The Gut Bacterial Community of Chlaenius pallipes (Coleoptera: Carabidae) Associates with Their Habitat and Morphology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
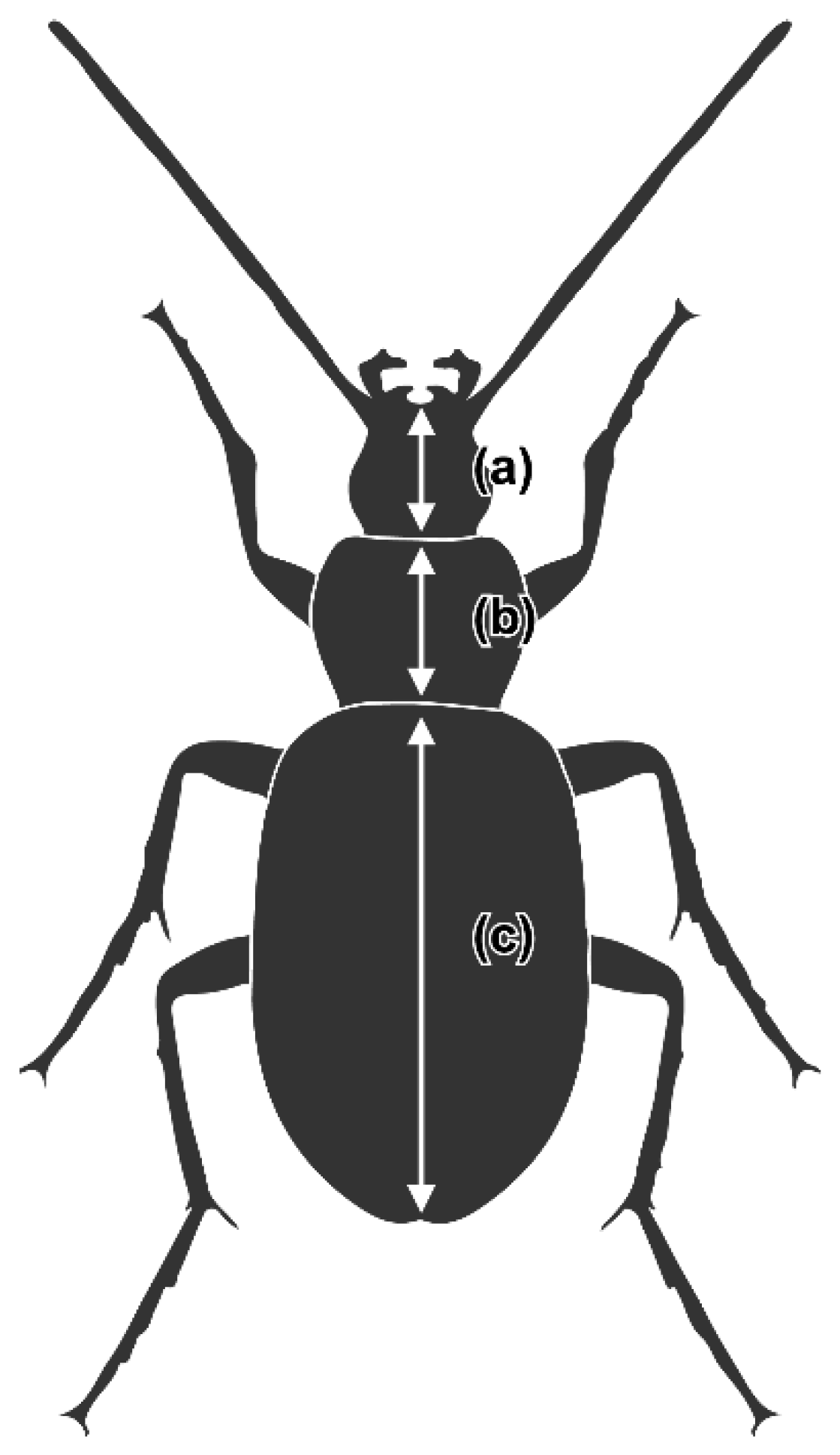
2.1. Carabid Beetle Sampling, Measurement, and Dissection
2.2. DNA Extraction, PCR, and Next Generation Sequencing
2.3. Landmark-Based Geometric Morphometrics
2.4. Nitrogen Stable Isotope Ratio Analyses
2.5. Statistical Analysis
3. Results
3.1. Difference in C. pallipes Food and Morphological Shape according to Habitat Types
3.2. Effect of Habitat Type on Gut Bacterial Community Composition
3.3. Correlation among Diet, Morphological Shape, and Bacterial Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lövei, G.L.; Sunderland, K. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Rainio, J.; Niemelä, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Pearce, J.L.; Venier, L.A. The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: A review. Ecology 2006, 6, 780–793. [Google Scholar] [CrossRef]
- Koivula, M.J. Useful model organisms, indicators, or both? Ground beetles (Coleoptera, Carabidae) reflecting environmental conditions. ZooKeys 2011, 100, 287–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giglio, A.; Giulianini, P.G.; Zetto, T.; Talarico, F. Effects of the pesticide dimethoate on a non-target generalist carabid, Pterostichus melas italicus (Dejean, 1828)(Coleoptera: Carabidae). Ital. J. Zool. 2011, 78, 471–477. [Google Scholar] [CrossRef] [Green Version]
- Giglio, A.; Cavaliere, F.; Giulianini, P.G.; Mazzei, A.; Talarico, F.; Vommaro, M.L.; Brandmayr, P. Impact of agrochemicals on non-target species: Calathus fuscipes Goeze 1777 (Coleoptera: Carabidae) as model. Ecotoxicol. Environ. Saf. 2017, 142, 522–529. [Google Scholar] [CrossRef]
- Simon, E.; Harangi, S.; Baranyai, E.; Braun, M.; Fábián, I.; Mizser, S.; Nagy, L.; Tóthmérész, B. Distribution of toxic elements between biotic and abiotic components of terrestrial ecosystem along an urbanization gradient: Soil, leaf litter and ground beetles. Ecol. Indic. 2016, 60, 258–264. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Lee, W.J.; Hase, K. Gut microbiota–generated metabolites in animal health and disease. Nat. Chem. Biol. 2014, 10, 416–424. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [Green Version]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, J.H.; Roh, S.W.; Whon, T.W.; Jung, M.J.; Kim, M.S.; Park, D.S.; Yoon, C.; Nam, Y.D.; Kim, Y.J.; Choi, J.H. Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, P.; Moran, N.A. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, H.R.; Tomberlin, J.K. Microbial influence on reproduction, conversion, and growth of mass produced insects. Curr. Opin. Insect Sci. 2021, 48, 57–63. [Google Scholar] [CrossRef]
- Syromyatnikov, M.Y.; Isuwa, M.M.; Savinkova, O.V.; Derevshchikova, M.I.; Popov, V.N. The effect of pesticides on the microbiome of animals. Agriculture 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Hyodo, F. Use of stable carbon and nitrogen isotopes in insect trophic ecology. Entomol. Sci. 2015, 18, 295–312. [Google Scholar] [CrossRef]
- Quinby, B.M.; Creighton, J.C.; Flaherty, E.A. Stable isotope ecology in insects: A review. Ecol. Entomol. 2020, 45, 1231–1246. [Google Scholar] [CrossRef]
- Woodcock, P.; Edwards, D.P.; Newton, R.J.; Vun Khen, C.; Bottrell, S.H.; Hamer, K.C. Impacts of intensive logging on the trophic organisation of ant communities in a biodiversity hotspot. PLoS ONE 2013, 8, e60756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbi, M.; Rossaro, B.; Vater, A.; De Bernardi, F.; Pelfini, M.; Brandmayr, P. Environmental features influencing Carabid beetle (Coleoptera) assemblages along a recently deglaciated area in the Alpine region. Ecol. Entomol. 2007, 32, 682–689. [Google Scholar] [CrossRef]
- Blake, S.; Foster, G.; Eyre, M.; Luff, M. Effects of habitat type and grassland management practices on the body size distribution of carabid beetles. Pedobiologia 1994, 38, 502–512. [Google Scholar]
- Magura, T.; Tóthmérész, B.; Lövei, G.L. Body size inequality of carabids along an urbanisation gradient. Basic Appl. Ecol. 2006, 7, 472–482. [Google Scholar] [CrossRef]
- Gray, J.S. Effects of environmental stress on species rich assemblages. Biol. J. Linn. Soc. 1989, 37, 19–32. [Google Scholar] [CrossRef]
- Desender, K. Heritability of wing development and body size in a carabid beetle, Pogonus chalceus Marsham, and its evolutionary significance. Oecologia 1989, 78, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Zaiewski, M.; Dudekgodeau, D.; Tiunov, A.V.; Godeau, J.F.; Okuzaki, Y.; Ikeda, H.; Sienkiewicz, P.; Ulrich, W. Wing morphology is linked to stable isotope composition of nitrogen and carbon in ground beetles (coleoptera: Carabidae). Eur. J. Entomol. 2015, 112, 810–817. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Fischer, C.; Schlinkert, H.; Ludwig, M.; Holzschuh, A.; Gallé, R.; Tscharntke, T.; Batáry, P. The impact of hedge-forest connectivity and microhabitat conditions on spider and carabid beetle assemblages in agricultural landscapes. J. Insect Conserv. 2013, 17, 1027–1038. [Google Scholar] [CrossRef]
- Aukema, B. The evolutionary significance of wing dimorphism in carabid beetles (Coleoptera: Carabidae). Popul. Ecol. 1995, 37, 105–110. [Google Scholar] [CrossRef]
- Yamashita, H.; Kiritani, K.; Togashi, K.; Kubota, K. Wing dimorphism in three carabid species living in the grasslands of Mt. Omuro, Shizuoka, Japan. Appl. Entomol. Zool. 2006, 41, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Maryanski, M.; Kramarz, P.; Laskowski, R.; Niklinska, M. Decreased energetic reserves, morphological changes and accumulation of metals in carabid beetles (Poecilus cupreus L.) exposed to zinc-or cadmium-contaminated food. Ecotoxicology 2002, 11, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Sasakawa, K.; Ikeda, H.; Kubota, T. Feeding ecology of granivorous carabid larvae: A stable isotope analysis. J. Appl. Entomol. 2010, 134, 116–122. [Google Scholar] [CrossRef]
- Venn, S. Morphological responses to disturbance in wing-polymorphic carabid species (Coleoptera: Carabidae) of managed urban grasslands. Balt. J. Coleopterol. 2007, 7, 51–59. [Google Scholar]
- Okuzaki, Y.; Tayasu, I.; Okuda, N.; Sota, T. Stable isotope analysis indicates trophic differences among forest floor carabids in Japan. Entomol. Exp. Appl. 2010, 135, 263–270. [Google Scholar] [CrossRef]
- Silver, A.; Perez, S.; Gee, M.; Xu, B.; Garg, S.; Will, K.; Gill, A. Persistence of the ground beetle (Coleoptera: Carabidae) microbiome to diet manipulation. PLoS ONE 2021, 16, e0241529. [Google Scholar] [CrossRef]
- Kudo, R.; Masuya, H.; Endoh, R.; Kikuchi, T.; Ikeda, H. Gut bacterial and fungal communities in ground-dwelling beetles are associated with host food habit and habitat. ISME J. 2019, 13, 676–685. [Google Scholar] [CrossRef] [Green Version]
- Kaltenpoth, M.; Steiger, S. Unearthing carrion beetles’ microbiome: Characterization of bacterial and fungal hindgut communities across the Silphidae. Mol. Ecol. 2014, 23, 1251–1267. [Google Scholar] [CrossRef]
- Lehman, R.M.; Lundgren, J.G.; Petzke, L.M. Bacterial communities associated with the digestive tract of the predatory ground beetle, Poecilus chalcites, and their modification by laboratory rearing and antibiotic treatment. Microb. Ecol. 2009, 57, 349–358. [Google Scholar] [CrossRef]
- Dillon, R.J.; Webster, G.; Weightman, A.J.; Keith Charnley, A. Diversity of gut microbiota increases with aging and starvation in the desert locust. Anton. Leeuw. 2010, 97, 69–77. [Google Scholar] [CrossRef]
- Paris, L.; Peghaire, E.; Moné, A.; Diogon, M.; Debroas, D.; Delbac, F.; El Alaoui, H. Honeybee gut microbiota dysbiosis in pesticide/parasite co-exposures is mainly induced by Nosema ceranae. J. Invertebr. Pathol. 2020, 172, 107348. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, Y.; Park, J.-K.; Park, W.-B.; Kim, M.-S. The Gut Bacterial Community of Chlaenius pallipes (Coleoptera: Carabidae) Associates with Their Habitat and Morphology. Insects 2022, 13, 1099. https://doi.org/10.3390/insects13121099
Do Y, Park J-K, Park W-B, Kim M-S. The Gut Bacterial Community of Chlaenius pallipes (Coleoptera: Carabidae) Associates with Their Habitat and Morphology. Insects. 2022; 13(12):1099. https://doi.org/10.3390/insects13121099
Chicago/Turabian StyleDo, Yuno, Jun-Kyu Park, Woong-Bae Park, and Min-Seob Kim. 2022. "The Gut Bacterial Community of Chlaenius pallipes (Coleoptera: Carabidae) Associates with Their Habitat and Morphology" Insects 13, no. 12: 1099. https://doi.org/10.3390/insects13121099




