Effect of Leaf Trichomes in Different Species of Cucurbitaceae on Attachment Ability of the Melon Ladybird Beetle Chnootriba elaterii
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Plants
2.3. Scanning Electron Microscopy
2.4. Light Microscopy
2.5. Contact Angle Measurements
2.6. Force Measurements
2.7. Statistical Analysis
3. Results
3.1. Adult and Larval Attachment Devices
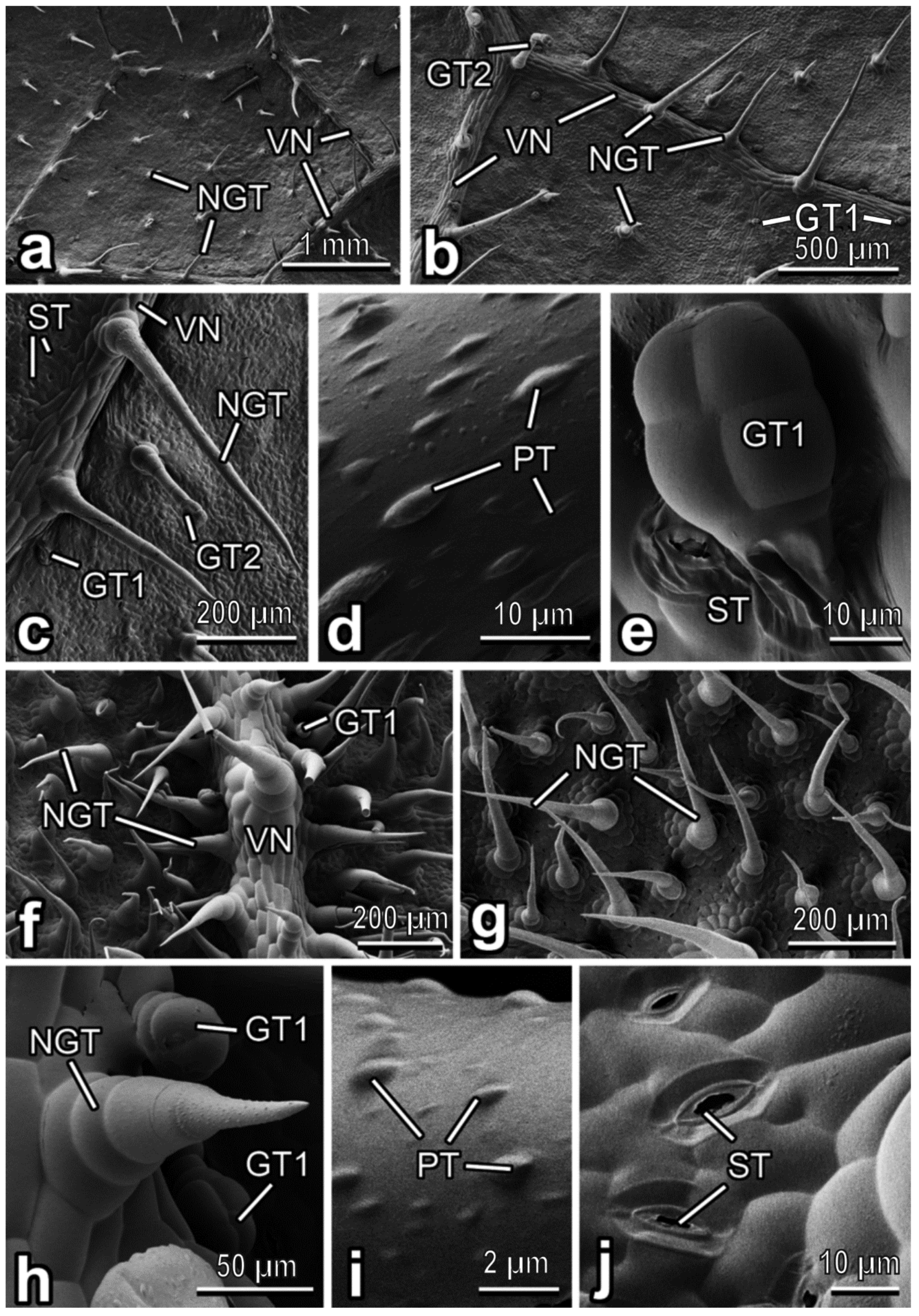
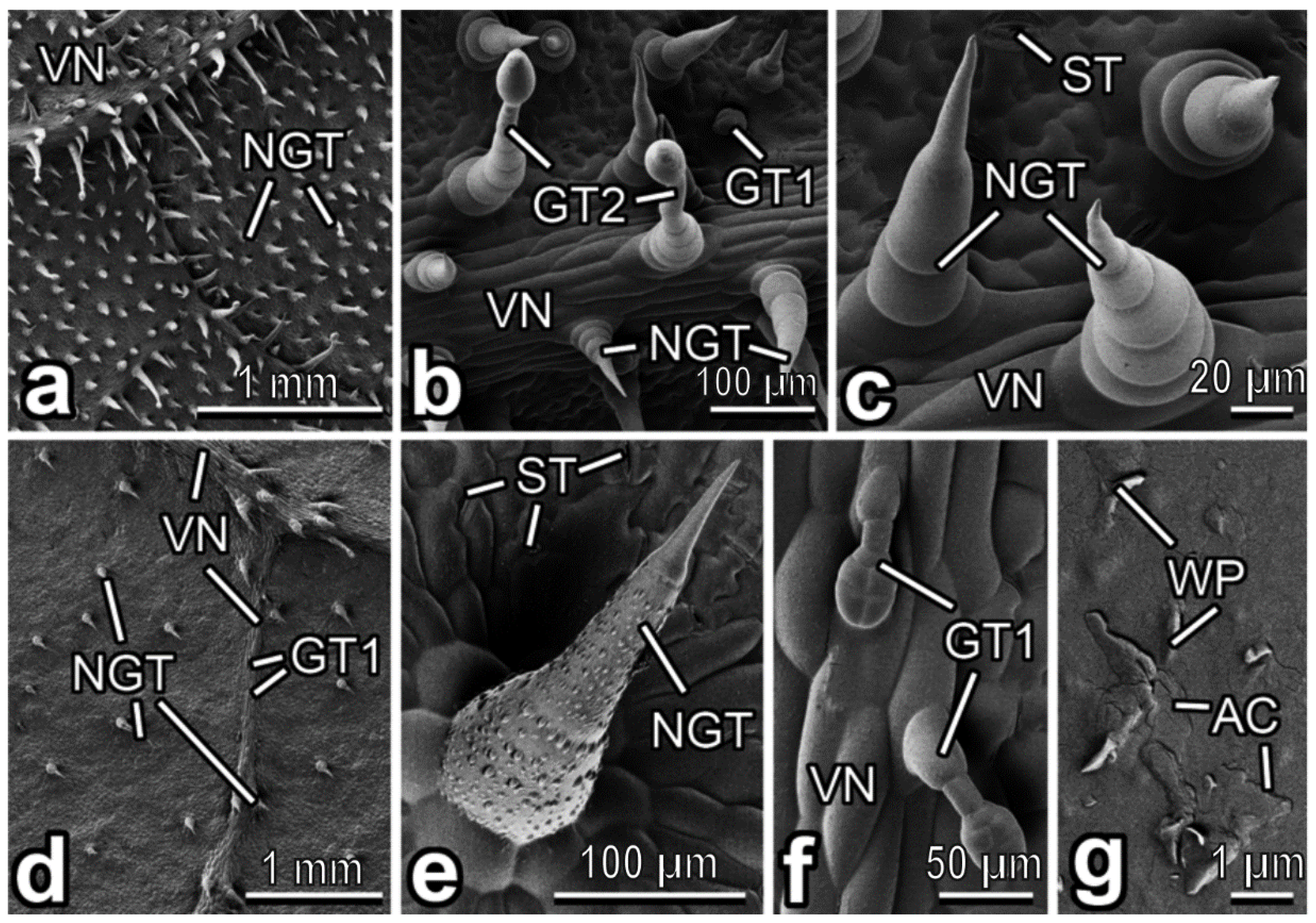
3.2. Characterization of Plant Surfaces
3.3. Adult and Larval Attachment Ability to Different Cucurbitaceae Species
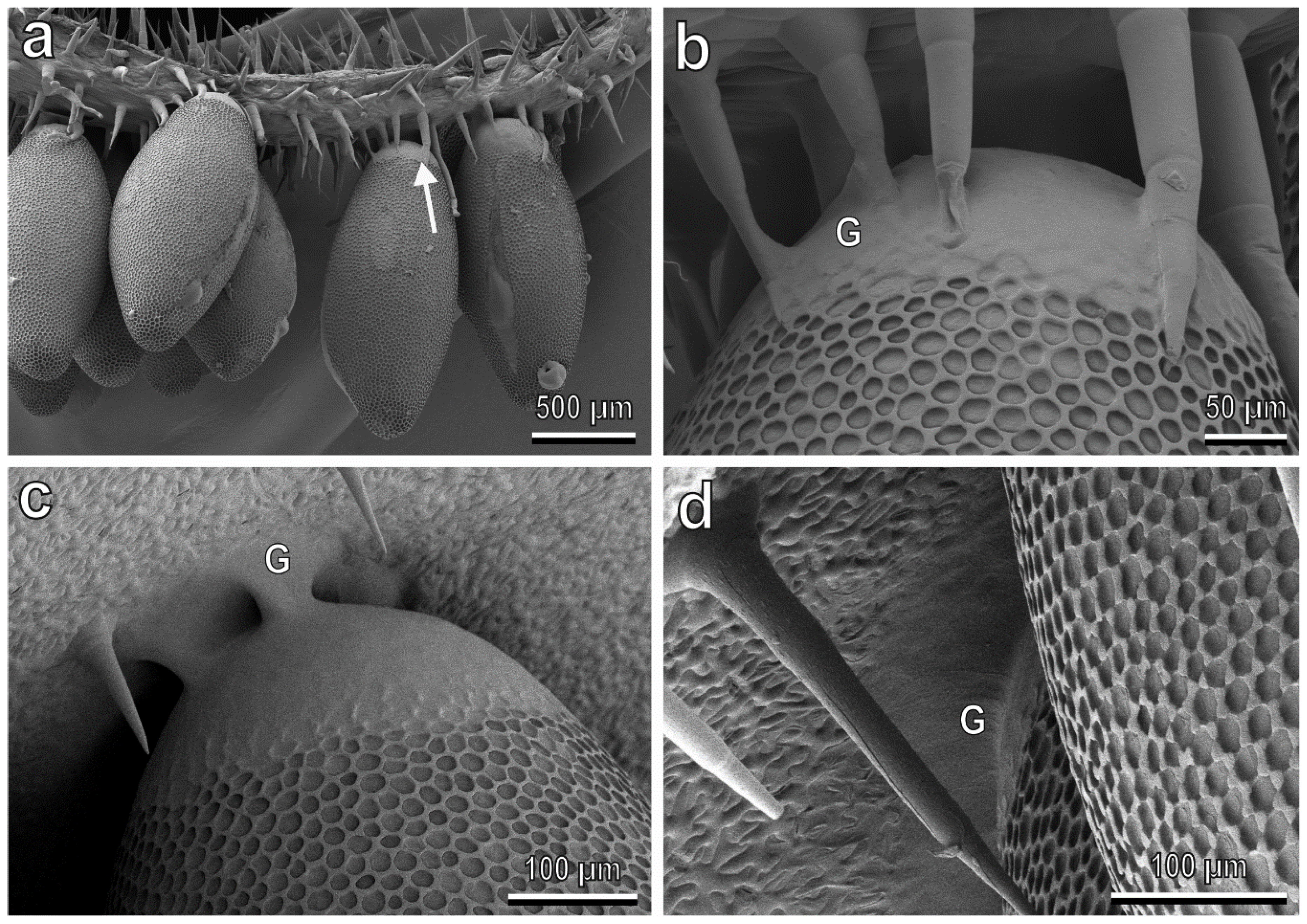
3.4. Egg-plant Surface Interaction
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beutel, R.; Gorb, S. Ultrastructure of attachment specializations of hexapods (Arthropoda): Evolutionary patterns inferred from a revised ordinal phylogeny. J. Zool. Syst. Evol. Res. 2001, 39, 117–207. [Google Scholar] [CrossRef]
- Gorb, E.; Gorb, S.N. Anti-adhesive surfaces in plants and their biomimetic potential. In Materials Design Inspired by Nature: Function through Inner Architecture; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 282–309. [Google Scholar]
- Prüm, B.; Florian Bohn, H.; Seidel, R.; Rubach, S.; Speck, T. Plant surfaces with cuticular folds and their replicas: Influence of microstructuring and surface chemistry on the attachment of a leaf beetle. Acta Biomater. 2013, 9, 6360–6368. [Google Scholar] [CrossRef] [PubMed]
- Bauer, U.; Bohn, H.F.; Federle, W. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proc. R. Soc. B Biol. Sci. 2008, 275, 259–265. [Google Scholar] [CrossRef]
- Prüm, B.; Seidel, R.; Bohn, H. Plant surfaces with cuticular folds are slippery for beetles. J. R. Soc. Interface 2012, 9, 127–135. [Google Scholar] [CrossRef]
- Stork, N.E. Role of waxblooms in preventing attachment to brassicas by the mustard beetle, Phaedon cochleariae. Entomol. Exp. Appl. 1980, 28, 100–107. [Google Scholar] [CrossRef]
- Gorb, E.; Gorb, S.N. Anti-adhesive effects of plant wax coverage on insect attachment. J. Exp. Bot. 2017, 68, 5323–5337. [Google Scholar] [CrossRef]
- Daltorio, K.; Horchler, A.; Gorb, S.; Ritzmann, R.; Quinn, R. A Small Wall-Walking Robot with Compliant, Adhesive Feet. In Proceedings of the 2005 IEEE/RSJ International Conference on Intelligent Robots and Systems, Edmonton, AB, Canada, 2–6 August 2005; pp. 3648–3653. [Google Scholar]
- Oelschlägel, B.; Gorb, S.; Wanke, S.; Neinhuis, C.; Oelschlä, B. Structure and biomechanics of trapping flower trichomes and their role in the pollination biology of Aristolochia plants (Aristolochiaceae). New Phytol. 2009, 184, 988–1002. [Google Scholar] [CrossRef]
- Poppinga, S.; Koch, K.; Bohn, H.; Barthlott, W. Comparative and functional morphology of hierarchically structured anti-adhesive surfaces in carnivorous plants and kettle trap flowers. Funct. Plant Biol. 2010, 37, 952–961. [Google Scholar] [CrossRef]
- Southwood, S. Plant surfaces and insects-an overview. Insects Plant Surf. 1986, 6, 1–22. [Google Scholar]
- Voigt, D.; Gorb, E.; Gorb, S. Plant surface–bug interactions: Dicyphus errans stalking along trichomes. Arthropod. Plant. Interact. 2007, 1, 221–243. [Google Scholar] [CrossRef]
- Levin, D.A. The Role of Trichomes in Plant Defense. Q. Rev. Biol. 1973, 48, 3–15. [Google Scholar] [CrossRef]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Baur, R.; Binder, S.; Benz, G. Nonglandular leaf trichomes as short-term inducible defense of the grey alder, Alnus incana (L.), against the chrysomelid beetle, Agelastica alni L. Oecologia 1991, 87, 219–226. [Google Scholar] [CrossRef]
- Fordyce, J.A.; Agrawal, A.A. The role of plant trichomes and caterpillar group size on growth and defence of the pipevine swallowtail Battus philenor. J. Anim. Ecol. 2001, 70, 997–1005. [Google Scholar] [CrossRef]
- Traw, B.; Dawson, T. Differential induction of trichomes by three herbivores of black mustard. Oecologia 2002, 131, 526–532. [Google Scholar] [CrossRef]
- Johnson, B. The injurious effects of the hooked epidermal hairs of French beans (Phaseolus vulgaris L.) on Aphis craccivora Koch. Bull. Entomol. Res. 1953, 44, 779–788. [Google Scholar] [CrossRef]
- Rebora, M.; Salerno, G.; Piersanti, S.; Gorb, E.; Gorb, S.N. Entrapment of Bradysia paupera (Diptera: Sciaridae) by Phaseolus vulgaris (Fabaceae) plant leaf. Arthropod. Plant. Interact. 2020, 14, 499–509. [Google Scholar] [CrossRef]
- Løe, G.; Toräng, P.; Gaudeul, M.; Ågren, J. Trichome production and spatiotemporal variation in herbivory in the perennial herb Arabidopsis lyrata. Oikos 2007, 116, 134–142. [Google Scholar] [CrossRef]
- Dalin, P.; Ågren, J.; Björkman, C.; Huttunen, P.; Kärkkäinen, K. Leaf trichome formation and plant resistance to herbivory. In Induced Plant Resistance to Herbivory; Springer: Dordrecht, The Netherlands, 2008; pp. 89–105. [Google Scholar]
- Hare, J.; Elle, E. Variable impact of diverse insect herbivores on dimorphic Datura wrightii. Ecology 2002, 83, 2711–2720. [Google Scholar] [CrossRef]
- Andres, M.R.; Connor, E.F. The community-wide and guild-specific effects of pubescence on the folivorous insects of manzanitas Arctostaphylos spp. Ecol. Entomol. 2003, 28, 383–396. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Gorb, E.; Gorb, S. Attachment ability of the polyphagous bug Nezara viridula (Heteroptera: Pentatomidae) to different host plant surfaces. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Haddad, N.; Hicks, W. Host Pubescence and the Behavior and Performance of the Butterfly Papilio Troilus (Lepidoptera: Papilionidae). Environ. Entomol. 2000, 29, 299–303. [Google Scholar] [CrossRef]
- Lill, J.; Marquis, R.; Forkner, R.; Le Corff, J.; Holmberg, N.; Barber, N. Leaf pubescence affects distribution and abundance of generalist slug caterpillars (Lepidoptera: Limacodidae). Environ. Entomol. 2006, 35, 797–806. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Piersanti, S.; Buescher, T.H.; Gorb, E.; Gorb, S.N. Oviposition site selection and attachment ability of Propylea quatuordecimpunctata and Harmonia axyridis from the egg to the adult stage. Physiol. Entomol. 2022, 47, 20–37. [Google Scholar] [CrossRef]
- Liotta, G. Contributo alla conoscenza della biologia dell’ Epilachna chrysomelina F. Sicilia (Col. Coccinellidae). Boll. Inst. Entomol. Agr. Osser. Fitopathol. Palermo 1964, 5, 235–262. [Google Scholar]
- Akandeh, M.; Shishehbor, P. Life history traits of melon ladybeetle, Epilachna chrysomelina (Col.: Coccinellidae), on four host plant species. J. Entomol. Soc. Iran 2011, 31, 17–27. [Google Scholar]
- Al-Digail, S.A.; Assagaf, A.I.; Mahyoub, J.A. Effect of Temperature and Humidity on the Population Abundance of Spotted Oriental Cucumber Beetle Epilachna chrysomelina (F.) (Coccinellidae: Coleoptera) In Al–Qunfudah Western Saudi Arabia. Curr. World Environ. 2012, 7, 7–12. [Google Scholar] [CrossRef]
- Jeffrey, C. A new system of Cucurbitaceae. Bot. Zhurn. 2005, 90, 332–335. [Google Scholar]
- Ali, M.; Al-Hemaid, F. Taxonomic significance of trichomes micromorphology in cucurbits. Saudi J. Biol. Sci. 2011, 18, 87–92. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Piersanti, S.; Saitta, V.; Gorb, E.V.; Gorb, S.N. Coleoptera claws and trichome interlocking. J. Comp. Physiol. A 2022, 208, 1–14. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- Ploem, J.S. Reflection-contrast microscopy as a tool for investigation of the attachment of living cells to a glass surface. In Mononuclear Phagocytes in Immunity, Infection and Pathology; Blackwell: Oxford, UK, 1975; pp. 405–421. [Google Scholar]
- Federle, W.; Riehle, M.; Curtis, A. An integrative study of insect adhesion: Mechanics and wet adhesion of pretarsal pads in ants. Integr. Comp. Biol. 2002, 42, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Heepe, L.; Kovalev, A.; Gorb, S. Direct observation of microcavitation in underwater adhesion of mushroom-shaped adhesive microstructure. Beilstein J. Nanotechnol. 2014, 5, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Gorb, S.; Gorb, E.; Kastner, V. Scale effects on the attachment pads and friction forces in syrphid flies (Diptera, Syrphidae). J. Exp. Biol. 2001, 204, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; W. H. Freeman: New York, NY, USA, 1998. [Google Scholar]
- Kolb, D.; Müller, A. Light, Conventional and Environmental Scanning Electron Microscopy of the Trichomes of Cucurbita pepo subsp. pepo var. styriaca and Histochemistry of Glandular secretory products. Ann. Bot. 2004, 94, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, J.; Gangadhara, M.; Shenoy, K.N. Structure, ontogeny, organographic distribution, and taxonomic significance of trichomes and stomata in the Cucurbitaceae. In Biology and utilization of the Cucurbitaceae; Bates, D.M., Robinson, R.W., Jeffrey, C., Eds.; Cornell University Press Ithaca: New York, NY, USA, 2019; Volume 17, pp. 1–209. [Google Scholar]
- Belcher, D.; Thurston, R. Inhibition of movement of larvae of the convergent lady beetle by leaf trichomes of tobacco. Environ. Entomol. 1982, 11, 91–94. [Google Scholar] [CrossRef]
- Voigt, D.; Gorb, S. An insect trap as habitat: Cohesion-failure mechanism prevents adhesion of Pameridea roridulae bugs to the sticky surface of the plant Roridula gorgonias. J. Exp. Biol. 2008, 211, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Gorb, E.; Gorb, S. Effects of surface topography and chemistry of Rumex obtusifolius leaves on the attachment of the beetle Gastrophysa viridula. Entomol. Exp. Appl. 2009, 130, 222–228. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Piersanti, S.; Gorb, E.; Gorb, S. Mechanical ecology of fruit-insect interaction in the adult Mediterranean fruit fly Ceratitis capitata (Diptera: Tephritidae). Zoology 2020, 139, 125748. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Piersanti, S.; Matsumura, Y.; Gorb, E.V.; Gorb, S.N. Variation of attachment ability of Nezara viridula (Hemiptera: Pentatomidae) during nymphal development and adult aging. J. Insect Physiol. 2020, 127, 104–117. [Google Scholar] [CrossRef]
- Rebora, M.; Salerno, G.; Piersanti, S.; Gorb, E.; Gorb, S. Role of Fruit Epicuticular Waxes in Preventing Bactrocera oleae (Diptera: Tephritidae) Attachment in Different Cultivars of Olea europaea. Insects 2020, 11, 189. [Google Scholar] [CrossRef]
- Gorb, E.; Gorb, S.N. Attachment ability of females and males of the ladybird beetle Cryptolaemus montrouzieri to different artificial surfaces. J. Insect Physiol. 2020, 121, 104011. [Google Scholar] [CrossRef] [PubMed]
- Lüken, D.; Voigt, D.; Gorb, S.; Zebitz, C.P.W. Die Tarsenmorphologie und die Haftfähigkeit des Schwarzen Batatenkäfers Cylas puncticollis (Boheman) auf glatten Oberflächen mit unterschiedlichen physiko-chemischen Eigenschaften. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2009, 17, 109–113. [Google Scholar]
- Gorb, E.V.; Hosoda, N.; Miksch, C.; Gorb, S.N. Slippery pores: Anti-adhesive effect of nanoporous substrates on the beetle attachment system. J. R. Soc. Interface 2010, 7, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, N.; Gorb, S.N. Underwater locomotion in a terrestrial beetle: Combination of surface de-wetting and capillary forces. Proc. R. Soc. B Biol. Sci. 2012, 279, 4236–4242. [Google Scholar] [CrossRef] [PubMed]
- Voigt, D.; Gorb, S. Attachment ability of sawfly larvae to smooth surfaces. Arthropod Struct. Dev. 2012, 41, 145–153. [Google Scholar] [CrossRef]
- Friedemann, K.; Kunert, G.; Gorb, E.; Gorb, S.; Beutel, R. Attachment forces of pea aphids (Acyrthosiphon pisum) on different legume species. Ecol. Entomol. 2015, 40, 732–740. [Google Scholar] [CrossRef]
- Zurek, D.; Gorb, S.; Voigt, D. Locomotion and attachment of leaf beetle larvae Gastrophysa viridula (Coleoptera, Chrysomelidae). Interface Focus 2015, 5, 20140055. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Gorb, E.; Kovalev, A.; Gorb, S. Attachment ability of the southern green stink bug Nezara viridula (Heteroptera: Pentatomidae). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2017, 203, 601–611. [Google Scholar] [CrossRef]
- Abe, J. Silicon deposition in leaf trichomes of Cucurbitaceae horticultural plants: A short report. Am. J. Plant Sci. 2019, 10, 486–490. [Google Scholar] [CrossRef]
- Piersanti, S.; Saitta, V.; Rebora, M.; Salerno, G. Olfaction in phytophagous ladybird beetles: Antennal sensilla and sensitivity to volatiles from host plants in Chnootriba elaterii (Coleoptera Coccinellidae). Arthropod. Plant. Interact. 2022, 16, 617–630. [Google Scholar] [CrossRef]
- Piersanti, S.; Saitta, V.; Rebora, M.; Salerno, G. Plant selection and development in the phytophagous ladybird Chnootriba elaterii. Physiol. Entomol. 2022. submitted. [Google Scholar]
- Yao, F.-L.; Lin, S.; Wang, L.-X.; Mei, W.-J.; Monticelli, L.S.; Zheng, Y.; Desneux, N.; He, Y.-X.; Weng, Q.-Y. Oviposition preference and adult performance of the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae): Effect of leaf microstructure associated with ladybeetle attachment ability. Pest Manag. Sci. 2021, 77, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Peeters, P. Correlations between leaf structural traits and the densities of herbivorous insect guilds. Biol. J. Linn. Soc. 2002, 77, 43–65. [Google Scholar] [CrossRef]
- Lambert, L.; Beach, R.M.; Kilen, T.C.; Todd, J.W. Soybean pubescence and its influence on larval development and oviposition preference of lepidopterous insects. Crop Sci. 1992, 32, 463–466. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitta, V.; Rebora, M.; Piersanti, S.; Gorb, E.; Gorb, S.; Salerno, G. Effect of Leaf Trichomes in Different Species of Cucurbitaceae on Attachment Ability of the Melon Ladybird Beetle Chnootriba elaterii. Insects 2022, 13, 1123. https://doi.org/10.3390/insects13121123
Saitta V, Rebora M, Piersanti S, Gorb E, Gorb S, Salerno G. Effect of Leaf Trichomes in Different Species of Cucurbitaceae on Attachment Ability of the Melon Ladybird Beetle Chnootriba elaterii. Insects. 2022; 13(12):1123. https://doi.org/10.3390/insects13121123
Chicago/Turabian StyleSaitta, Valerio, Manuela Rebora, Silvana Piersanti, Elena Gorb, Stanislav Gorb, and Gianandrea Salerno. 2022. "Effect of Leaf Trichomes in Different Species of Cucurbitaceae on Attachment Ability of the Melon Ladybird Beetle Chnootriba elaterii" Insects 13, no. 12: 1123. https://doi.org/10.3390/insects13121123
APA StyleSaitta, V., Rebora, M., Piersanti, S., Gorb, E., Gorb, S., & Salerno, G. (2022). Effect of Leaf Trichomes in Different Species of Cucurbitaceae on Attachment Ability of the Melon Ladybird Beetle Chnootriba elaterii. Insects, 13(12), 1123. https://doi.org/10.3390/insects13121123









