Diversity but Not Overall Abundance of Moths and Butterflies (Insecta: Lepidoptera) Decreases around Two Arctic Polluters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
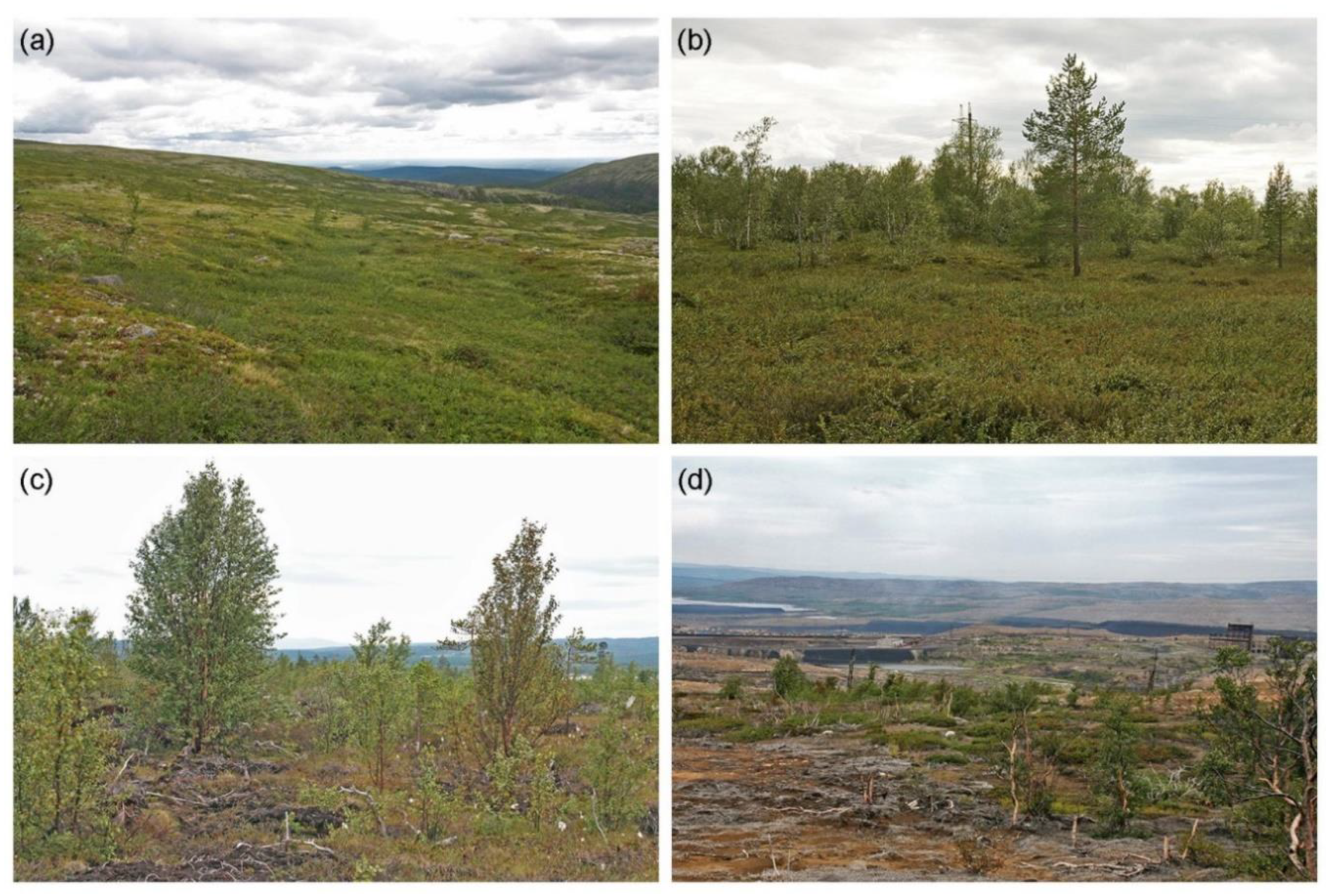
2.1. Study Area and Study Sites
2.2. Collection and Processing
2.3. Data Analysis
3. Results
3.1. Data Overview
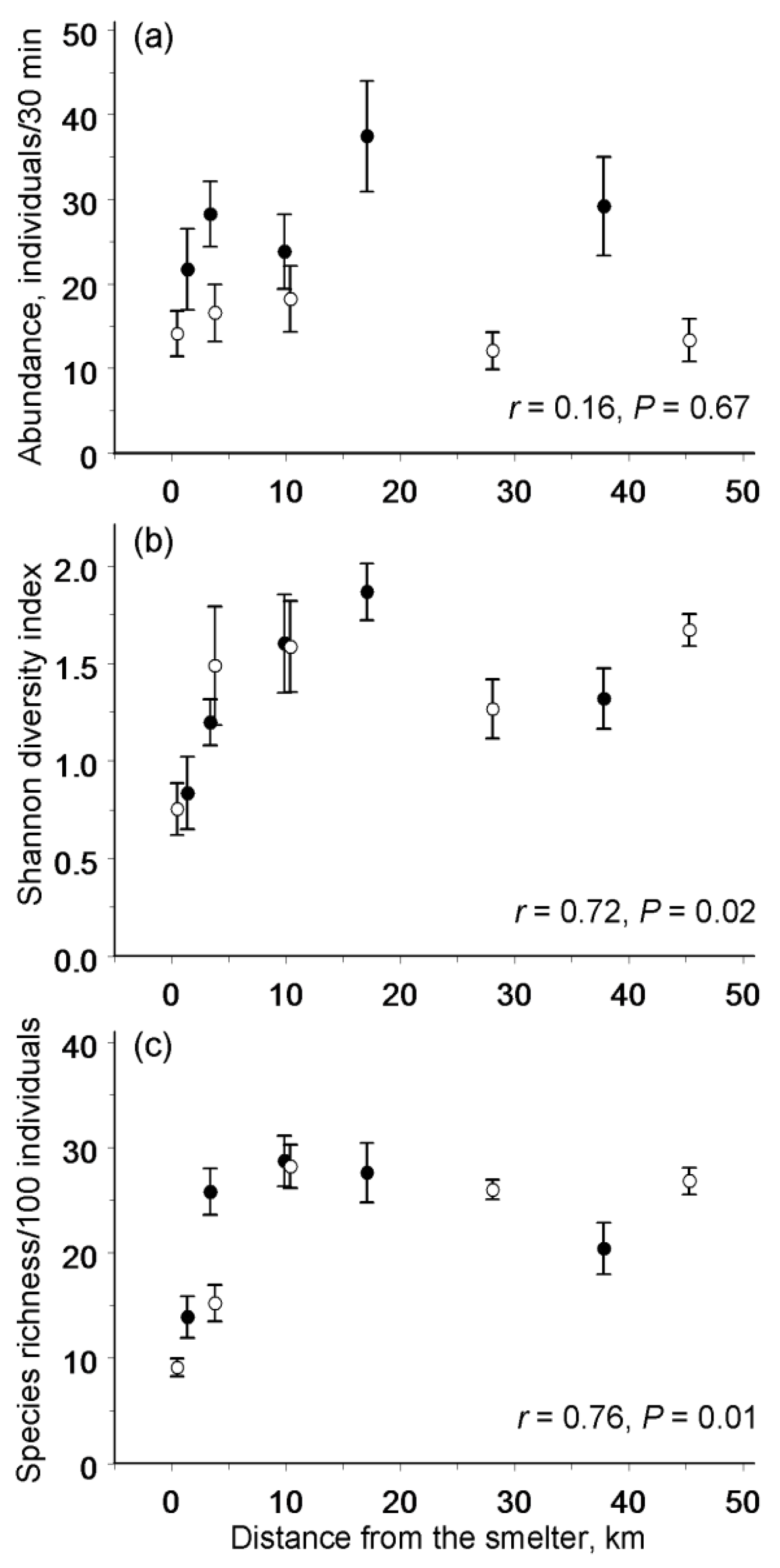
3.2. Spatial Patterns in Abundance and Diversity
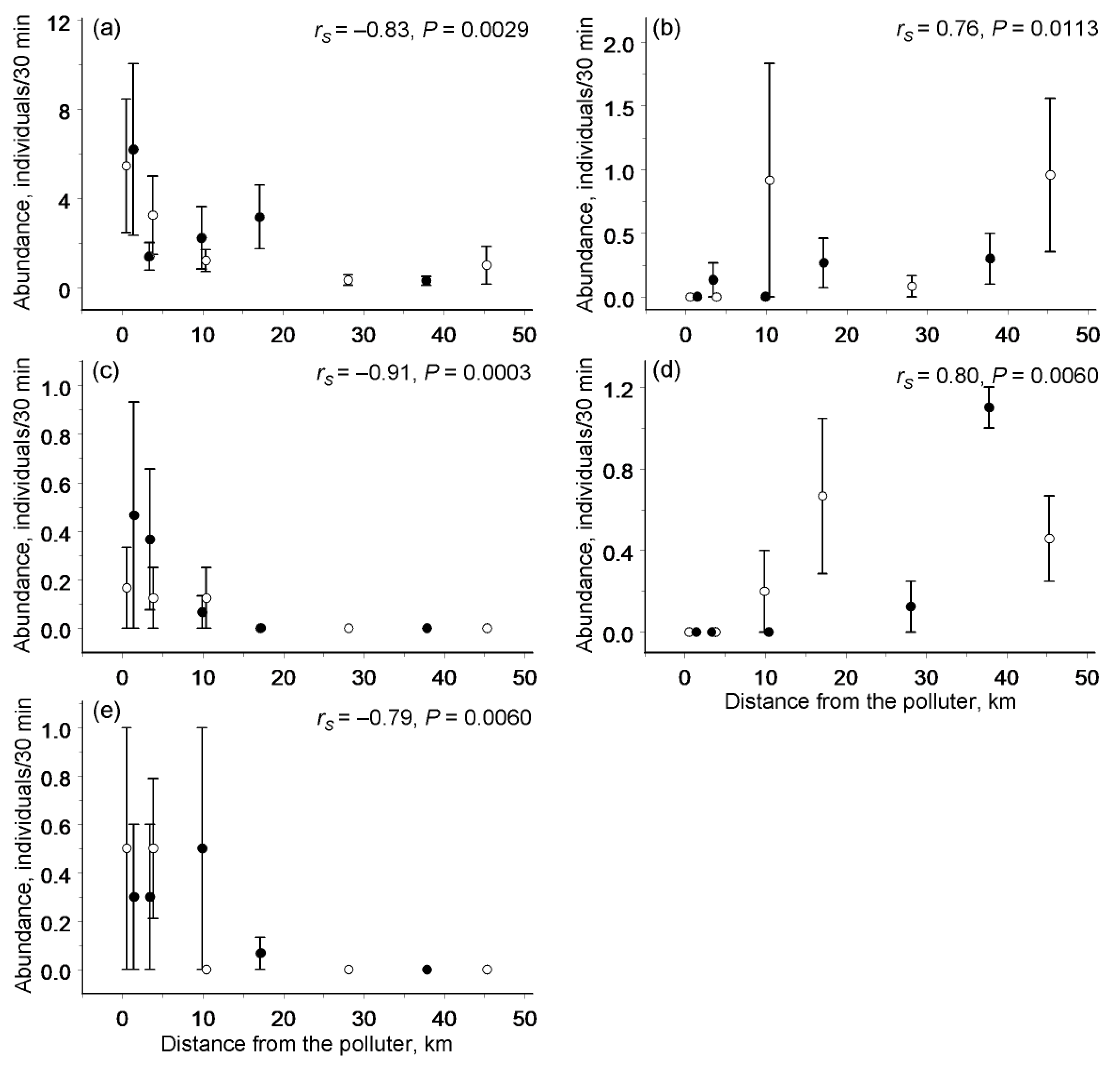
3.3. Abundances of Individual Species
3.4. Research Methodology
4. Discussion
4.1. Spatial Patterns in Abundance and Diversity
4.2. Research Methodology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haywood, J.K. Injury to vegetation and animal life by smelter fumes. J. Am. Chem. Soc. 1907, 29, 998–1009. [Google Scholar] [CrossRef] [Green Version]
- National Research Council of Canada. Effect of Sulphur Dioxide on Vegetation, Prepared for the Associate Committee on Trail Smelter Smoke; Publ. No. 815; National Research Council of Canada: Ottawa, ON, Canada, 1939.
- Gorham, E.; Gordon, A.G. Some effects of smelter pollution northeast of Falconbridge, Ontario. Can. J. Bot. 1960, 38, 307–312. [Google Scholar] [CrossRef]
- Gorham, E.; Gordon, A.G. The influence of smelter fumes upon the chemical composition of lake waters near Sudbury, Ontario, and upon the surrounding vegetation. Can. J. Bot. 1960, 38, 477–487. [Google Scholar] [CrossRef]
- Hutchinson, T.C.; Whitby, L.M. Heavy-metal pollution in the Sudbury mining and smelting region of Canada, I. Soil and vegetation contamination by nickel, copper, and other metals. Environ. Conserv. 1974, 1, 123–132. [Google Scholar] [CrossRef]
- Jordan, M.J. Effects of zinc smelter emissions and fire on a chestnut-oak woodland. Ecology 1975, 56, 78–91. [Google Scholar] [CrossRef]
- Wood, C.W.; Nash, T.N.I. Copper smelter effluent effects on Sonoran desert vegetation. Ecology 1976, 57, 1311–1316. [Google Scholar] [CrossRef]
- Freedman, B.; Hutchinson, T.C. Smelter pollution near Sudbury, Ontario, Canada, and effects on forest litter decomposition. In Effects of Acid Precipitation on Terrestrial Ecosystems; Hutchinson, T.C., Havas, M., Eds.; Plenum Press: New York, NY, USA; London, UK, 1980; pp. 395–434. [Google Scholar]
- Freedman, B.; Hutchinson, T.C. Long-term effects of smelter pollution at Sudbury, Ontario, on forest community composition. Can. J. Bot. 1980, 58, 2123–2140. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L. Industrial barrens: Extreme habitats created by non-ferrous metallurgy. Rev. Environ. Sci. Biotechnol. 2007, 6, 231–259. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L.; Zverev, V. Impacts of Point Polluters on Terrestrial Biota: Comparative Analysis of 18 Contaminated Areas; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Paoletti, E.; Schaub, M.; Matyssek, R.; Wieser, G.; Augustaitis, A.; Bastrup-Birk, A.M.; Bytnerowicz, A.; Günthardt-Goerg, M.S.; Müller-Starck, G.; Serengil, Y. Advances of air pollution science: From forest decline to multiple-stress effects on forest ecosystem services. Environ. Pollut. 2010, 158, 1986–1989. [Google Scholar] [CrossRef]
- De Marco, A.; Sicard, P.; Feng, Z.; Agathokleous, E.; Alonso, R.; Araminiene, V.; Augustatis, A.; Badea, O.; Beasley, J.C.; Branquinho, C.; et al. Strategic roadmap to assess forest vulnerability under air pollution and climate change. Glob. Change Biol. 2022, 28, 5062–5085. [Google Scholar] [CrossRef]
- Vorobeichik, E.L.; Kozlov, M.V. Impact of point polluters on terrestrial ecosystems: Methodology of research, experimental design, and typical errors. Russ. J. Ecol. 2012, 43, 89–96. [Google Scholar] [CrossRef]
- Liebhold, A.M. Air pollution as an experimental probe of insect population dynamics. J. Anim. Ecol. 2019, 88, 662–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairns, J.; Niederlehner, B.R. Developing a field of landscape ecotoxicology. Ecol. Appl. 1996, 6, 790–796. [Google Scholar] [CrossRef] [Green Version]
- Matthews, R.A.; Landis, W.G.; Matthews, G.B. The community conditioning hypothesis and its application to environmental toxicology. Environ. Toxicol. Chem. 1996, 15, 597–603. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Castagneyrol, B.; Zverev, V.; Zvereva, E.L. Recovery of moth and butterfly (Lepidoptera) communities in a polluted region following emission decline. Sci. Total Environ. 2022, 838, 155800. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Responses of terrestrial arthropods to air pollution: A meta-analysis. Environ. Sci. Pollut. Res. 2010, 17, 297–311. [Google Scholar] [CrossRef]
- Conrad, K.F.; Warren, M.S.; Fox, R.; Parsons, M.S.; Woiwod, I.P. Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol. Conserv. 2006, 132, 279–291. [Google Scholar] [CrossRef]
- Warren, M.S.; Maes, D.; van Swaay, C.A.M.; Goffart, P.; van Dyck, H.; Bourn, N.A.D.; Wynhoff, I.; Hoare, D.; Ellis, S. The decline of butterflies in Europe: Problems, significance, and possible solutions. Proc. Natl. Acad. Sci. USA 2021, 118, e2002551117. [Google Scholar] [CrossRef]
- Wepprich, T.; Adrion, J.R.; Ries, L.; Wiedmann, J.; Haddad, N.M. Butterfly abundance declines over 20 years of systematic monitoring in Ohio, USA. PLoS ONE 2019, 14, e0216270. [Google Scholar] [CrossRef] [Green Version]
- Bezuglaya, E.Y. (Ed.) Annual Report: The State of Air Pollution in Cities of Russia in 2007; Main Geophysical Observatory: St. Petersburg, Russia, 2009. (In Russian) [Google Scholar]
- Bezuglaya, E.Y. (Ed.) Annual Report: The State of Air Pollution in Cities of Russia in 2008; Main Geophysical Observatory: St. Petersburg, Russia, 2009. (In Russian) [Google Scholar]
- Bezuglaya, E.Y. (Ed.) Annual Report: The State of Air Pollution in Cities of Russia in 2009; Main Geophysical Observatory: St. Petersburg, Russia, 2010. (In Russian) [Google Scholar]
- Häyrén, E. Pummanginniemi in Petsamo as a nature reserve. Silva Fenn. 1927, 3, 1–26. (In Finnish) [Google Scholar]
- Valle, K.J. Die Lepidopterenfauna des Petsamogebietes under faunistisch-zoogeographischer Berücksichtigung der Fauna ganz N-Fennoscandias. Ann. Zool. Soc. Vanamo 1933, 1, 1–262. [Google Scholar]
- Høgda, K.A.; Tømmervik, H.; Solheim, I.; Marhaud, Ø. Use of Multitemporal Landsat Image Data for Mapping the Effects from Air Pollution in the Kirkenes-Pechenga Area in the Period 1973–1994; NORUT-IT Report, IT2039/1–95; University of Tromsø: Tromsø, Norway, 1995. [Google Scholar]
- Tømmervik, H.; Johansen, B.E.; Pedersen, J.P. Monitoring the effects of air pollution on terrestrial ecosystems in Varanger (Norway) and Nikel-Pechenga (Russia) using remote sensing. Sci. Total Environ. 1995, 161, 753–767. [Google Scholar] [CrossRef]
- Tømmervik, H.; Høgda, K.A.; Solheim, L. Monitoring vegetation changes in Pasvik (Norway) and Pechenga in Kola Peninsula (Russia) using multitemporal Landsat MSS/TM data. Remote Sens. Environ. 2003, 85, 370–388. [Google Scholar] [CrossRef]
- Aarvik, L.; Bengtsson, B.Å.; Elven, H.; Ivinskis, P.; Jürivete, U.; Karsholt, O.; Mutanen, M.; Savenkov, N. Nordic-Baltic checklist of Lepidoptera. Nor. J. Entomol. 2017, 64 (Suppl. S3), 1–236. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- SAS Institute. SAS/STAT. User’s Guide, Version 9.2; SAS Institute: Cary, NC, USA, 2009. [Google Scholar]
- Kenward, M.G.; Roger, J.H. An improved approximation to the precision of fixed effects from restricted maximum likelihood. Computat. Stat. Data Anal. 2009, 53, 2583–2595. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute: Cary, NC, USA, 2006. [Google Scholar]
- Koricheva, J.; Gurevitch, J.; Mengersen, K. (Eds.) Handbook of Meta-Analysis in Ecology and Evolution; Princeton University Press: Princeton, NJ, USA; Oxford, UK, 2013. [Google Scholar]
- Bowler, D.E.; Eichenberg, D.; Conze, K.-J.; Suhling, F.; Baumann, K.; Benken, T.; Bönsel, A.; Bittner, T.; Drews, A.; Günther, A.; et al. Winners and losers over 35 years of dragonfly and damselfly distributional change in Germany. Divers. Distrib. 2021, 27, 1353–1366. [Google Scholar] [CrossRef]
- Wagner, D.L.; Fox, R.; Salcido, D.M.; Dyer, L.A. A window to the world of global insect declines: Moth biodiversity trends are complex and heterogeneous. Proc. Natl. Acad. Sci. USA 2021, 118, e2002549117. [Google Scholar] [CrossRef]
- Jackson, H.M.; Johnson, S.A.; Morandin, L.A.; Richardson, L.L.; Guzman, L.M.; M’Gonigle, L.K. Climate change winners and losers among North American bumblebees. Biol. Lett. 2022, 18, 20210551. [Google Scholar] [CrossRef]
- Odum, E.P. Trends expected in stressed ecosystems. Bioscience 1985, 35, 419–422. [Google Scholar] [CrossRef]
- Rapport, D.J.; Regier, H.A.; Hutchinson, T.C. Ecosystem behavior under stress. Am. Nat. 1985, 125, 617–640. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L. A second life for old data: Global patterns in pollution ecology revealed from published observational studies. Environ. Pollut. 2011, 159, 1067–1075. [Google Scholar] [CrossRef]
- Manzano, S.; Julier, A.C.M. How FAIR are plant sciences in the twenty-first century? The pressing need for reproducibility in plant ecology and evolution. Proc. R. Soc. B 2021, 288, 20202597. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR guiding principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef]
- Pollard, E. A method for assessing changes in the abundance of butterflies. Biol. Conserv. 1977, 12, 115–134. [Google Scholar] [CrossRef]
- Van Swaay, C.; Regan, E.; Ling, M.; Bozhinovska, E.; Fernandez, M.; Marini-Filho, O.J.; Huertas, B.; Phon, C.-K.; Kőorösi, A.; Meerman, J.; et al. Guidelines for Standardised Global Butterfly Monitoring; GEO BON Technical Series 1; Group on Earth Observations Biodiversity Observation Network: Leipzig, Germany, 2015. [Google Scholar]
- Sevilleja, C.G.; van Swaay, C.A.M.; Bourn, N.; Collins, S.; Settele, J.; Warren, M.S.; Wynhoff, I.; Roy, D.B. Butterfly Transect Counts: Manual to Monitor Butterflies; Report VS2019.016; Butterfly Conservation Europe & De Vlinderstichting/Dutch Butterfly Conservation: Wageningen, The Netherlands, 2019. [Google Scholar]
- Baker, M. Is there a reproducibility crisis? Nature 2016, 533, 452–454. [Google Scholar] [CrossRef] [PubMed]


| Year | Nikel | Zapolyarnyy | ||||
|---|---|---|---|---|---|---|
| SO2 | Ni | Cu | SO2 | Ni | Cu | |
| 2003 | 60,600 | 149 | 83 | 63,700 | 180 | 84 |
| 2004 | 56,400 | 154 | 86 | 56,000 | 175 | 82 |
| 2005 | 55,500 | 157 | 88 | 51,400 | 171 | 84 |
| 2006 | 57,600 | . | . | 50,800 | . | . |
| 2007 | 62,700 | . | . | 44,600 | . | . |
| 2008 | 53,800 | . | . | 45,800 | . | . |
| Polluter | Site a | Latitude, N | Longitude, E | Distance from the Polluter, km | Ni in Birch Leaves, μg g−1 | Stand Basal Area, m2 ha−1 | Cover of Field Layer Plants, % |
|---|---|---|---|---|---|---|---|
| Nikel | 1.4 E | 69°25′ | 30°17′ | 1.4 | 273 | 0.7 | 1.1 |
| 3 SW | 69°24′ | 30°11′ | 3.4 | 97 | 1.0 | 7.4 | |
| 10 SW | 69°21′ | 30°03′ | 9.9 | 48 | 4.3 | 19.7 | |
| 17 S | 69°16′ | 30°05′ | 17.1 | 29 | 2.0 | 49.0 | |
| 38 S | 69°04′ | 30°12′ | 37.8 | 33 | 11.7 | 25.3 | |
| Zapolyarnyy | 0.5 NE | 69°25′ | 30°48′ | 0.5 | 366 | 0 | 0 |
| 4 NE | 69°26′ | 30°53′ | 3.8 | 68 | 0.3 | 2.5 | |
| 10 E | 69°27′ | 31°02′ | 10.4 | 36 | 2.0 | 45.2 | |
| 28 E | 69°28′ | 31°30′ | 28.1 | 15 | 0.7 | 33.0 | |
| 45 E | 69°25′ | 31°57′ | 45.3 | 8 | 0 | 39.0 |
| Effect Type | Explanatory Variable | Diversity | Abundance | ||
|---|---|---|---|---|---|
| Test Statistics | p | Test Statistics | p | ||
| Fixed | Polluter (P) | F1, 7.50 = 0.21 | 0.66 | F1, 9.34 = 1.05 | 0.33 |
| Collector (C) | F2, 78.3 = 0.15 | 0.86 | F2, 78.4 = 1.39 | 0.26 | |
| Distance (D) | F1, 7.48 = 5.30 | 0.05 | F1, 9.31 = 1.16 | 0.31 | |
| P × C | F2, 78.1 = 1.23 | 0.380 | F2, 78.3 = 0.06 | 0.95 | |
| C × D | F2, 78.1 = 0.16 | 0.85 | F2, 78.3 = 0.39 | 0.68 | |
| P × D | F1, 7.49 = 0.08 | 0.79 | F1, 9.33 = 2.42 | 0.15 | |
| P × C × D | F2, 78.1 = 1.13 | 0.33 | F2, 78.3 = 0.33 | 0.72 | |
| Random | Site | χ21 = 13.6 | 0.0001 | χ21 = 3.63 | 0.03 |
| Year | χ21 = 59.3 | <0.0001 | χ21 = 92.6 | <0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, M.V.; Zverev, V.; Zvereva, E.L. Diversity but Not Overall Abundance of Moths and Butterflies (Insecta: Lepidoptera) Decreases around Two Arctic Polluters. Insects 2022, 13, 1124. https://doi.org/10.3390/insects13121124
Kozlov MV, Zverev V, Zvereva EL. Diversity but Not Overall Abundance of Moths and Butterflies (Insecta: Lepidoptera) Decreases around Two Arctic Polluters. Insects. 2022; 13(12):1124. https://doi.org/10.3390/insects13121124
Chicago/Turabian StyleKozlov, Mikhail V., Vitali Zverev, and Elena L. Zvereva. 2022. "Diversity but Not Overall Abundance of Moths and Butterflies (Insecta: Lepidoptera) Decreases around Two Arctic Polluters" Insects 13, no. 12: 1124. https://doi.org/10.3390/insects13121124
APA StyleKozlov, M. V., Zverev, V., & Zvereva, E. L. (2022). Diversity but Not Overall Abundance of Moths and Butterflies (Insecta: Lepidoptera) Decreases around Two Arctic Polluters. Insects, 13(12), 1124. https://doi.org/10.3390/insects13121124







