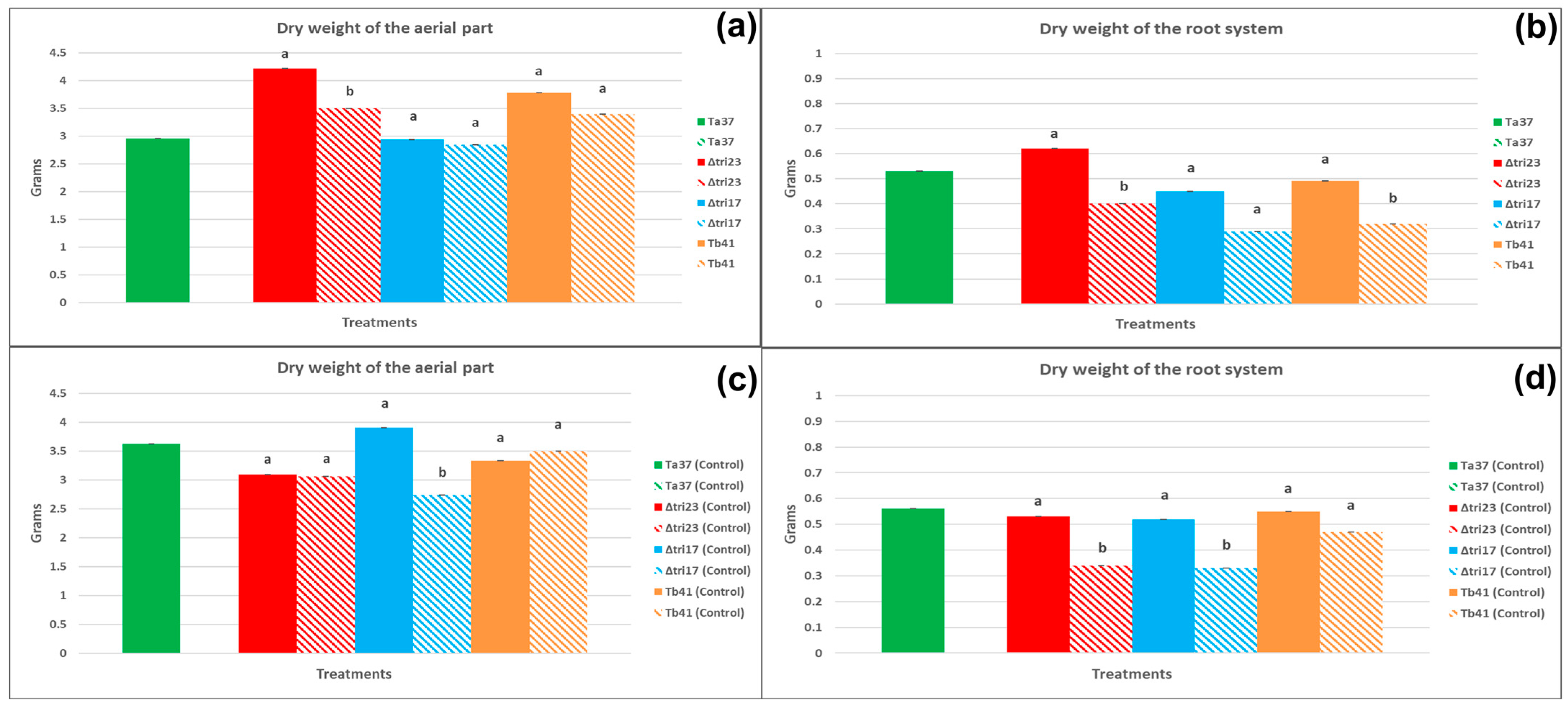
The undamaged beans sprayed with the Δtri23 spores had the greatest dry weights for the aerial part and root system of their plants (4.22 g and 0.62 g, respectively), significantly higher than those from the plants grown from the beans treated with the rest of the fungal strains (Ta37, Δtri17, and Tb41). The plants grown from the undamaged Δtri23-sprayed beans had a greater weight for the aerial part than its control (Δtri23 control), whereas the undamaged Ta37- and Δtri17-sprayed beans had weights for the aerial part of the plant significantly lower than those obtained for their control treatments. Only the undamaged beans sprayed with Δtri23 resulted in plants with a dry weight for the root system that was significantly higher than those achieved for its control treatment (Δtri23 control) (Table 1).

Table 1.
The effect of fungal strains on the agronomic parameters (mean in grams ± SE) of the plants (45 days) grown from the beans sprayed with Trichoderma strains and their controls.
Regarding the damaged beans, no significant differences were found for any of the agronomic parameters evaluated, either among treatments or between each of the fungal strains and their respective controls (Table 1).
3.4.2. The Effect of Seed Condition on the Agronomic Parameters of the Plants Obtained from the Treated Beans
The plants grown from the undamaged beans sprayed with Δtri23 spores had significantly greater dry weights for the aerial part and the root system (4.22 and 0.62 g, respectively) than those obtained from the plants whose beans were sprayed with the same strain but had previously been damaged by insects (Figure 3a). Additionally, the undamaged beans sprayed with Tb41 produced plants with a significantly greater dry weight for the root system (0.49 g) than the plants obtained from the beans sprayed with the same strain but had previously been damaged by the larvae (Figure 3b).
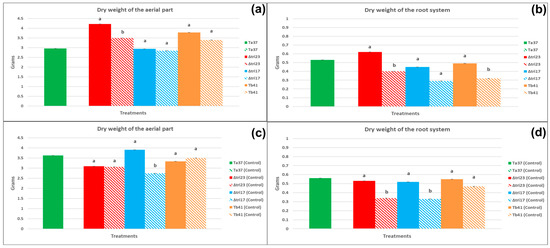
Figure 3.
The effect of seed condition on the agronomic parameters (mean in grams ± SE) of the plants (45 days) grown from beans sprayed with different Trichoderma strains and their controls. Bars with pure color represent undamaged beans and bars with faded color represent damaged beans: (a) the dry weight of the aerial part of the plants grown from beans treated with Trichoderma strains; (b) the dry weight of the root system of the plants grown from beans treated with Trichoderma strains; (c) the dry weight of the aerial part of plants grown from beans treated with sterile water; (d) the dry weight of the root system of plants grown from beans treated with sterile water. Different lowercase letters indicate significant differences between a fungal isolate, in pure color, and its control, in faded color (=beans sprayed with sterile water), within each treatment and parameter evaluated; LSD test at 0.05.
With regard to the beans sprayed with sterile water (control), the results indicated that the undamaged beans sprayed with the Δtri17-control treatment produced plants with significantly greater dry weights for the aerial part and the root system (3.91 and 0.52 g, respectively), than those obtained from the plants whose beans were sprayed with the Δtri17-control treatment but had previously been damaged by the larvae (Figure 3c). In addition, the undamaged beans sprayed with sterile water (Δtri23 control) produced plants with a significantly heavier dry weight for the root system (0.53 g), than the plants obtained from the beans sprayed with the Δtri23-control treatment but that had previously been damaged by the larvae (Figure 3d).
4. Discussion
The application of Trichoderma strains on beans affected the survival rate of the insects in contact with those seeds. The Tb41 strain significantly reduced the insects’ survival rate to 17 days, whereas the insects in contact with the Δtri23 strain had the greatest survival rate (more than 21 days). This longer insect life span implies that they had a longer reproduction period, which resulted in a greater emergence of insects in the next generation, so the insect emergence from the beans treated with Δtri23 was significantly greater (in 5 out of the 18 days in which it was evaluated) than that reached by its respective control. Other reports showed a modification of insect development because their hosts (seeds) were treated with fungi. Thus, Akello and Siroka [57] reported that the inoculation of bean seeds with fungal isolates (one of them being T. asperellum M2RT4) reduced the population of Acyrthosiphon pisum (Homoptera: Aphididae) by 33-fold, compared with the population growth observed in the untreated seeds. Menjivar Barahona [58] found that there was a reduction in the whitefly population in tomato seeds inoculated with T. atroviride. Most of the biopesticides and biofertilizers currently available on the market are based on the beneficial symbionts of the Trichoderma genus [59]. Several reports have also proven the potential of Trichoderma spp. as a natural control agent against some targeted insects, such as Spodoptera littoralis (Lepidoptera: Noctuidae), Tenebrio molitor (Coleoptera: Tenebrionidae) and Xylosandrus crassiusculus (Coleoptera: Curculionidae) [60,61,62]. More recently, Rodríguez-González et al. [42,47] described the reduction in insect survival with T. citrinoviride and T. harzianum, and T. brevicompactum (Tb41), one of the wild-type strains evaluated in their work, which is based on the results of this work. Therefore, it might be suitable for the control of this insect pest, due to the reduction in the survival rate of adult insects and the consequent reduction in the number of insects that emerge from the beans in the next generation.
The insect cadavers showed external growth of the Trichoderma strains used for the bean inoculation. The insect cadavers that were previously in contact with the Δtri23 strain showed the greatest percentage of fungal growth (86.10%). This strain, which accumulates trichodermol, was the one that attracted the greatest number of insects (22 adults). The movement of the insects to the beans treated with the spores of Trichoderma strains or to the volatiles produced by them has recently been discovered [43,45,47]. Furthermore, the insects in contact with the untreated beans (controls) did not show any external growth of the fungal strains when their cadavers were evaluated. The fungal growth observed in the cadavers of A. obtectus in the previous contact with the treated beans would support the idea that the Trichoderma was the cause of death. These fungi have unique invasion mechanisms that allow them to penetrate the cuticle or the wall of the digestive tract of the insects directly, which makes them excellent biological control agents that act as contact insecticides [63]. Trichoderma, unlike other agents, does not need to be ingested by the insect, but its infection occurs through the contact and adhesion of the spores to the buccal parts, intersegmental membranes, or through the spiracles of the insects [64]. The fungal spores germinate in the host’s cuticle, penetrate, and spread throughout the body. After the fungus kills the insect, it can grow and sporulate out of the corpse, increasing the likelihood that other insects may be infected [65]. There are numerous studies on the use of entomopathogenic microorganisms, such as fungi, which have great potential as controlling agents because of their ability to cause disease and insect death [66].
The capacity of the germination of the beans varied depending on the Trichoderma strains that were applied and on the seed condition. Thus, the undamaged beans treated with Ta37, Δtri23, and Δtri17 exhibited a greater capacity of germination than their respective controls, while the damaged Tb41-treated beans had a greater capacity of germination than their control at the same stages. In both cases, Δtri17 significantly protected the beans, which was achieved by lowering the survival rate of the insects in contact with the Δtri17-treated beans, and subsequently favored the germination of the damaged beans, as long as the seed’s embryo was not damaged by the A. obtectus larvae.
The use of biological control agents, e.g., T. harzianum strains, is one of the alternatives for seed treatment while aiming for greater sustainability in agriculture [67,68]. A wide variety of T. harzianum strains have been used in seed treatment but little is known about the possible interactions between Trichoderma spp. and the early stages of seed germination [69] as well as the dosage needed. To avoid the effect of antagonists on seed germination and vigor, Dalzotto et al. [68] obtained a rate of germination 4% higher in the P. vulgaris seeds treated with Trichoderma, than the percentage of the germination observed in the control treatment. Mastouri et al. [69] showed that the inoculation of tomato seeds with T. harzianum T-22 (Rifai) conidia favored seed germination and growth in in vitro cultures. The increase in the germination of the seeds treated with Trichoderma strains has also been attributed to the production of phytohormones from Trichoderma, which would be responsible for that effect [68]. According to López-Bucio et al. [70], T. harzianum produces harzianic acid and isoharzianic acid, which promote plant growth. On the other hand, the excessive production of indole acetic acid (IAA), ethylene [71], auxins, and cytokinin hormones [72] inhibit plant cell division and elongation, impairing germination and the development of seedlings.
Finally, the application of Trichoderma strains on the bean seeds had a diverse effect on the dry weight of the plants grown from seed after 45 days. Thus, the application of the Δtri23 strain on the undamaged beans resulted in plants with significantly higher dry weights for the aerial part and the root system than their respective controls and to the other Trichoderma strains that were analyzed in this work. Moreover, the weights of the plants grown from the beans previously damaged by A. obtectus larvae and treated with the Δtri23 strain were significantly higher in comparison to the rest of the treatments. Previous studies have also described the effect of the application of Trichoderma on the improvement in seed germination, vegetative growth, and flowering in horticultural crops such as cucumber, periwinkle, chrysanthemum, and lettuce [73,74,75]. The application of the Trichoderma inoculum at an early stage of crop growth maximizes the benefits in terms of root development and nutrient uptake. Furthermore, increases in plant growth following Trichoderma treatment depend on the specific crop or plant genotype [70]. Inoculation with Trichoderma spp. in crops such as cucumber, maize, bean, and tomato [76,77,78,79,80,81,82] showed increases in root growth and shoot biomass production (increases in dry weight, shoot length, and leaf area), whereas T. harzianum (T-969 isolate) and Trichoderma spp. applied directly to tomato seeds produced plants with a great shoot height, shoot diameter, and fresh and dry shoot weights [81,82].
5. Conclusions
Δtri23 growth was observed in 86.10% of the insect cadavers that had previously been in contact with this strain, and it was the treatment that attracted the greatest number of insects. The daily emergence of the insects was reduced in the beans treated with Ta37, Δtri17, and Tb41. The undamaged beans treated with Ta37 or Δtri23 provided a great germination capacity, whereas the Δtri17 and Tb41 treatments increased the germination capacity of damaged beans. The undamaged beans treated with Δtri23 had the greatest dry weights for the aerial part and root system in the plants. More studies on the mechanisms of A. obtectus control, P. vulgaris plant growth promotion, and trichodermol and trichodermin production by Δtri23 and Tb41 strains should be explored for these fungal strains to be commercialized on a massive scale without causing any harmful effects on human health and environment.
Author Contributions
Conceptualization, Á.R.-G., P.A.C. and S.G.; methodology, Á.R.-G., G.C.-H. and S.M.-P.; formal analysis, Á.R.-G., M.G., P.A.C. and S.G.; investigation, A.J.P.-Á., A.L., M.P.C. and A.F.-M.; resources, A.L. and M.P.C.; data curation, Á.R.-G., M.G. and P.A.C.; writing—original draft preparation, Á.R.-G. and G.C.-H.; writing—review and editing, M.G., S.M.-P., P.A.C. and S.G.; project administration, P.A.C. and S.G.; funding acquisition, P.A.C. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Education Department of the Junta de Castilla y León for the project entitled “Aplicación de cepas de Trichoderma en la producción sostenible de judía de calidad” (Reference: LE251P18), and by the Ministry of Science, Innovation and Universities for the Grants entitled “Aislamiento de cepas de Trichoderma productoras de trichotecenos a partir de cultivos de judía y estudio de su efecto en la defensa de la planta frente a enfermedades fúngicas” (RTI2018-099600-B-I00), and “Aislamiento de cepas bacterianas capaces de de-epoxidar trichotecenos a partir de cultivos de judía y lúpulo colonizados por cepas de Trichoderma productoras de estas micotoxinas” (PID2021-123874OB-I00). The two latest Grants have been financed by the MCIN/AEI/10.13039/501100011033.
Data Availability Statement
The data presented in this study are available in this manuscript.
Acknowledgments
We would like to thank the Ministry of Science, Innovation, and Universities (Spain) (Resolution of 27 July 2018, BOE No.184, of July 31) for giving a grant to Álvaro Rodríguez González (PTA2017-14403-I) through the program Technical Support Staff (Call 2017). All authors included in this section have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Yang, H.; Qin, C.S.; Chen, Y.M.; Zhang, G.Y.; Dong, L.H.; Wan, S.Q. Persistence of Metarhizium (Hypocreales: Clavicipitaceae) and Beauveria bassiana (Hypocreales: Clavicipitaceae) in tobacco soils and potential as Biocontrol Agents of Spodoptera litura (Lepidoptera: Noctuidae). Environ. Entomol. 2019, 48, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Barra, P.; Rosso, L.; Nesci, A.; Etcheverry, M. Isolation and identification of entomopathogenic fungi and their evaluation against Tribolium confusum, Sitophilus zeamais, and Rhyzopertha dominica in stored maize. J. Pest Sci. 2013, 86, 217–226. [Google Scholar] [CrossRef]
- Dal Bello, G.; Padín, S.; Juárez, P.; Pedrini, N.; De Giusto, M. Biocontrol of Acanthoscelides obtectus and Sitophilus oryzae with diatomaceous earth and Beauveria bassiana on stored grains. Bio. Sci. Technol. 2006, 16, 215–220. [Google Scholar] [CrossRef]
- Cherry, A.J.; Abalo, P.; Hell, K. A laboratory assessment of the potential of different strains of the entomopathogenic fungi Beauveria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metschnikoff) to control Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in stored cowpea. J. Stored Prod. Res. 2005, 41, 295–309. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Vayias, B.J.; Tsakiri, J.B.; Mikeli, N.H.; Meletsis, C.M.; Tomanovic´, Z. Persistence and efficacy of Metarhizium anisopliae (Metschnikoff) Sorokin (Deuteromycotina: Hyphomycetes) and diatomaceous earth against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) and Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae) on wheat and maize. Crop Prot. 2008, 27, 1303–1311. [Google Scholar]
- Batta, Y.A. Control of rice weevil (Sitophilus oryzae L., Coleoptera: Curculionidae) with various formulations of Metarhizium anisopliae. Crop Prot. 2004, 23, 103–108. [Google Scholar] [CrossRef]
- Atanasova, L.; Le Crom, S.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genom. 2013, 14, 121. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Komon-Zelazowska, M.; Druzhinina, I.S. Fungal genus Hypocrea/Trichoderma: From barcodes to biodiversity. J. Zhejiang Univ. Sci. B 2008, 9, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Ann. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, R.; Hermosa, R.; Vizcaíno, J.; Sanz, L.; Monte, E.; Gutiérrez, S. Secondary metabolites produced by Trichoderma and their importance in the biocontrol process. Res. Signpost Indian 2005, 661, 207. [Google Scholar]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galan, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Keszler, A.; Forgács, E.; Kótai, L.; Vizcaíno, J.A.; Monte, E.; García-Acha, I. Separation and identification of volatile components in the fermentation broth of Trichoderma atroviride by solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. Sci. 2000, 38, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.B.; Hermosa, R.; Reino, J.L.; Collado, I.G.; Monte, E. Thctf1 transcription factor of Trichoderma harzianum is involved in 6-pentyl-2H-pyran-2-one production and antifungal activity. Fungal Genet. Biol. 2009, 46, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Bioch. 2013, 73, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Vizcaino, J.A.; Sanz, L.; Basilio, A.; Vicente, F.; Gutierrez, S.; Hermosa, M.R.; Monte, E. Screening of antimicrobial activities in Trichoderma isolates representing three Trichoderma sections. Mycol. Res. 2005, 109, 1397–1406. [Google Scholar] [CrossRef]
- Vizcaíno, J.A.; González, F.J.; Suárez, M.B.; Redondo, J.; Heinrich, J.; Delgado-Jarana, J.; Hermosa, R.; Gutiérrez, S.; Monte, E.; Llobell, A.; et al. Generation, annotation and analysis of ESTs from Trichoderma harzianum CECT 2413. BMC Genom. 2006, 7, 193. [Google Scholar] [CrossRef]
- Cardoza, R.E.; Malmierca, M.G.; Hermosa, M.R.; Alexander, N.J.; McCormick, S.P.; Proctor, R.H.; Tijerino, A.M.; Rumbero, A.; Monte, E.; Gutiérrez, S. Identification of loci and functional characterization of trichothecene biosynthesis genes in filamentous fungi of the genus Trichoderma. Appl. Environ. Microbiol. 2011, 77, 4867–4877. [Google Scholar] [CrossRef]
- Malmierca, M.G.; Cardoza, R.E.; Alexander, N.J.; McCormick, S.P.; Hermosa, R.; Monte, E.; Gutiérrez, S. Involvement of Trichoderma trichothecenes in the biocontrol activity and induction of plant defense-related genes. Appl. Environ. Microbiol. 2012, 78, 4856–4868. [Google Scholar] [CrossRef]
- Malmierca, M.G.; Cardoza, R.E.; Alexander, N.J.; McCormick, S.P.; Collado, I.G.; Hermosa, R.; Monte, E.; Gutiérrez, S. Relevance of trichothecenes in fungal physiology: Disruption of tri5 in Trichoderma arundinaceum. Fungal Genet. Biol. 2013, 53, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; McCormick, S.P.; Kim, H.-S.; Cardozar, R.E.; Stanley, A.M.; Lindo, L.; Kelly, A.; Brown, D.W.; Lee, T.; Vaughan, M.M.; et al. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Pathog. 2018, 14, e1006946. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-plant-pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Drakulic, J.; Kahar, M.; Ajigboye, O.; Bruce, T.; Ray, R. Contrasting roles of deoxynivalenol and nivalenol in host-mediated interactions between Fusarium graminearum and Sitobion avenae. Toxins 2016, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Malmierca, M.G.; Mccormick, S.P.; Cardoza, R.E.; Alexander, N.J.; Monte, E.; Gutiérrez, S. Production of trichodiene by Trichoderma harzianum alters the perception of this biocontrol strain by plants and antagonized fungi. Environ. Microbiol. 2015, 17, 2628–2646. [Google Scholar] [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Rocha, O.; Ansari, K.; Doohan, F.M. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam. 2005, 22, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Masuda, D.; Ishida, M.; Yamaguchi, K.; Yamaguchi, I.; Kimura, M.; Nishiuchi, T. Phytotoxic effects of trichothecenes on the growth and morphology of Arabidopsis thaliana. J. Exp. Bot. 2007, 58, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Paul, U.V.; Lossini, J.S.; Edwards, P.J.; Hilbeck, A. Effectiveness of products from four locally grown plants for the management of Acanthoscelides obtectus (Say) and Zabrotes subfasciatus (Boheman) (Coleoptera: Bruchidae) in stored beans under laboratory and farm conditions in Northern Tanzania. J. Stored Prod. Res. 2009, 45, 97–107. [Google Scholar] [CrossRef]
- Thakur, D.R. Taxonomy, distribution and pest status of indian biotypes of Acanthoscelides obtectus (Coleoptera: Chrysomelidae: Bruchinae)—A new record. Pak. J. Zool. 2012, 44, 189–195. [Google Scholar]
- Vilca-Mallqui, K.S.; Oliveira, E.E.; Guedes, R.N.C. Competition between the bean weevils Acanthoscelides obtectus and Zabrotes subfasciatus in common beans. J. Stored Prod. Res. 2013, 55, 32–35. [Google Scholar] [CrossRef]
- Baier, A.H.; Webster, B.D. Control of Acanthoscelides obtectus Say (Coleoptera: Bruchidae) in Phaseolus vulgaris L. seed stored on small farms—I. Evaluation of damage. J. Stored Prod. Res. 1992, 28, 289–293. [Google Scholar] [CrossRef]
- Gołebiowski, M.; Maliński, E.; Nawrot, J.; Stepnowski, P. Identification and characterization of surface lipid components of the dried-bean beetle Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2008, 44, 386–388. [Google Scholar] [CrossRef]
- Daglish, G.J.; Hall, E.A.; Zorzetto, M.J.; Lambkin, T.M.; Erbacher, J.M. Evaluation of protectants for control of Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) in navy beans (Phaseolus vulgaris (L.)). J. Stored Prod. Res. 1993, 29, 215–219. [Google Scholar] [CrossRef]
- Freitas, R.S.; Faroni, L.R.A.; Sousa, A.H. Hermetic storage for control of common bean weevil, Acanthoscelides obtectus (Say). J. Stored Prod. Res. 2016, 66, 1–5. [Google Scholar] [CrossRef]
- Daglish, G.J. Impact of resistance on the efficacy of binary combinations of spinosad, chlorpyrifos-methyl and s-methoprene against five stored-grain beetles. J. Stored Prod. Res. 2008, 44, 71–76. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Álvarez-García, S.; González-López, Ó.; Da Silva, F.; Casquero, P.A. Insecticidal Properties of Ocimum basilicum and Cymbopogon winterianus against Acanthoscelides obtectus, insect pest of the common bean (Phaseolus vulgaris, L.). Insects 2019, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Wenda-Piesik, A.; Piesik, D.; Nowak, A.; Wawrzyniak, M. Tribolium confusum responses to blends of cereal kernels and plant volátiles. J. Appl. Entomol. 2016, 140, 558–563. [Google Scholar] [CrossRef]
- Gantner, M.; Najda, A.; Piesik, D. Effect of phenolic acid content on acceptance of hazel cultivars by filbert aphid. Plant Protect. Sci. 2019, 55, 116–122. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Mayo, S.; González-López, Ó.; Reinoso, B.; Gutierrez, S.; Casquero, P.A. Inhibitory activity of Beauveria bassiana and Trichoderma spp. on the insect pests Xylotrechus arvicola (Coleoptera: Cerambycidae) and Acanthoscelides obtectus (Coleoptera: Chrisomelidae: Bruchinae). Environ. Monit. Assess. 2017, 189, 12. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Casquero, P.A.; Suárez-Villanueva, V.; Carro-Huerga, G.; Álvarez-García, S.; Mayo-Prieto, S.; Lorenzana, A.; Cardoza, R.E.; Gutiérrez, S. Effect of trichodiene production by Trichoderma harzianum on Acanthoscelides obtectus. J. Stored Prod. Res. 2018, 77, 231–239. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Carro-Huerga, G.; Mayo-Prieto, S.; Lorenzana, A.; Gutiérrez, S.; Peláez, H.J.; Casquero, P.A. Investigations of Trichoderma spp. and Beauveria bassiana as biological control agent for Xylotrechus arvicola, a major insect pest in Spanish vineyards. J. Econ. Entomol. 2018, 111, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, Á.; Casquero, P.A.; Cardoza, R.E.; Gutiérrez, S. Effect of trichodiene synthase encoding gene expresion in Trichoderma strain on their effectiveness in the control of Acanthoscelides obtectus. J. Stored Prod. Res. 2019, 83, 275–280. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Porteous-Álvarez, A.J.; Del Val, M.; Casquero, P.A.; Escriche, B. Toxicity of five Cry proteins against the insect pest Acanthoscelides obtectus (Coleoptera: Chrisomelidae: Bruchinae). J. Invertebr. Pathol. 2020, 169, 107295. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, Á.; Campelo, M.P.; Lorenzana, A.; Mayo-Prieto, A.; González-López, Ó.; Álvarez-García, S.; Gutiérrez, S.; Casquero, P.A. Spores of Trichoderma strains sprayed over Acanthoscelides obtectus and Phaseolus vulgaris L. beans: Effects in the biology of the bean weevil. J. Stored Prod. Res. 2020, 88, 101666. [Google Scholar] [CrossRef]
- Abdul-Wahid, O.A.; Elbanna, S.M. Evaluation of the insecticidal activity of Fusarium solani and Trichoderma harzianum against cockroaches; Periplaneta americana. Afr. J. Microbiol. Res. 2012, 6, 1024–1032. [Google Scholar] [CrossRef]
- Degenkolb, T.; Dieckmann, R.; Nielsen, K.F.; Gräfenhan, T.; Theis, C.; Zafari, D.; Chaverri, P.; Isamaiel, A.; Brückner, H.; von Döhren, H.; et al. The Trichoderma brevicompactum clade: A separate lineage with new species, new peptabiotics, and mycotoxins. Mycol. Prog. 2008, 7, 177–219. [Google Scholar] [CrossRef]
- Cardoza, R.E.; McCormick, S.P.; Lindo, L.; Kim, H.-S.; Olivera, E.R.; Nelson, D.R.; Proctor, R.H.; Gutierrez, S. A cytochrome P450 monooxygenase gene required for biosynthesis of the trichothecene toxin harzianum A in Trichoderma. Appl. Microbiol. Biotechnol. 2019, 103, 8087–8103. [Google Scholar] [CrossRef]
- Potter, C. An Improved laboratory apparatus for applying direct sprays and surface films, with data on the electrostatic charge on atomized spray fluids. Ann. Appl. Biol. 1952, 39, 1–28. [Google Scholar] [CrossRef]
- Mazzonetto, F.; Vendramim, J.D. Efeito de pos de origem vegetal sobre Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) em feijao armazenado. Neotrop. Entomol. 2003, 32, 145–149. [Google Scholar] [CrossRef]
- Fouad, H.A.; Faroni, L.R.D.; Ribeiro, R.C.; Tavares, W.D.S.; Petacci, F. Extraction and repellent activity of Lepidoploa aurea and Memora nodosa against stored grain and by product pests. Vie Milieu 2012, 62, 11–15. [Google Scholar]
- Mayo, S.; Gutiérrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Front. Plant Sci. 2015, 6, 685. [Google Scholar] [CrossRef] [PubMed]
- Mayo, S.; Cominelli, E.; Sparvoli, F.; González-López, O.; Rodríguez-González, A.; Gutiérrez, S.; Casquero, P.A. Development of a qPCR strategy to select bean genes involved in plant defense response and regulated by the Trichoderma velutinum–Rhizoctonia solani interaction. Front. Plant Sci. 2016, 7, 1109. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, J.R.; Puppo, A. Indole 3 acetic acid catabolism by soybean bacteroids. J. Gen. Microbiol. 1975, 88, 223–228. [Google Scholar] [CrossRef]
- Akello, J.; Sikora, R. Systemic acropedal influence of endophyte seed treatment on Acyrthosiphon pisum and Aphis fabae offspring development and reproductive fitness. Biol. Control 2012, 61, 215–221. [Google Scholar] [CrossRef]
- Menjivar Barahona, R.D. The Systemic Activity of Mutualistic Endophytic Fungi in Solanaceae and Cucurbitaceae Plants on the Behaviour of the Phloem-Feeding Insects Trialeurodes vaporariorum, Aphis gossypii and Myzus persicae; Institut für Nutzpflanzenwissenschaften und Ressourcenschutz (INRES): Bonn, Germany, 2010. [Google Scholar]
- Woo, S.L.; Scala, F.; Ruocco, M.; Lorito, M. The molecular biology of the interactions between Trichoderma spp., Phytopathogenic Fungi, and plants. Phytopathology 2006, 96, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, J.; Foster, H.A. Proteolytic activity and antibiotic production by Trichoderma harzianum in relation to pathogenicity to insects. Enzyme Microb. Technol. 2007, 40, 961–968. [Google Scholar] [CrossRef]
- El-Katatny, M.H. Virulence potential of some fungal isolates and their control-promise against the Egyptian cotton leaf worm, Spodoptera littoralis. Arch. Phytopathol. Plant Prot. 2010, 43, 332–356. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Griggs, M.H.; Vandenberg, J.D. Granulate ambrosia beetle, Xylosandrus crassiusculus (Coleoptera: Curculionidae), survival and brood production following exposure to entomopathogenic and mycoparasitic fungi. Biol. Control 2013, 67, 220–226. [Google Scholar] [CrossRef]
- Charnley, A.; Collins, S. Entomopathogenic fungi and their role in pest control. In Environmental and Microbial Relationships; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4, pp. 159–187. [Google Scholar]
- Pucheta Díaz, M.; Flores-Macías, A.; Rodríguez-Navarro, S.; De La Torre, M. Mecanismo de acción de los hongos entomopatógenos. Interciencia 2006, 31, 856–860. [Google Scholar]
- Kaoud, H.A.; Saeid, S.; El-dahshan, A.R.; El-behary, A.M. New Methods for the Control of Lesser Grain Borer, Rhyzopertha dominica. Int. J. Eng. 2013, 3, 285–289. [Google Scholar]
- Motta-Delgado, P.A.; Murcia-Ordoñez, B. Hongos entomopatógenos como alternativa para el control biológico de plagas. Rev. Ambiente Agua—Interdiscip. J. Appl. Sci. 2011, 6, 76–90. [Google Scholar]
- Xu, X.-M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. Combined use of biocontrol agentes to manage plant diseases in theory and practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef]
- Dalzotto, L.; Tortelli, B.; Spitza, F.; Sacon, S.D.; Neumann Silva, V.; Mendes Milanesi, P. Creole bean seeds microbiolization with doses of Trichoderma harzianum. Ciênc. Rural 2020, 50, e20190542. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 4th ed.; Artmed: Porto Alegre, Brazil, 2009; p. 848. [Google Scholar]
- Brotman, Y.; Gupta Kapuganti, J.; Viterbo, A. Trichoderma. Curr. Biol. 2010, 20, 390–391. [Google Scholar] [CrossRef]
- Chang, Y.C.; Baker, R.; Klefield, O.; Chet, I. Increased growth of plants in the presence of the biological control agent Trichoderma harzianum. Plant Dis. 1986, 70, 145–148. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Studholme, D.J.; Harris, B.; LeCocq, K.; Winsbury, R.; Perer, A.V.; Ryder, L.; Ward, J.L.; Beale, M.H.; Thornton, C.R.; Grant, M. Investigating the beneficial traits of Trichoderma hamatum GD 12 for sustainable agriculture-insights from genomics. Front. Plant Sci. 2013, 4, 258. [Google Scholar] [CrossRef] [PubMed]
- Björkman, T.; Blanchard, L.M.; Harman, G.E. Growth enhancement ofshrunken-2 sweet corn when colonized with Trichoderma harzianum 1295-22: Effect of environmental stress. ASHS J. 1998, 123, 35–40. [Google Scholar]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Björkman, T. Effect of Trichoderma colonization on auxin-mediated regulation of root elongation. Plant Growth Regul. 2004, 43, 89–92. [Google Scholar] [CrossRef]
- Harman, G.E. Multifunctional fungal plant symbionts: New tools to enhance plant growth and productivity. New Phytol. 2011, 189, 647–649. [Google Scholar] [CrossRef]
- Vargas, W.A.; Mandawe, J.C.; Kenerley, C.M. Plant-derived sucrose is a key element in the symbiotic association between Trichoderma virens and maize plants. Plant Physiol. 2009, 151, 792–808. [Google Scholar] [CrossRef]
- Azarmi, R.; Hajieghrari, B.; Giglou, A. Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr. J. Biotechnol. 2011, 10, 5850–5855. [Google Scholar]
- Pereira, J.L.; Queiroz, R.M.L.; Charneau, S.; Felix, C.R.; Ricart, C.A.; Lopes da Silva, F.; Stecca, A.; Steindorff, C.; Ulhoa, C.J.; Noronha, E.F. Analysis of Phaseolus vulgaris response to its association with Trichoderma harzianum (ALL-42) in the presence or absence of the phytopathogenic fungi Rhizoctonia solani and Fusarium solani. PLoS ONE 2014, 9, e98234. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).