Transcriptomic Analysis Insight into the Immune Modulation during the Interaction of Ophiocordyceps sinensis and Hepialus xiaojinensis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection, RNA Extraction
2.2. Sequencing and Assembly of Transcriptome Data
2.3. Analysis and Annotation of Differentially Expressed Genes
2.4. Identification of Immune-Related Genes from the Comparative Transcriptomes of H. xiaojinensis
2.5. Quantitative Real-Time PCR
2.6. Data Availability
3. Results and Discussion
3.1. RNA Sequencing Analysis
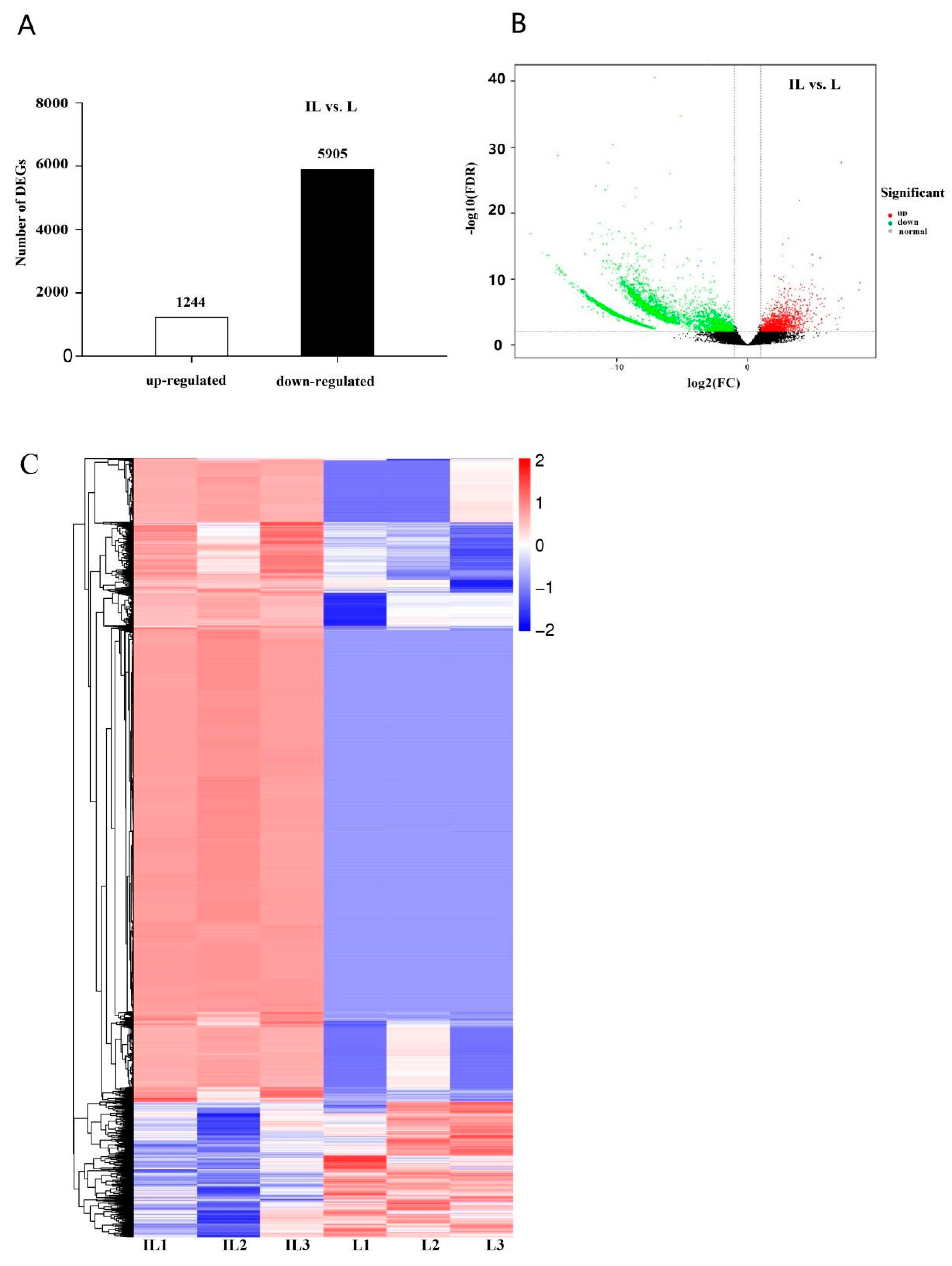
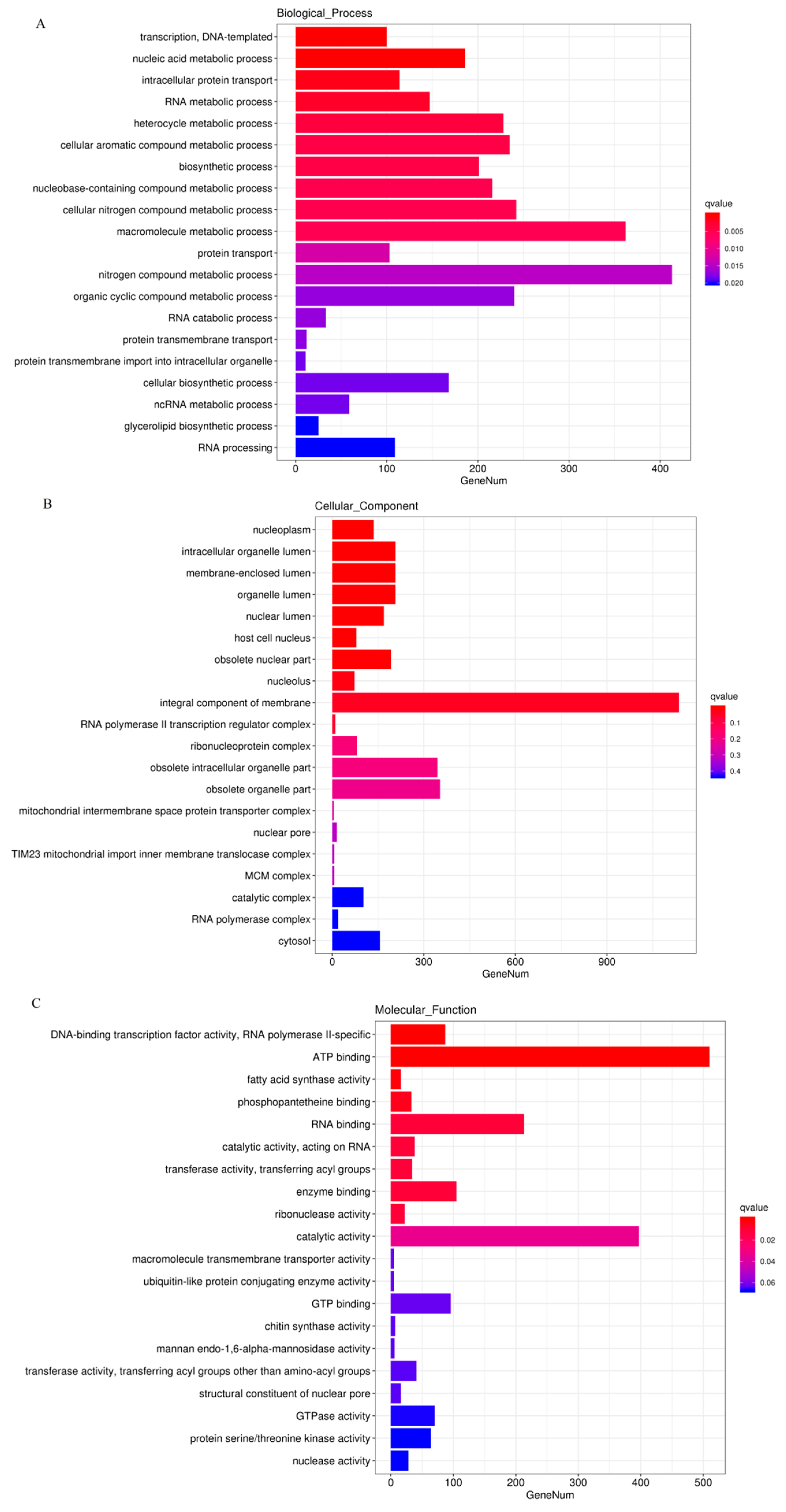
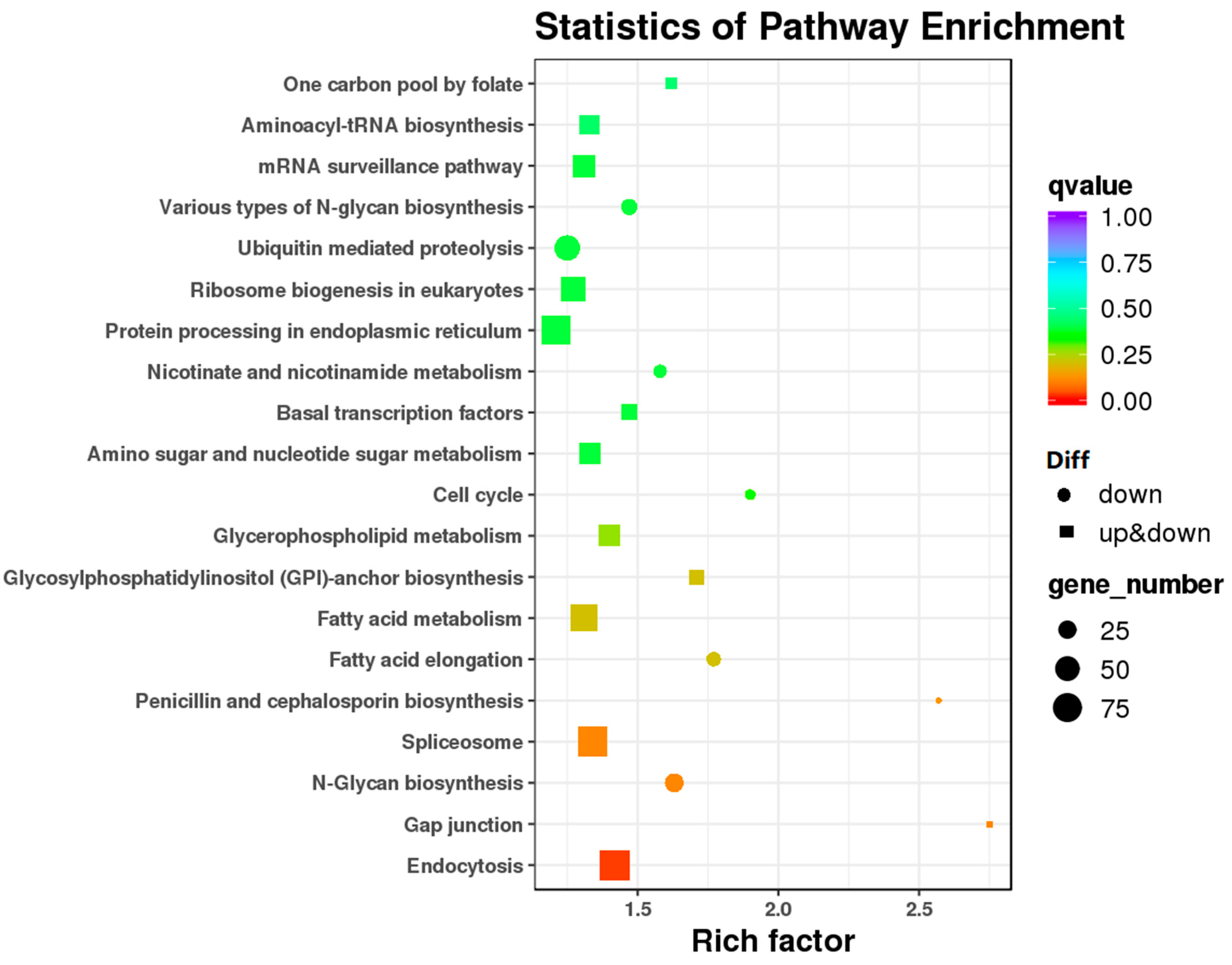
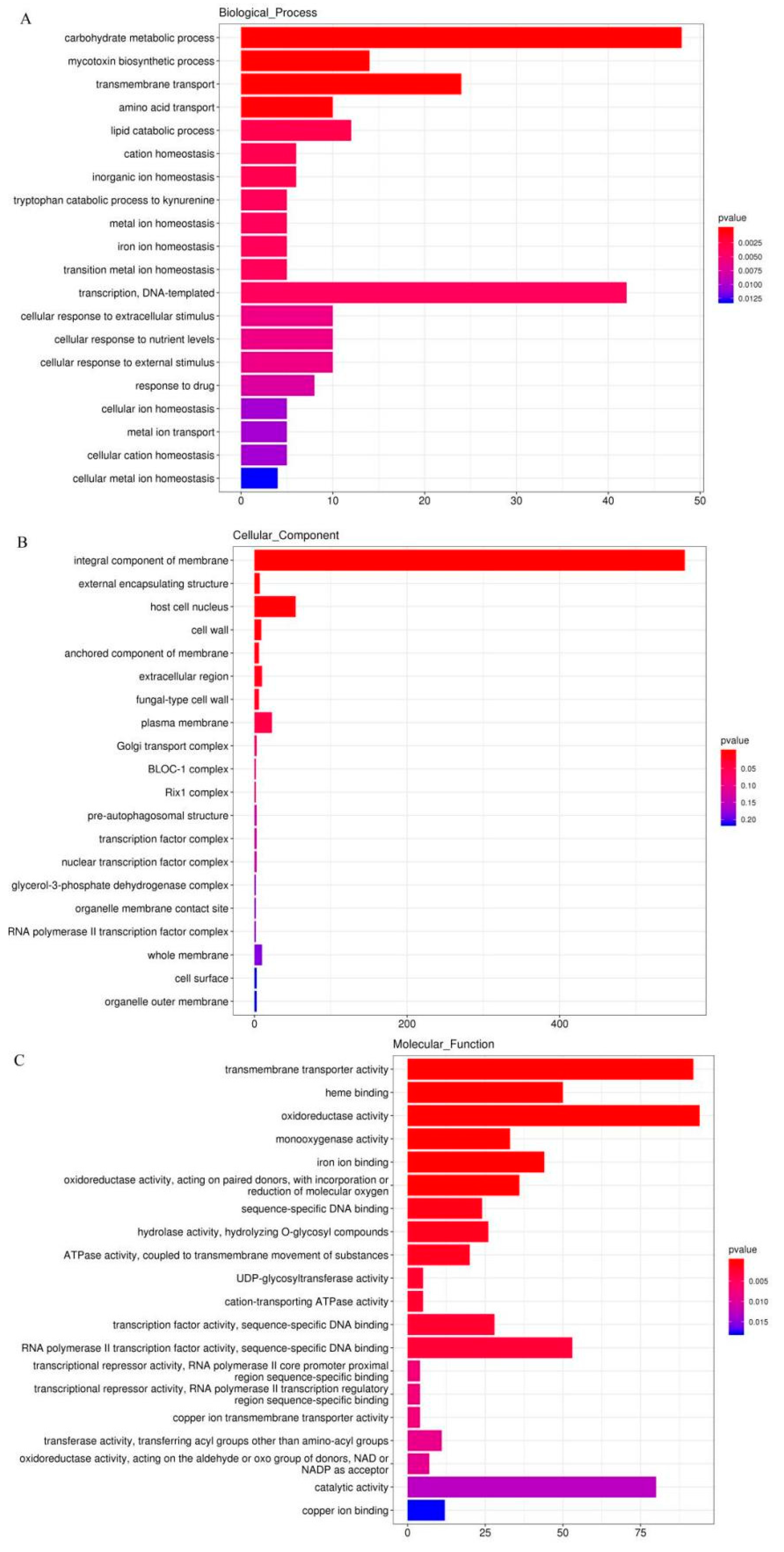
3.2. Analysis of Gene Differential Expression and Functional Enrichment in the Comparison of IL and L
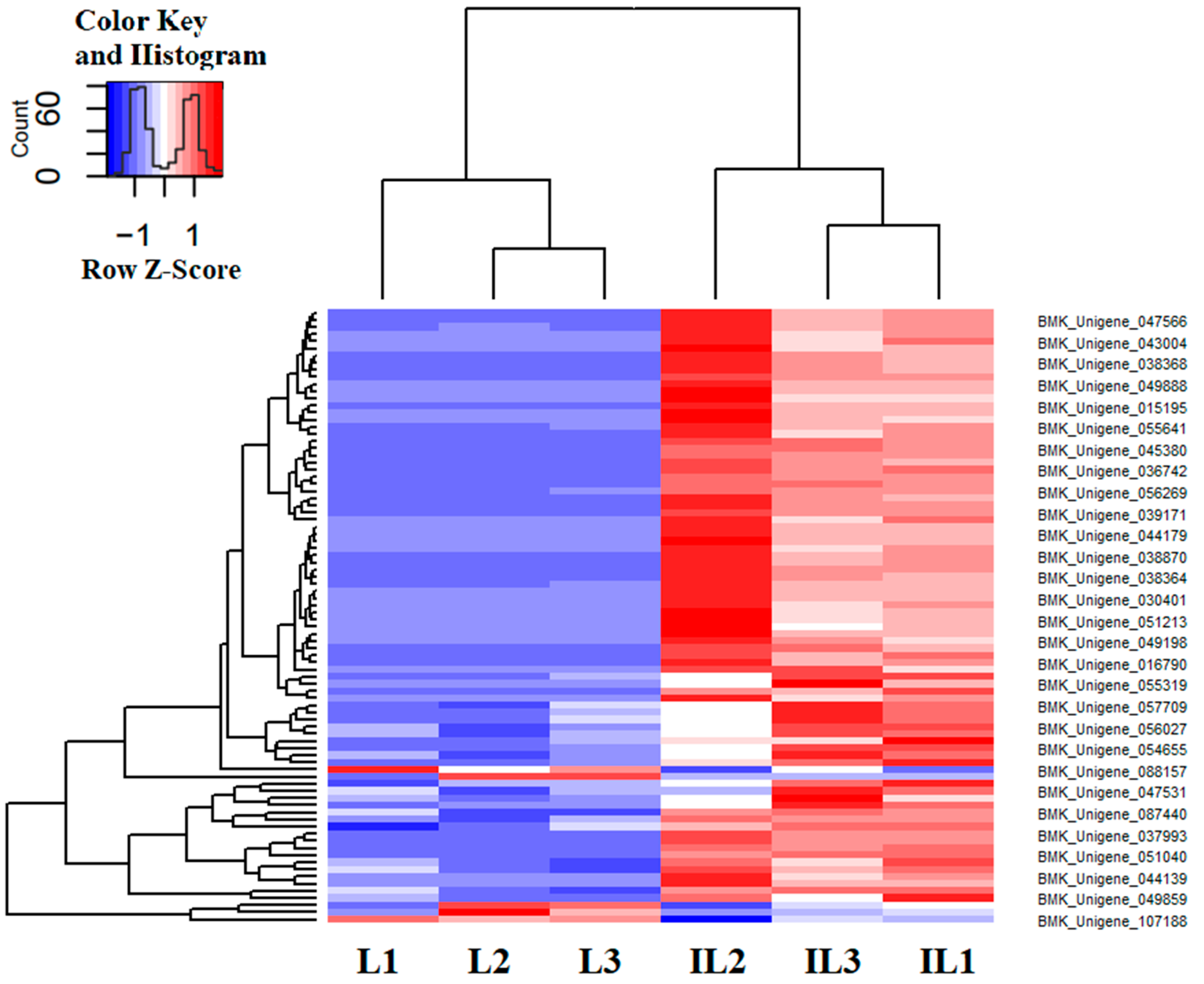
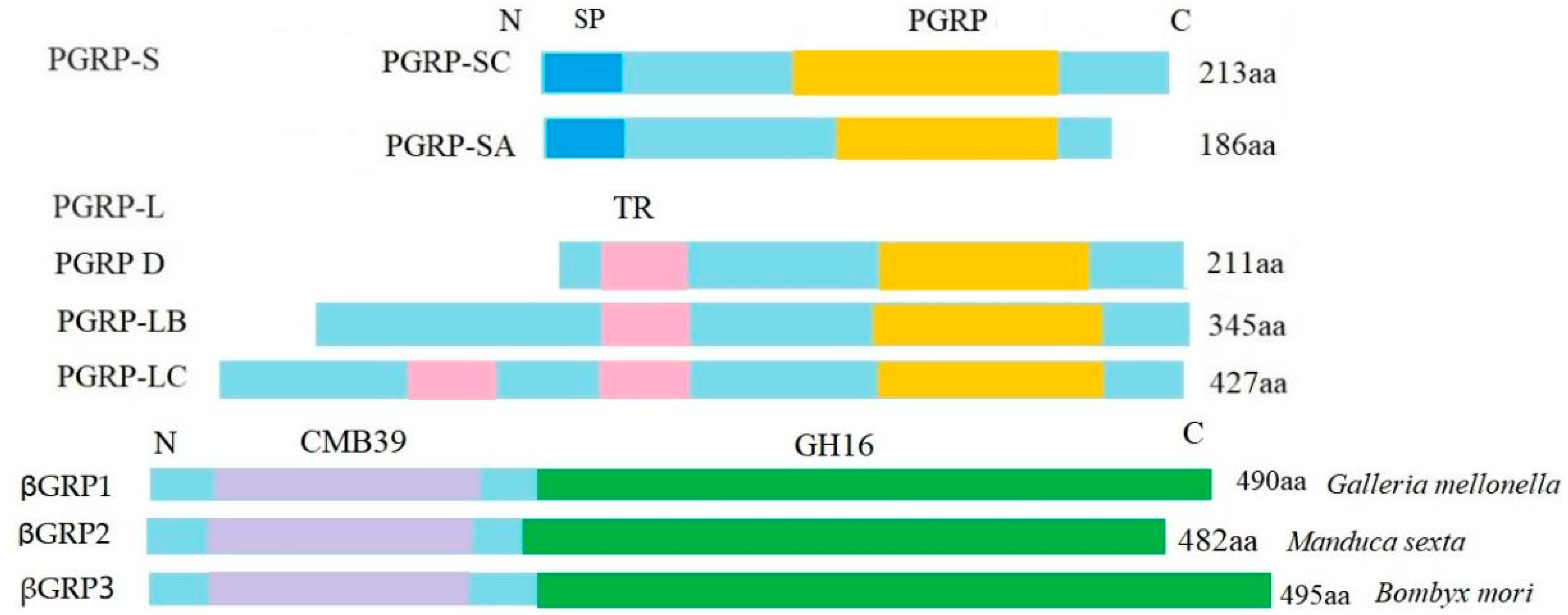
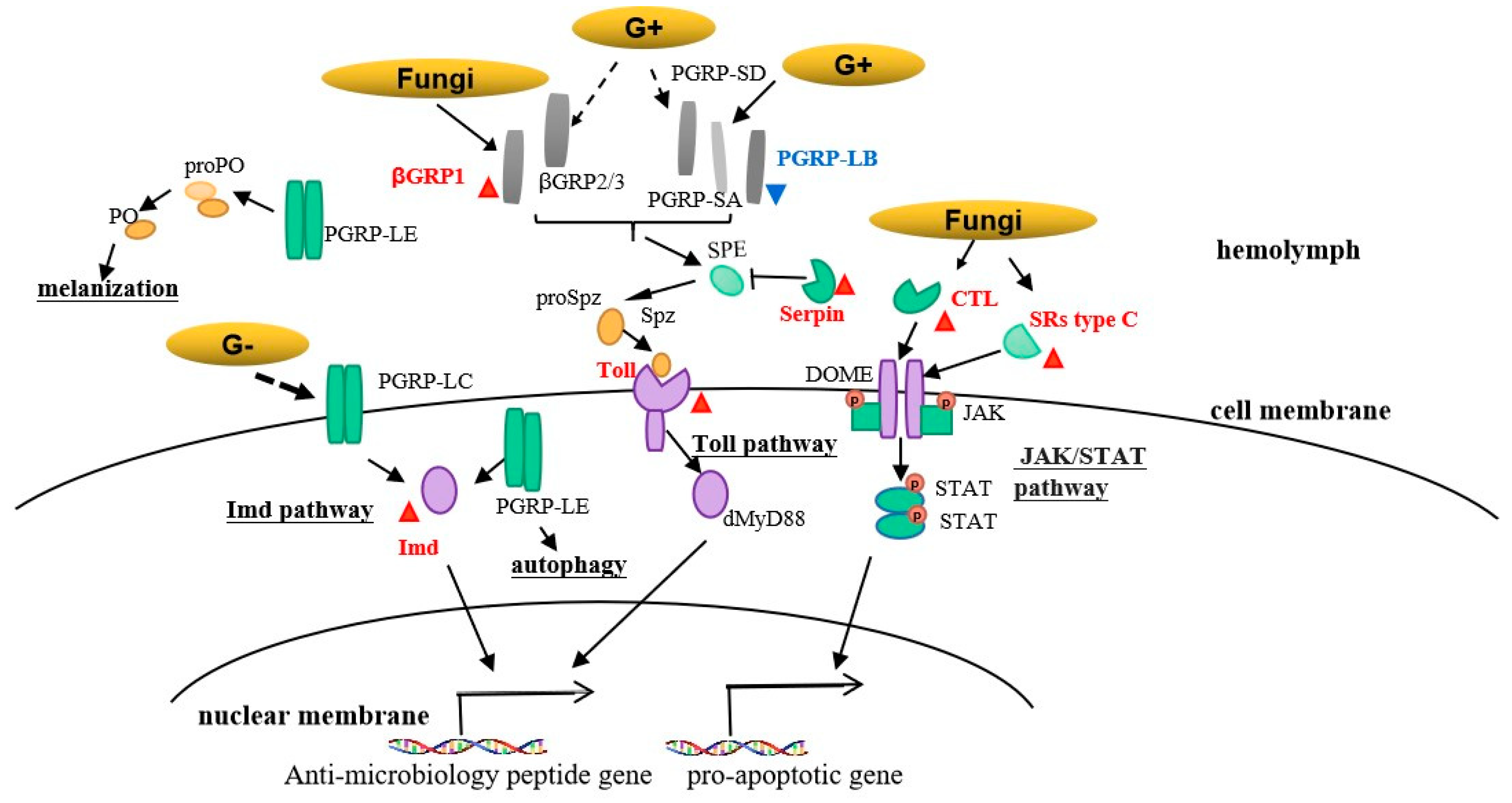
3.3. Immune-Related DEGs Analyses
3.4. Differential Gene Expression and Functional Enrichment Analysis in the Comparison of IL and ML
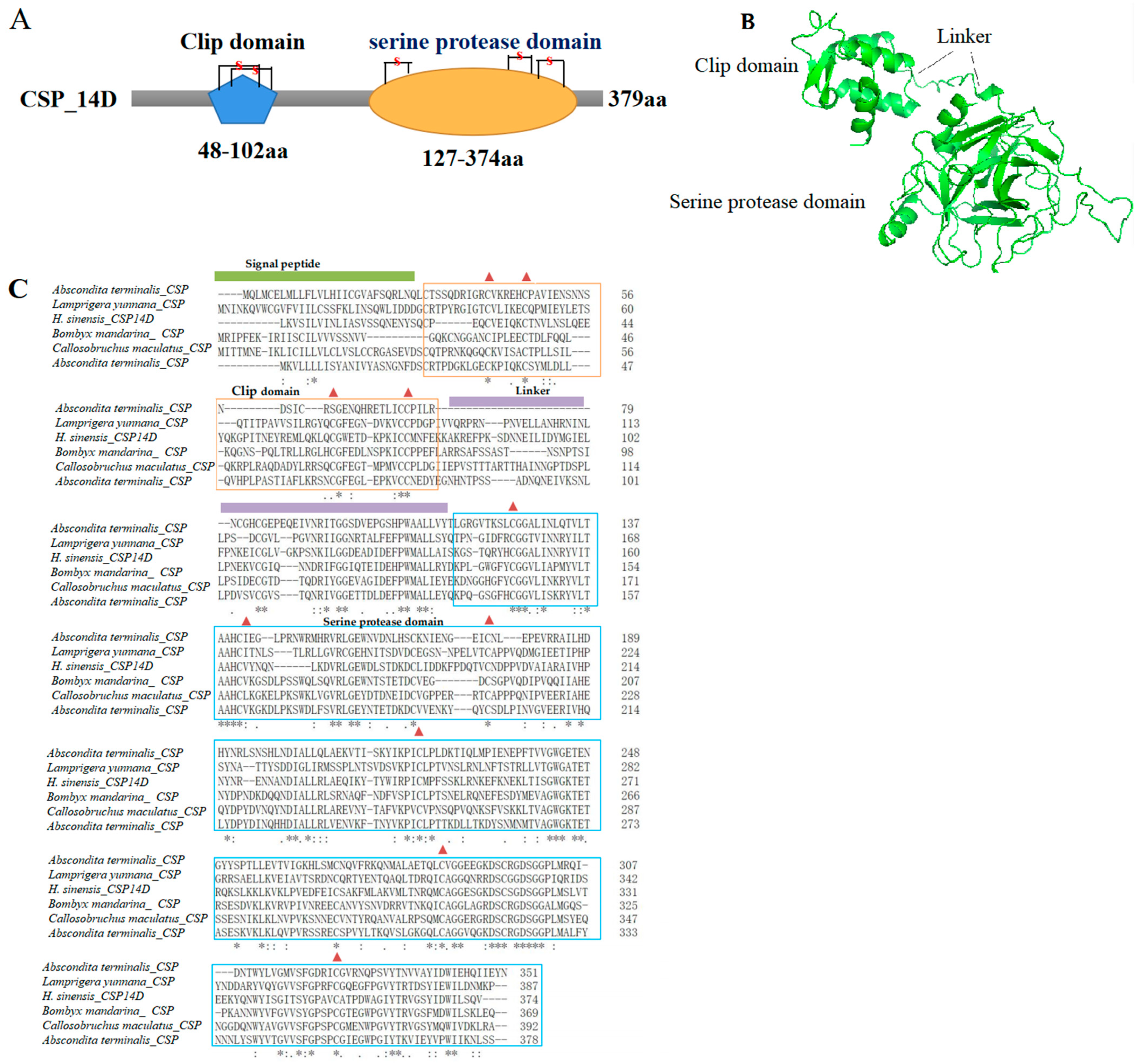
3.5. Analysis of the Gene Families Involved in Fungal Pathogenicity
3.6. The Results of qPCR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| L | the pre-infected larva of H. xiaojinensis |
| IL | the one-year post-infected larva of H. xiaojinensis |
| ML | the mummified larva of H. xiaojinensis |
| TCM | Traditional Chinese Medicine |
| RNA-seq | RNA sequencing |
| log2FC | log 2-fold-change |
| DEGs | differentially expressed genes |
| ROS | reactive oxygen species |
| qPCR | quantitative real-time PCR |
| PDA | Potato Dextrose Agar |
| FDR | false discovery rate |
| GO | Gene Ontology |
| IMD | immune deficiency |
| FPKM | fragments per kilobase per million mapped reads |
| CTL | C-type lectins |
| PAMP | pathogen-associated molecular pattern |
| PRRs | pathogen recognition receptor |
| βGRP | β-1,3-glucan recognition protein |
| PGRP | peptidoglycan recognition protein |
| SR | scavenger receptors |
| m | meter |
| RH | Relative Humidity |
| proPO | prophenoloxidase |
| SP | signal peptide |
| TM | transmembrane domain |
| CSP | clip domain serine proteases |
| MAPK | mitogen-activated protein kinase |
| SCPL | serine carboxypeptidase-like protein |
References
- Wei, Y.; Zhang, L.; Wang, J.; Wang, W.; Niyati, N.; Guo, Y.; Wang, X. Chinese caterpillar fungus (Ophiocordyceps sinensis) in China: Current distribution, trading, and futures under climate change and overexploitation. Sci. Total Environ. 2021, 755, 142548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Li, E.; Wang, C.S.; Li, Y.L.; Liu, X.Z. Ophiocordyceps sinensis, the flagship fungus of China: Terminology, life strategy and ecology. Mycology 2012, 3, 2–10. [Google Scholar]
- Xu, J.; Huang, Y.; Chen, X.X.; Zheng, S.C.; Chen, P.; Mo, M.H. The Mechanisms of Pharmacological Activities of Ophiocordyceps sinensis Fungi. Phytother. Res. 2016, 30, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Liu, X.Y.; Kanari, K. Economic aspects of harvesting and trading the Chinese Caterpillar Fungus Ophiocordyceps sinensis and Southern Schisandra Schisandra sphenanthera in China’s Upper Yangtze Ecoregion. Traffic Bull. 2012, 24, 15–24. [Google Scholar]
- Li, X.; Liu, Q.; Li, W.; Li, Q.; Qian, Z.; Liu, X.; Dong, C. A breakthrough in the artificial cultivation of Chinese cordyceps on a large-scale and its impact on science, the economy, and industry. Crit. Rev. Biotechnol. 2019, 39, 181–191. [Google Scholar] [CrossRef]
- Li, M.; Meng, Q.; Zhang, H.; Ni, R.; Zhou, G.; Zhao, Y.; Wu, P.; Shu, R.; Qin, Q.; Zhang, J. Vegetative development and host immune interaction of Ophiocordyceps sinensis within the hemocoel of the ghost moth larva, Thitarodes xiaojinensis. J. Invertebr. Pathol. 2020, 170, 107331. [Google Scholar] [CrossRef]
- Xia, E.H.; Yang, D.R.; Jiang, J.J.; Zhang, Q.J.; Liu, Y.; Liu, Y.L.; Zhang, Y.; Zhang, H.B.; Shi, C.; Tong, Y.; et al. The caterpillar fungus, Ophiocordyceps sinensis, genome provides insights into highland adaptation of fungal pathogenicity. Sci. Rep. 2017, 7, 1806. [Google Scholar] [CrossRef] [Green Version]
- Etxebeste, O.; Herrero-García, E.; Cortese, M.S.; Garzia, A.; Oiartzabal-Arano, E.; Ríos, V.; Ugalde, U.; Espeso, E.A. GmcA is a putative glucose-methanol-choline oxidoreductase required for the induction of asexual development in Aspergillus nidulans. PLoS ONE 2012, 7, e40292. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Czymmek, K.J.; Caplan, J.L.; Sweigard, J.A.; Donofrio, N.M. HYR1-mediated detoxification of reactive oxygen species is required for full virulence in the rice blast fungus. PLoS Pathog. 2011, 7, e1001335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.L.; Zeng, W.; Chen, S.J.; Xu, Y.Q. Study on biological characteristic of Hepialus xiaojinensis in Sichuan. Chin. J. Appl. Entomol. 2011, 48, 990–996. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Chor, B.; Horn, D.; Goldman, N.; Levy, Y.; Massingham, T. Genomic DNA k-mer spectra: Models and modalities. Genome Biol. 2009, 10, R108. [Google Scholar] [CrossRef]
- Kamal, M.S.; Parvin, S.; Ashour, A.S.; Fuqian Shi, F.Q.; Dey, N. De-Bruijn graph with MapReduce framework towards metagenomic data classification. Int. J. Inf. Technol. 2017, 9, 59–75. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [Green Version]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, Y.; Nakagawa, H.; Koike, K. Lipid Metabolism in Oncology: Why It Matters, How to Research, and How to Treat. Cancers 2021, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, P.; Calder, P.C. Fatty acids and immune function: New insights into mechanisms. Br. J. Nutr. 2007, 1, S41–S45. [Google Scholar] [CrossRef] [Green Version]
- Di, C.F.; Andreoletti, P.; Trompier, D.; Vejux, A.; Bülow, M.H.; Sellin, J.; Lizard, G.; Cherkaoui-Malki, M.; Savary, S. Peroxisomes in Immune Response and Inflammation. Int. J. Mol. Sci. 2019, 20, 3877. [Google Scholar]
- Li, L.; Wang, J.; Chen, H.; Chai, R.; Zhang, Z.; Mao, X.; Qiu, H.; Jiang, H.; Wang, Y.; Sun, G. Pex14/17, a filamentous fungus-specific peroxin, is required for the import of peroxisomal matrix proteins and full virulence of Magnaporthe oryzae. Mol. Plant Pathol. 2017, 18, 1238–1252. [Google Scholar] [CrossRef] [Green Version]
- Di, C.F.; Sheshachalam, A.; Braverman, N.E.; Rachubinski, R.A.; Simmonds, A.J. Peroxisome-Mediated Metabolism Is Required for Immune Response to Microbial Infection. Immunity 2017, 47, 93–106. [Google Scholar]
- Vijayan, V.; Srinu, T.; Karnati, S.; Garikapati, V.; Linke, M.; Kamalyan, L.; Mali, S.R.; Sudan, K.; Kollas, A.; Schmid, T.; et al. A New Immunomodulatory Role for Peroxisomes in Macrophages Activated by the TLR4 Ligand Lipopolysaccharide. J. Immunol. 2017, 198, 2414–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in Lepidopteran Insects, Invertebrate Immunity; Springer: Berlin/Heidelberg, Germany, 2010; pp. 181–204. [Google Scholar]
- Yoshida, H.; Kinoshita, K.; Ashida, M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 1996, 271, 13854–13860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziarski, R. Peptidoglycan recognition proteins (PGRPs). Mol. Immunol. 2004, 40, 877–886. [Google Scholar] [CrossRef] [Green Version]
- Zaidman-Rémy, A.; Hervé, M.; Poidevin, M.; Pili-Floury, S.; Kim, M.S.; Blanot, D.; Oh, B.H.; Ueda, R.; Mengin-Lecreulx, D.; Lemaitre, B. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity 2006, 24, 463–473. [Google Scholar] [CrossRef]
- Kurata, S. Extracellular and intracellular pathogen recognition by Drosophila PGRP-LE and PGRP-LC. Int. Immunol. 2010, 22, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Matskevich, A.A.; Quintin, J.; Ferrandon, D. The Drosophila PRR GNBP3 assembles effector complexes involved in antifungal defenses independently of its Toll-pathway activation function. Eur. J. Immunol. 2010, 40, 1244–1254. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Yu, H.Y.; Zhang, H.; Zhu, W.; Wang, M.L.; Zhang, J.H.; Zhou, G.L.; Li, X.; Qin, Q.L.; Hu, S.N.; et al. Transcriptomic insight into the immune defenses in the ghost moth, Hepialus xiaojinensis, during an Ophiocordyceps sinensis fungal infection. Insect Biochem. Mol. Biol. 2015, 64, 1–15. [Google Scholar] [CrossRef]
- Geng, T.; Lu, F.; Wu, H.; Wang, Y.; Lou, D.; Tu, N.; Zhu, F.; Wang, S. C-type lectin 5, a novel pattern recognition receptor for the JAK/STAT signaling pathway in Bombyx mori. J. Invertebr. Pathol. 2021, 179, 107473. [Google Scholar] [CrossRef]
- Pombinho, R.; Sousa, S.; Cabanes, D. Scavenger Receptors: Promiscuous Players during Microbial Pathogenesis. Crit. Rev. Microbiol. 2018, 44, 685–700. [Google Scholar] [CrossRef]
- Jiang, H.; Kanost, M.R. The clip-domain family of serine proteinases in arthropods. Insect Biochem. Mol. Biol. 2000, 30, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Y.; Yu, X.Q.; Zhu, Y.; Kanost, M. Prophenoloxidase-activating proteinase-3 (PAP-3) from Manduca sexta hemolymph: A clip-domain serine proteinase regulated by serpin-1J and serine proteinase homologs. Insect Biochem. Mol. Biol. 2003, 33, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Forneris, F.; Wu, J.; Gros, P. The modular serine proteases of the complement cascade. Curr. Opin. Struct. Biol. 2012, 22, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.G.; Pinto, S.B.; Ghosh, A.; Vanlandingham, D.L.; Budd, A.; Higgs, S.; Kafatos, F.C.; Jacobs-Lorena, M.; Michel, K. An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc. Natl. Acad. Sci. USA 2005, 102, 16327–16332. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Picheng, Z.; Weng, H.; Mita, K.; Jiang, H. A comparative analysis of serpin genes in the silkworm genome. Genomics 2009, 93, 367–375. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Soares-Silva, M.; Diniz, F.F.; Gomes, G.N.; Bahia, D. The Mitogen-Activated Protein Kinase (MAPK) Pathway: Role in Immune Evasion by Trypanosomatids. Front. Microbiol. 2016, 7, 183. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef]
- Wileman, T. Autophagy as a defence against intracellular pathogens. Essays Biochem. 2013, 55, 153–163. [Google Scholar]
- Meng, X.; Liao, Z.; Liu, T.; Hussain, K.; Chen, J.; Fang, Q.; Wang, J. Vital roles of Pks11, a highly reducing polyketide synthase, in fungal conidiation, antioxidant activity, conidial cell wall integrity, and UV tolerance of Beauveria bassiana. J. Invertebr. Pathol. 2021, 181, 107588. [Google Scholar] [CrossRef]
- Feng, P.; Shang, Y.; Cen, K.; Wang, C. Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 11365–11370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; de Waard, A.A.; Wuhrer, M.; Spaapen, R.M. The Role of Glycosphingolipids in Immune Cell Functions. Front. Immunol. 2019, 10, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, H.; Nagafuku, M.; Suzuki, A.; Iwabuchi, K.; Inokuchi, J.I. The regulatory roles of glycosphingolipid-enriched lipid rafts in immune systems. FEBS Lett. 2018, 592, 3921–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Cai, M.; Xia, W.; Liu, J.; Zhang, Q.; Xie, H.; Wang, C.; Wang, X.; Zheng, S. FTY720, a synthetic compound from Isaria sinclairii, inhibits proliferation and induces apoptosis in pancreatic cancer cells. Cancer Lett. 2007, 254, 288–297. [Google Scholar] [CrossRef]
- Nishimura, T.; Duereh, M.; Sugita, Y.; Yoshida, Y.; Higuchi, K.; Tomi, M.; Nakashima, E. Protective effect of hypotaurine against oxidative stress-induced cytotoxicity in rat placental trophoblasts. Placenta 2015, 36, 693–698. [Google Scholar] [CrossRef]
- Cozzone, A.J. Role of protein phosphorylation on serine/threonine and tyrosine in the virulence of bacterial pathogens. J. Mol. Microbiol. Biotechnol. 2005, 9, 198–213. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.J.; Xiao, G.H.; Zheng, P.; Xia, Y.L.; Zhang, X.Y.; St Leger, R.J.; Liu, X.Z.; Wang, C.S. Genome survey uncovers the secrets of sex and lifestyle in caterpillar fungus. Chin. Sci. Bull. 2013, 58, 2846–2854. [Google Scholar] [CrossRef] [Green Version]
- Chandor-Proust, A.; Bibby, J.; Régent-Kloeckner, M.; Roux, J.; Guittard-Crilat, E.; Poupardin, R.; Riaz, M.A.; Paine, M.; Dauphin-Villemant, C.; Reynaud, S.; et al. The central role of mosquito cytochrome P450 CYP6Zs in insecticide detoxification revealed by functional expression and structural modelling. Biochem. J. 2013, 455, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, P.A.; Shen, A.L.; Paschke, R.; Kasper, C.B.; Kim, J.J. NADPH-cytochrome P450 oxidoreductase. Structural basis for hydride and electron transfer. Biochem. J. 2001, 276, 29163–29170. [Google Scholar]
- Yike, I. Fungal proteases and their pathophysiological effects. Mycopathologia 2011, 171, 299–323. [Google Scholar] [CrossRef]
- Mucha, A.; Drag, M.; Dalton, J.P.; Kafarski, P. Metallo-aminopeptidase inhibitors. Biochimie 2010, 92, 1509–1529. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, L.; Tian, Y.; Zhang, K.Q. Molecular evolution of the deuterolysin (M35) family genes in Coccidioides. PLoS ONE 2012, 7, e31536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, K.Q. Independent expansion of zincin metalloproteinases in Onygenales fungi may be associated with their pathogenicity. PLoS ONE 2014, 9, e90225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilly, W.W.; Stajich, J.E.; Pukkila, P.J.; Wilke, S.K.; Inoguchi, N.; Gathman, A.C. An expanded family of fungalysin extracellular metallopeptidases of Coprinopsis cinerea. Mycol. Res. 2008, 112, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, L.; Zhao, W.; Fu, L.; Han, Y.; Wang, K.; Yan, L.; Li, Y.; Zhang, X.H.; Min, D.H. Genome-wide analysis of the serine carboxypeptidase-like protein family in Triticum aestivum reveals TaSCPL184-6D is involved in abiotic stress response. BMC Genom. 2021, 22, 350. [Google Scholar] [CrossRef]
- Kenney, E.; Yaparla, A.; Hawdon, J.M.; O’ Halloran, D.M.; Grayfer, L.; Eleftherianos, I. A putative lysozyme and serine carboxypeptidase from Heterorhabditis bacteriophora show differential virulence capacities in Drosophila melanogaster. Dev. Comp. Immunol. 2021, 114, 103820. [Google Scholar] [CrossRef]


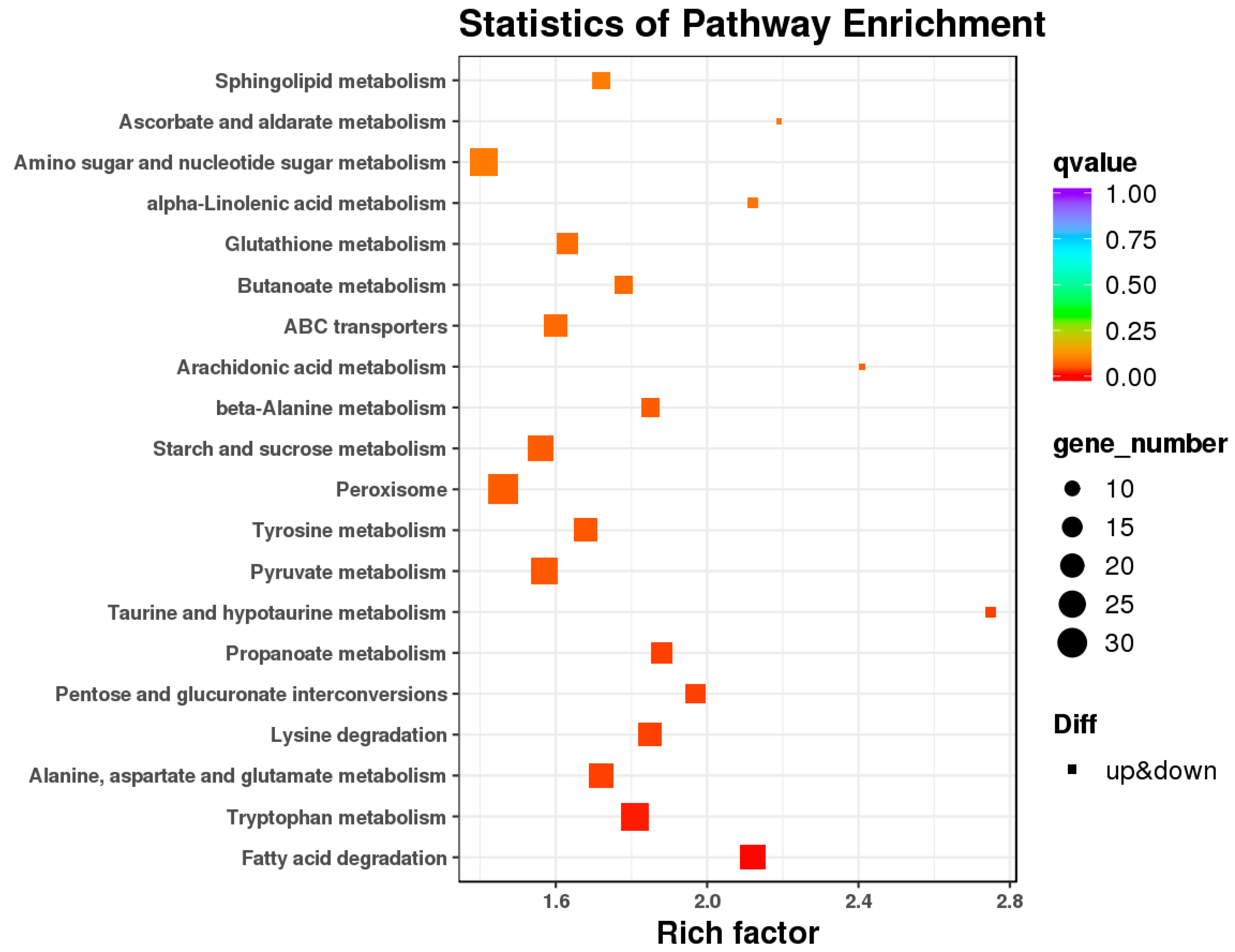
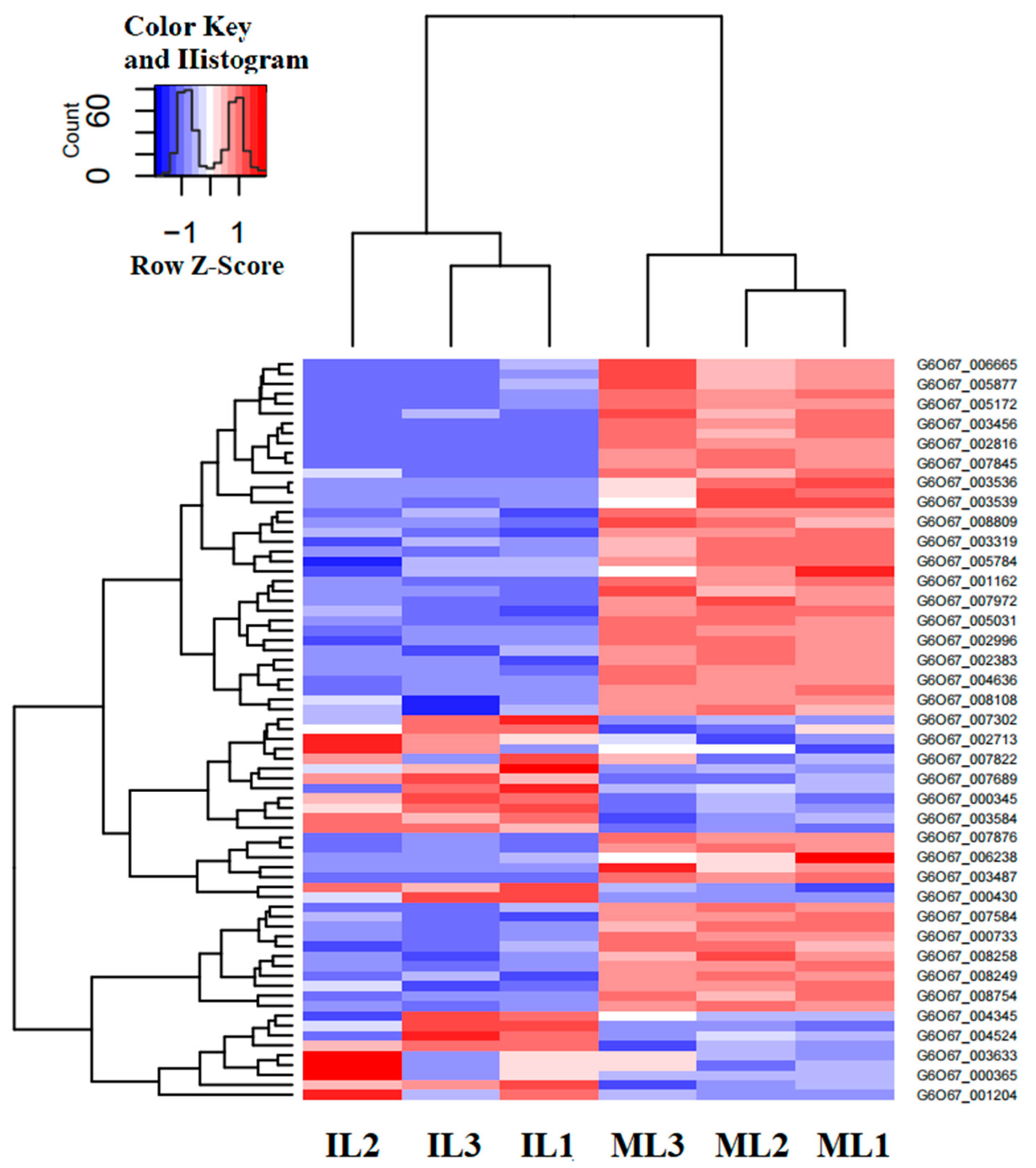
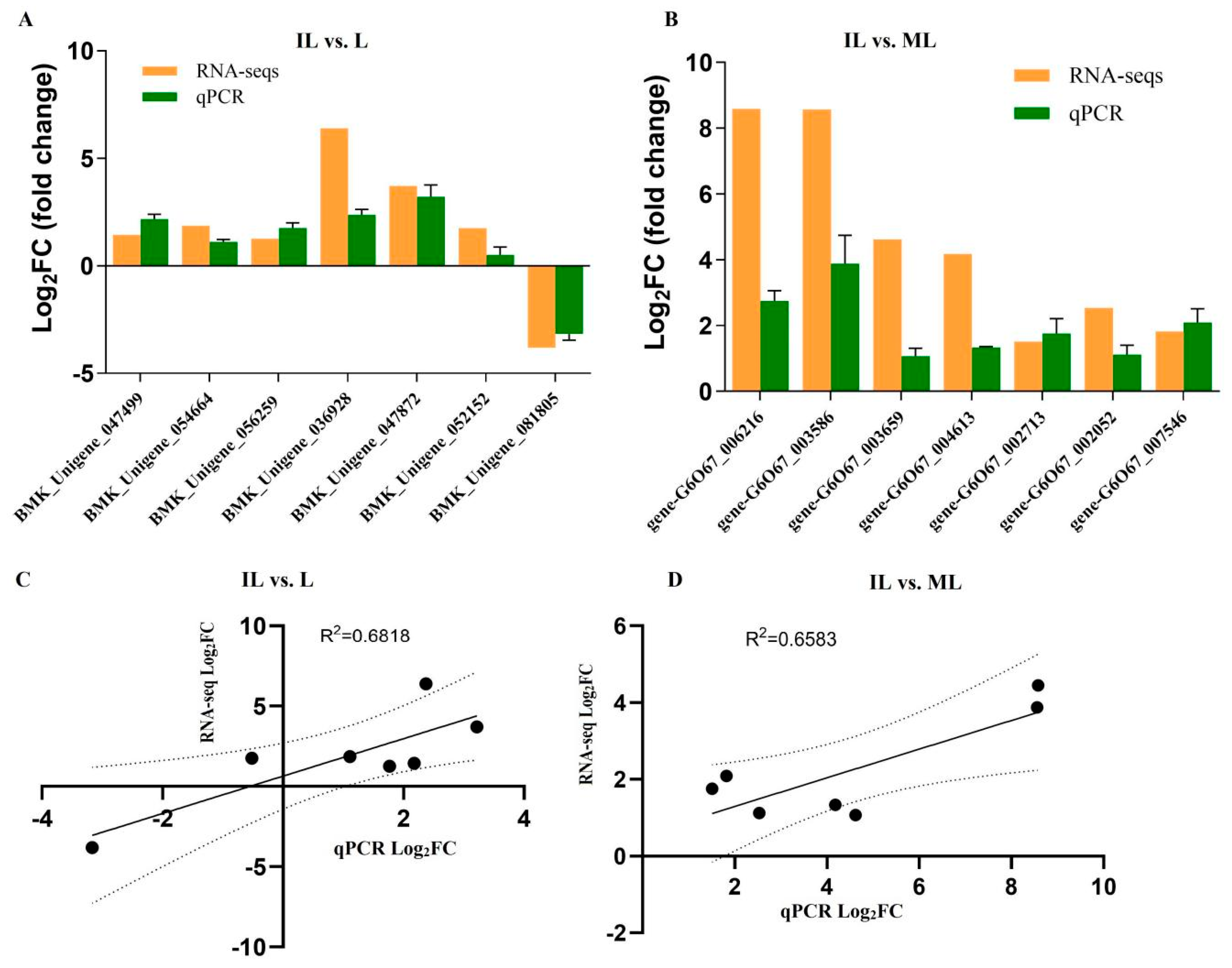
| Gene ID | log2FC | FDR | Pfam_Annotation | Swiss_Prot_Annotation | Organisms |
|---|---|---|---|---|---|
| gene-G6O67_000650 | 2.55 | 2.18 × 10−7 | Cytochrome P450 | Cytochrome P450 monooxygenase | Penicillium roqueforti |
| gene-G6O67_004524 | 2.43 | 0.000694338 | Cytochrome P450 | Benzoate 4-monooxygenase | Aspergillus niger |
| gene-G6O67_001204 | 5.67 | 2.05 × 10−6 | Cytochrome P450 | Cytochrome P450 52-M1 | Starmerella bombicola |
| gene-G6O67_007863 | 2.50 | 2.22 × 10−13 | NADH:flavin oxidoreductase | NADPH dehydrogenase afvA | Aspergillus flavus |
| gene-G6O67_008258 | 1.73 | 8.12 × 10−9 | ESSS subunit of NADH:ubiquinone oxidoreductase (complex I) | -- | Ophiocordyceps sinensis |
| gene-G6O67_000430 | 8.34 | 1.27 × 10−14 | Fungalysin metallopeptidase (M36) | Extracellular metalloproteinase | Aspergillus flavus |
| gene-G6O67_002713 | 1.51 | 1.89 × 10−6 | Fungalysin metallopeptidase (M36) | Extracellular metalloproteinase 5 | Arthrodermaotae |
| gene-G6O67_007546 | 1.82 | 3.44 × 10−13 | Serine aminopeptidase, S33 | Protein bem46 | Schizosaccharomyces pombe |
| gene-G6O67_006297 | 1.61 | 0.002749971 | Serine carboxypeptidase | Carboxypeptidase S1 homolog A | Trichophyton rubrum |
| gene-G6O67_007302 | 1.82 | 4.01 × 10−5 | peptidase_M16 | Putative zinc protease mug138 | Schizosaccharomyces pombe |
| gene-G6O67_002052 | 2.53 | 2.37 × 10−12 | Redoxin | Peroxiredoxin Asp f3 | Neosartorya fumigata |
| gene-G6O67_006384 | 1.83 | 8.15 × 10−7 | CPBP intramembrane metalloprotease | Probable CAAX prenyl protease 2 | Schizosaccharomyces |
| gene-G6O67_005828 | 2.60 | 7.97 × 10−6 | Eukaryotic aspartyl protease | Endothiapepsin | Cryphonectria parasitica |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Peng, T.; Liu, S.; Zhang, D.; Guo, J. Transcriptomic Analysis Insight into the Immune Modulation during the Interaction of Ophiocordyceps sinensis and Hepialus xiaojinensis. Insects 2022, 13, 1119. https://doi.org/10.3390/insects13121119
Tong X, Peng T, Liu S, Zhang D, Guo J. Transcriptomic Analysis Insight into the Immune Modulation during the Interaction of Ophiocordyceps sinensis and Hepialus xiaojinensis. Insects. 2022; 13(12):1119. https://doi.org/10.3390/insects13121119
Chicago/Turabian StyleTong, Xinxin, Ting Peng, Sukun Liu, Daixi Zhang, and Jinlin Guo. 2022. "Transcriptomic Analysis Insight into the Immune Modulation during the Interaction of Ophiocordyceps sinensis and Hepialus xiaojinensis" Insects 13, no. 12: 1119. https://doi.org/10.3390/insects13121119




