The Influence of Body Weight on Semen Parameters in Apis mellifera Drones
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
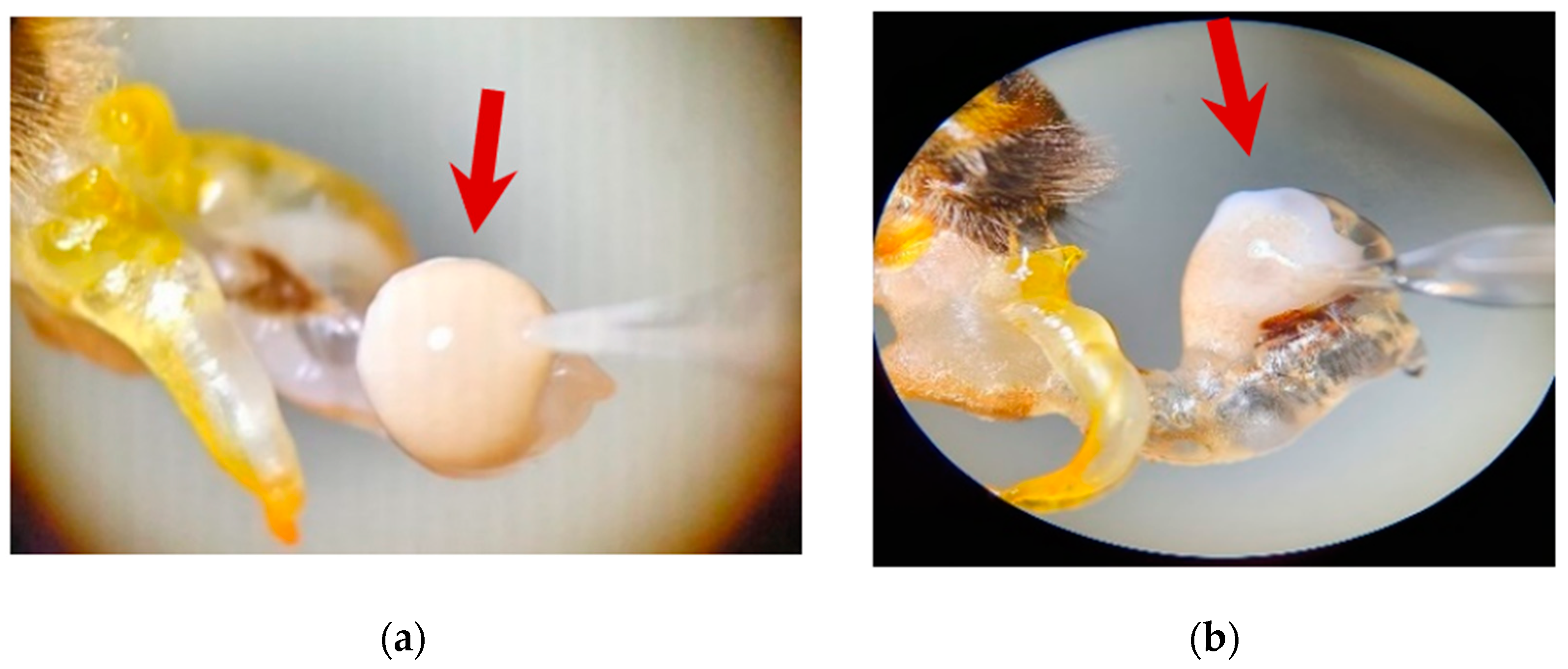
2.1. Collection and Determination of the Collected Semen Volume
2.2. Evaluation of Spermatozoa Motility
2.3. Determination of Semen Concentration
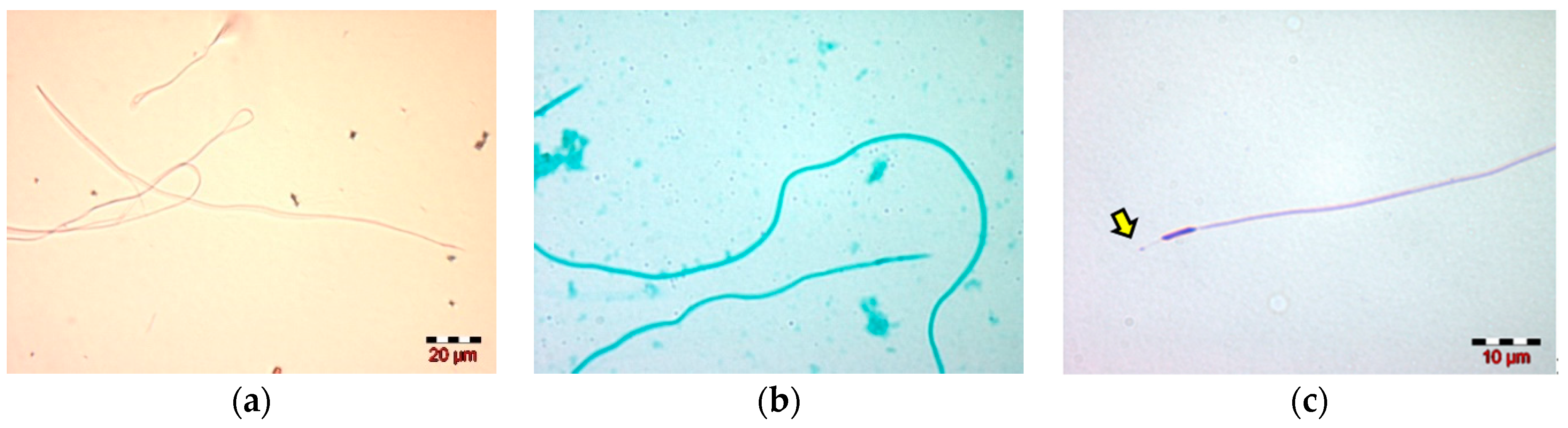
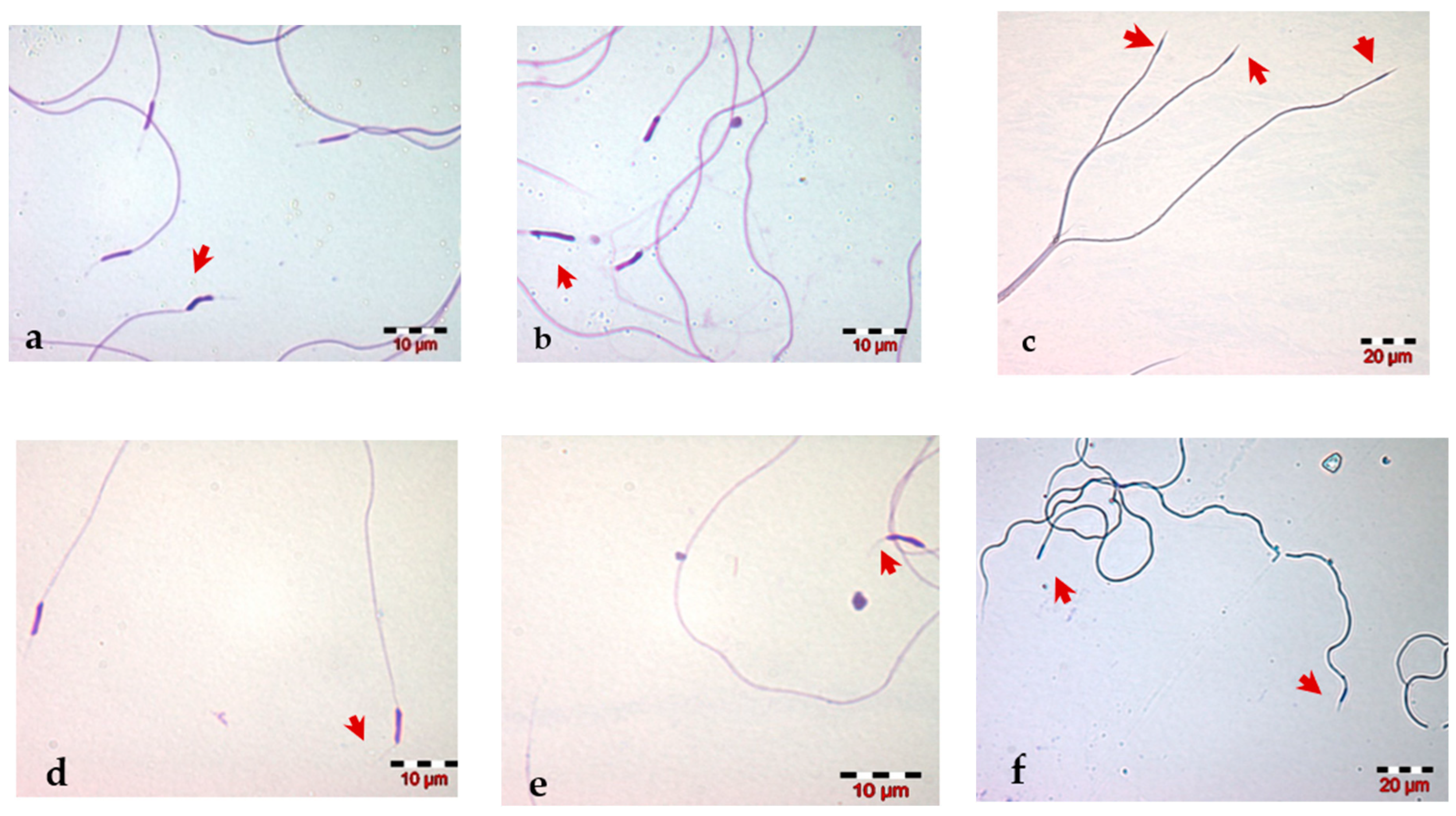
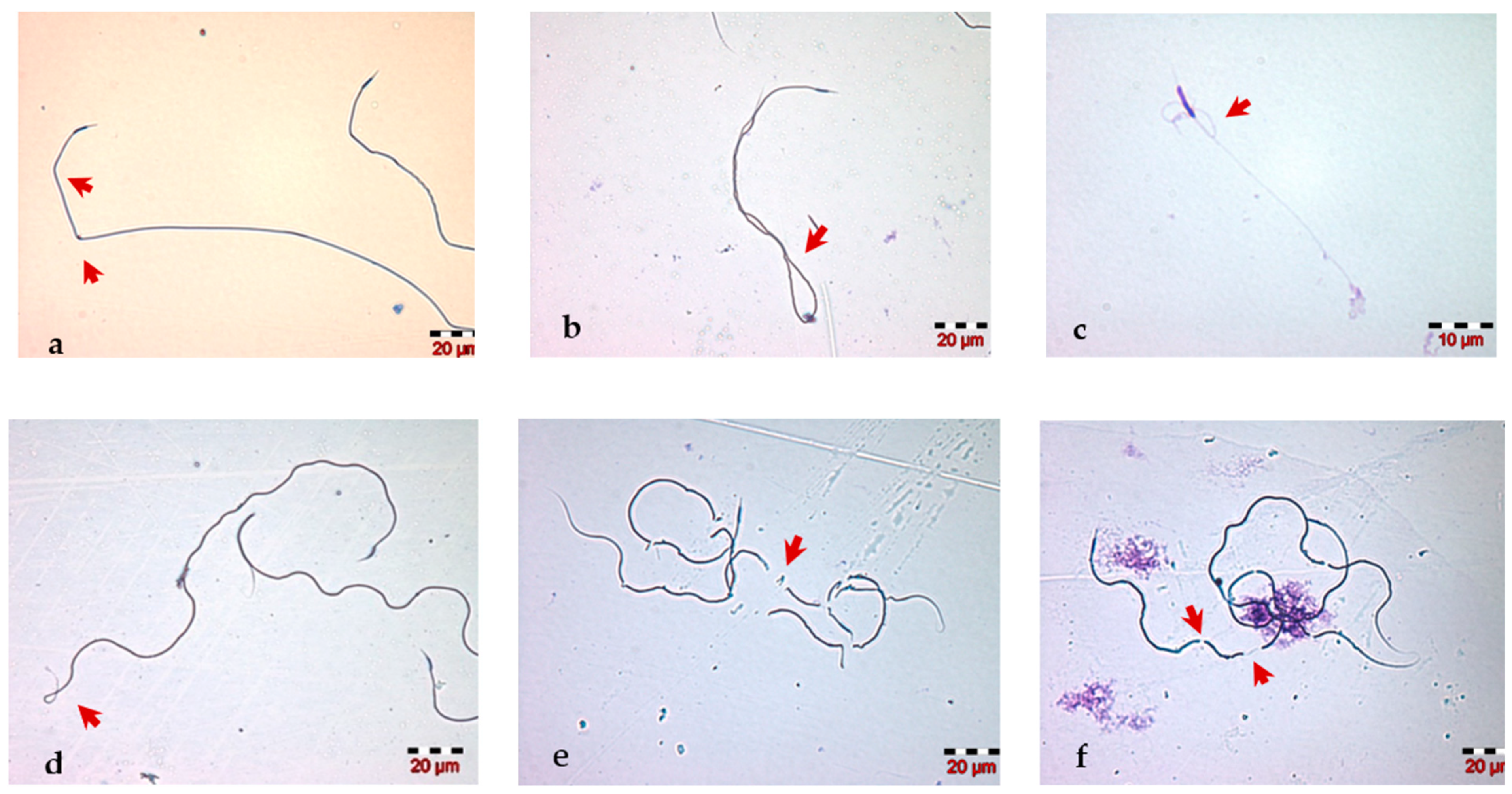
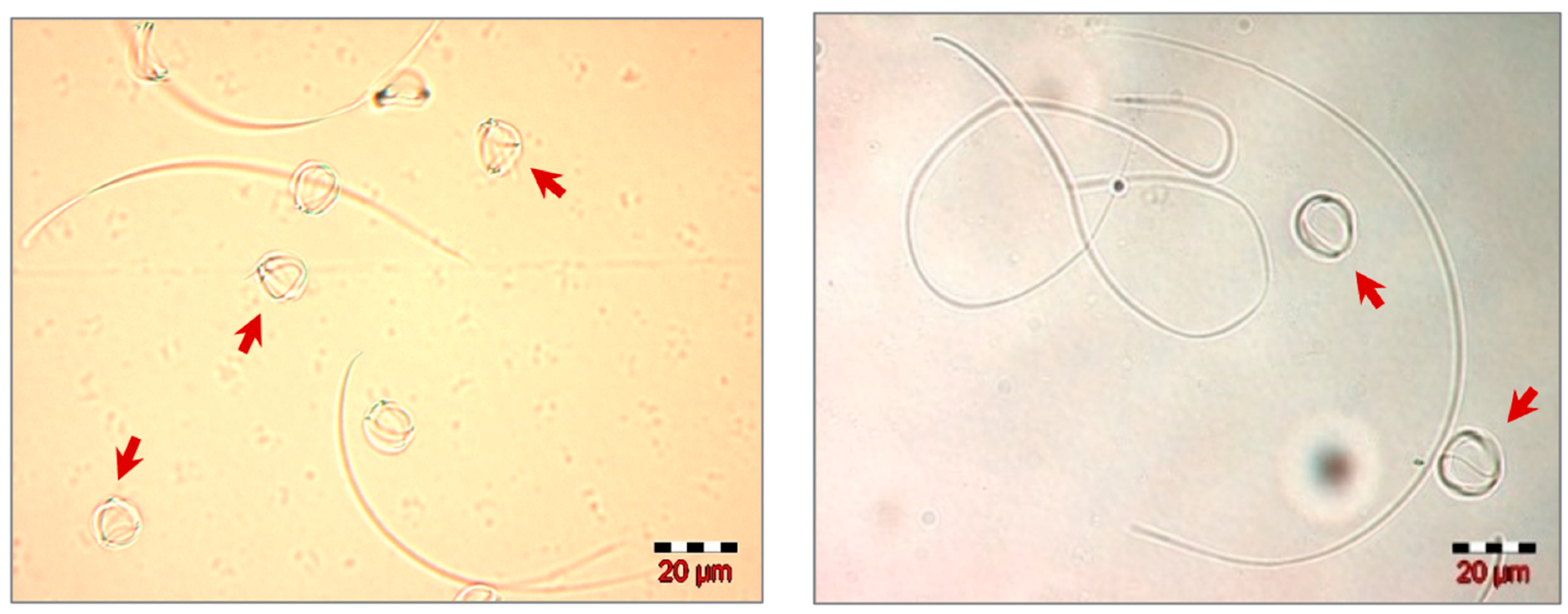
2.4. Morphological Evaluation of Spermatozoa
2.5. Morphometric Analysis of Spermatozoa
2.6. Evaluation of the Functional Integrity of the Spermatozoa Membrane
2.7. Data Collection and Statistical Processing
3. Results
3.1. Semen Volume and Spermatozoa Count
3.2. Spermatozoa Motility, Morphology and Membrane Integrity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aizen, M.A.; Harder, L.D. The Global Stock of Domesticated Honey Bees Is Growing Slower Than Agricultural Demand for Pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Owen, E.L.; Bale, J.S.; Hayward, S.A.L. Establishment risk of the commercially imported bumblebee Bombus terrestris dalmatinus—Can they survive UK winters? Apidologie 2015, 47, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardi, T.; Berta, F.; Fabbri, C.A.; Marchetti, C. Operation Pollinator: A New Way for the Protection and Implementation of Insect Pollinators in Different Agroecosystem—Results of Seven Years of Experiment in Italy. AgroLife Sci. J. 2015, 4, 70–73. [Google Scholar]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Pătruică, S.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazăr, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania. Molecules 2022, 27, 4179. [Google Scholar] [CrossRef]
- Dégi, J.; Herman, V.; Igna, V.; Dégi, D.M.; Hulea, A.; Muselin, F.; Cristina, R.T. Antibacterial Activity of Romanian Propolis against Staphylococcus aureus Isolated from Dogs with Superficial Pyoderma: In Vitro Test. Vet. Sci. 2022, 9, 299. [Google Scholar] [CrossRef]
- Hulea, A.; Obiștioiu, D.; Cocan, I.; Alexa, E.; Negrea, M.; Neacșu, A.-G.; Hulea, C.; Pascu, C.; Costinar, L.; Iancu, I.; et al. Diversity of Monofloral Honey Based on the Antimicrobial and Antioxidant Potential. Antibiotics 2022, 11, 595. [Google Scholar] [CrossRef]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive Markers of Honey Bee Colony Collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef] [Green Version]
- Clermont, A.; Eickermann, M.; Kraus, F.; Georges, C.; Hoffmann, L.; Beyer, M. A survey on some factors potentially affecting losses of managed honey bee colonies in Luxembourg over the winters 2010/2011 and 2011/2012. J. Apic. Res. 2014, 53, 43–56. [Google Scholar] [CrossRef]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, Pests, and Economics: Drivers of Honey Bee Colony Declines and Losses. EcoHealth 2013, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Honey bee colony losses: Why are honey bees disappearing? Sociobiology 2021, 68, 5851. [Google Scholar] [CrossRef]
- Rowland, B.W.; Rushton, S.P.; Shirley, M.D.F.; Brown, M.A.; Budge, G.E. Identifying the climatic drivers of honey bee disease in England and Wales. Sci. Rep. 2021, 11, 21953. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.R.; Artz, D.R.; Biddinger, D.; Bobiwash, K.; Boyle, N.K.; Brittain, C.; Brokaw, J.; Campbell, J.W.; Daniels, J.; Elle, E.; et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc. R. Soc. B: Boil. Sci. 2020, 287, 20200922. [Google Scholar] [CrossRef] [PubMed]
- Pettis, J.S.; Rice, N.; Joselow, K.; Vanengelsdorp, D.; Chaimanee, V. Colony Failure Linked to Low Sperm Viability in Honey Bee (Apis mellifera) Queens and an Exploration of Potential Causative Factors. PLoS ONE 2016, 11, e0147220. [Google Scholar] [CrossRef]
- Yániz, J.L.; Silvestre, M.A.; Santolaria, P. Sperm Quality Assessment in Honey Bee Drones. Biology 2020, 9, 174. [Google Scholar] [CrossRef]
- Schlüns, H.; Schlüns, E.A.; van Praagh, J.; Moritz, R.F. Sperm numbers in drone honeybees (Apis mellifera) depend on body size. Apidologie 2003, 34, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Simone-Finstrom, M.; Tarpy, D.R. Honey Bee Queens Do Not Count Mates to Assess their Mating Success. J. Insect Behav. 2018, 31, 200–209. [Google Scholar] [CrossRef]
- Ellis, J.; Lawrence, J.C.; Koeniger, N.; Koeniger, G. Mating Biology of Honey Bees (Apis mellifera); Wicwas Press: Kalamazoo, MI, USA, 2015. [Google Scholar]
- Brutscher, L.M.; Baer, B.; Niño, E.L. Putative Drone Copulation Factors Regulating Honey Bee (Apis mellifera) Queen Reproduction and Health: A Review. Insects 2019, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Czekońska, K.; Chuda-Mickiewicz, B.; Chorbiński, P. The Influence of Honey Bee (Apis mellifera) Drone Age on Volume of Semen and Viability of Spermatozoa. J. Apic. Sci. 2013, 57, 61–66. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Keller, J.J.; Caren, J.R.; Delaney, D.A. Assessing the Mating ‘Health’ of Commercial Honey Bee Queens. J. Econ. Èntomol. 2012, 105, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Baer, B.; Collins, J.; Maalaps, K.; Boer, S.P.A.D. Sperm use economy of honeybee (Apis mellifera) queens. Ecol. Evol. 2016, 6, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Mattila, H.R.; Seeley, T.D. Genetic Diversity in Honey Bee Colonies Enhances Productivity and Fitness. Science 2007, 317, 362–364. [Google Scholar] [CrossRef] [Green Version]
- Kairo, G.; Provost, B.; Tchamitchian, S.; Ben Abdelkader, F.; Bonnet, M.; Cousin, M.; Sénéchal, J.; Benet, P.; Kretzschmar, A.; Belzunces, L.P.; et al. Drone exposure to the systemic insecticide Fipronil indirectly impairs queen reproductive potential. Sci. Rep. 2016, 6, 31904. [Google Scholar] [CrossRef] [Green Version]
- Boes, K.E. Honeybee colony drone production and maintenance in accordance with environmental factors: An interplay of queen and worker decisions. Insectes Sociaux 2009, 57, 1–9. [Google Scholar] [CrossRef]
- Rowland, C.; McLellan, A. Seasonal changes of drone numbers in a colony of the honeybee, Apis mellifera. Ecol. Model. 1987, 37, 155–166. [Google Scholar] [CrossRef]
- Gençer, H.V.; Kahya, Y. Sperm competition in honey bees (Apis mellifera L.): The role of body size dimorphism in drones. Apidologie 2019, 51, 1–17. [Google Scholar] [CrossRef]
- Couvillon, M.J.; Hughes, W.; Pérez-Sato, J.A.; Martin, S.J.; Roy, G.G.; Ratnieks, F.L. Sexual selection in honey bees: Colony variation and the importance of size in male mating success. Behav. Ecol. 2010, 21, 520–525. [Google Scholar] [CrossRef] [Green Version]
- Slone, J.D.; Stout, T.L.; Huang, Z.Y.; Schneider, S.S. The influence of drone physical condition on the likelihood of receiving vibration signals from worker honey bees, Apis mellifera. Insectes Sociaux 2011, 59, 101–107. [Google Scholar] [CrossRef]
- Zhao, H.; Mashilingi, S.; Liu, Y.; An, J. Factors Influencing the Reproductive Ability of Male Bees: Current Knowledge and Further Directions. Insects 2021, 12, 529. [Google Scholar] [CrossRef] [PubMed]
- Free, J.; Williams, I.H. Factors determining the rearing and rejection of drones by the honeybee colony. Anim. Behav. 1975, 23, 650–675. [Google Scholar] [CrossRef]
- Smith, M.L.; Ostwald, M.M.; Seeley, T.D. Honey bee sociometry: Tracking honey bee colonies and their nest contents from colony founding until death. Insectes Sociaux 2016, 63, 553–563. [Google Scholar] [CrossRef]
- Smith, M.L.; Koenig, P.A.; Peters, J.M. The cues of colony size: How honey bees sense that their colony is large enough to begin to invest in reproduction. J. Exp. Biol. 2017, 220, 1597–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, B.N.; Tarpy, D.R. Reproductive Senescence in Drones of the Honey Bee (Apis mellifera). Insects 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrassnigg, N.; Crailsheim, K. Differences in drone and worker physiology in honeybees (Apis mellifera). Apidologie 2005, 36, 255–277. [Google Scholar] [CrossRef] [Green Version]
- Ben Abdelkader, F.; Kairo, G.; Tchamitchian, S.; Cousin, M.; Senechal, J.; Crauser, D.; Vermandere, J.P.; Alaux, C.; Le Conte, Y.; Belzunces, L.P.; et al. Semen quality of honey bee drones maintained from emergence to sexual maturity under laboratory, semi-field and field conditions. Apidologie 2013, 45, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Cobey, S.W.; Tarpy, D.R.; Woyke, J. Standard methods for instrumental insemination of Apis mellifera queens. J. Apic. Res. 2013, 52, 1–18. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010; Available online: https://apps.who.int/iris/handle/10665/44261 (accessed on 30 September 2022).
- Jeyendran, R.S.; Van Der Ven, H.H.; Perez-Pelaez, M.; Crabo, B.G.; Zaneveld, L.J.D. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. Reproduction 1984, 70, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Gençer, H.V.; Kahya, Y. Are sperm traits of drones (Apis mellifera L.) from laying worker colonies noteworthy? J. Apic. Res. 2011, 50, 130–137. [Google Scholar] [CrossRef]
- Collins, A.M.; Pettis, J.S. Effect of Varroa Infestation on Semen Quality. Am. Bee J. 2001, 141, 29–38. [Google Scholar]
- Rhodes, J.W.; Harden, S.; Spooner-Hart, R.; Anderson, D.L.; Wheen, G. Effects of age, season and genetics on semen and sperm production in Apis mellifera drones. Apidologie 2011, 42, 29–38. [Google Scholar] [CrossRef]
- Woyke, J. Natural and Artificial Insemination of Queen Honeybees. Bee World 1962, 43, 21–25. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, T.; Zhao, F.; Feng, G.; Liu, J.; Yang, G.; Zhang, L.; Zhuang, P. Effects of Cryopreservation on Acrosin Activity and DNA Damage of Russian Sturgeon (Acipenser gueldenstaedtii) Semen. Cryo Lett. 2021, 42, 129–136. [Google Scholar]
- Schlüns, H.; Koeniger, G.; Koeniger, N.; Moritz, R.F.A. Sperm utilization pattern in the honeybee (Apis mellifera). Behav. Ecol. Sociobiol. 2004, 56, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Koeniger, G.; Koeniger, N.; Tingek, S.; Phiancharoen, M. Variance in spermatozoa number among Apis dorsata drones and among Apis mellifera drones. Apidologie 2005, 36, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, A.; Fournier, V.; Giovenazzo, P. Apis mellifera (Hymenoptera: Apidae) drone sperm quality in relation to age, genetic line, and time of breeding. Can. Èntomol. 2015, 147, 702–711. [Google Scholar] [CrossRef]
- Rhodes, J.; Rural Industries, R.; Honeybee, R.; Development Corporation. Development Semen Production in Drone Honeybees; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bieńkowska, M.; Panasiuk, B.; Wegrzynowicz, P.; Gerula, D. The Effect of Different Thermal Conditions on Drone Semen Quality and Number of Spermatozoa Entering the Spermatheca of Queen Bee. J. Apic. Sci. 2011, 55, 2. [Google Scholar]
- Yániz, J.; Palacín, I.; Santolaria, P. Effect of chamber characteristics, incubation, and diluent on motility of honey bee (Apis mellifera) drone sperm. Apidologie 2019, 50, 472–481. [Google Scholar] [CrossRef]
- Wegener, J.; May, T.; Knollmann, U.; Kamp, G.; Müller, K.; Bienefeld, K. In vivo validation of in vitro quality tests for cryopreserved honey bee semen. Cryobiology 2012, 65, 126–131. [Google Scholar] [CrossRef]
- Peng, C.Y.-S.; Yin, C.; Yin, L.R.S. Ultrastructure of honey bee, Apis mellifera, sperm with special emphasis on the acrosomal complex following high-pressure freezing fixation. Physiol. Èntomol. 1993, 18, 93–101. [Google Scholar] [CrossRef]
- Gontarz, A.; Banaszewska, D.; Gryzinska, M.; Andraszek, K. Differences in drone sperm morphometry and activity at the beginning and end of the season. Turk. J. Vet. Anim. Sci. 2016, 40, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Tarliyah, L.; Boedino, A.; Walujo, D. Motility of Honeybee Apis mellifera L. (Hymenoptera: Apidae) Spermatozoa in Various Storage Temperature in Dilution Media Containing Different Glucose Levels. Media Vet. 1999, 6, 15–20. [Google Scholar]
- Gomendio, M.; Roldan, E.R. Sperm competition influences sperm size in mammals. Proc. R. Soc. B: Boil. Sci. 1991, 243, 181–185. [Google Scholar] [CrossRef]
- Zawadzka, J.; Lukaszewicz, E.T. NR 591 Sperm Morphometry of Six Polish Duck. Zesz. Nauk. UP Wroc. Biol. Hod. Zwierz. 2012, 67, 41–48. [Google Scholar]
- Tourmente, M.; Gomendio, M.; Roldán, E.R.S. Sperm competition and the evolution of sperm design in mammals. BMC Evol. Biol. 2011, 11, 12. [Google Scholar] [CrossRef]
- Chenoweth, P.J.; Lorton, S.P. Animal Andrology: Theories and Applications; CABI: Wallingford, UK, 2014. [Google Scholar] [CrossRef] [Green Version]
- Lodesani, M.; Balduzzi, D.; Galli, A. Functional characterisation of semen in honeybee queen (A.m.ligustica S.) spermatheca and efficiency of the diluted semen technique in instrumental insemination. Ital. J. Anim. Sci. 2004, 3, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Kondracki, S.; Banaszewska, D.; Mielnicka, C. The effect of age on the morphometric sperm traits of domestic pigs (Sus scrofa domestica). Cell. Mol. Biol. Lett. 2005, 10, 3–13. [Google Scholar]
- Andraszek, K.; Walczak-Jedrzejowska, R. The Role of Staining Techniques in Seminological Analysis of Mamalian Semen. Folia Pomer Univ. Stetin. Agric. Aliment. Pisc. Zootech. 2015, 320, 5–20. [Google Scholar]
- Collins, A.; Donoghue, A. Viability assessment of honey bee, Apis mellifera, sperm using dual fluorescent staining. Theriogenology 1999, 51, 1513–1523. [Google Scholar] [CrossRef]
- Nur, Z.; Seven-Cakmak, S.; Ustuner, B.; Cakmak, I.; Erturk, M.; Abramson, C.I.; Sağirkaya, H.; Soylu, M.K. The use of the hypo-osmotic swelling test, water test, and supravital staining in the evaluation of drone sperm. Apidologie 2011, 43, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Fabbrocini, A.; D’Adamo, R.; Del Prete, F.; Langellotti, A.L.; Barone, C.M.; Rinna, F.; Sessa, R.; Silvestri, F.; Villani, G.; Vitiello, V.; et al. Motility of cryopreserved spermatozoa for the ecotoxicological evaluation of aquatic environments. Chem. Ecol. 2013, 29, 660–667. [Google Scholar] [CrossRef] [Green Version]
- Alçay, S.; Çakmak, S.; Çakmak, I.; Mülkpinar, E.; Toker, M.B.; Üstüner, B.; Şen, H.; Nur, Z. Arı Spermasının Protein Eklenmiş TL-Hepes Bazlı Sulandırıcı İle Dondurulması. KAFKAS Univ. Vet. Fak. Derg. 2019, 25, 4. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Waberski, D.; Günzel-Apel, A.R.; Töpfer-Petersen, E. Determinants of sperm quality and fertility in domestic species. Reproduction 2007, 134, 3–17. [Google Scholar] [CrossRef] [PubMed]
| Sperm Parameters | Group | n | Mean | SD | Values | p Values | |
|---|---|---|---|---|---|---|---|
| Min | Max | ||||||
| Semen volume (μL) | G1 1 | 30 | 0.70 a | 0.12 | 0.50 | 0.90 | 0.991 |
| G2 2 | 30 | 0.70 a | 0.10 | 0.50 | 0.85 | ||
| Semen concentration (nr. spz/μL) | G1 | 30 | 8.38 × 106 a | 4.09 × 106 | 3.8 × 106 | 14.6 × 106 | 0.163 |
| G2 | 30 | 9.16 × 106 a | 4.54 × 106 | 3.20 × 106 | 14.9 × 106 | ||
| Total number spermatozoa/ejaculate | G1 | 30 | 5.73 × 106 a | 2.72 × 106 | 2.28 × 106 | 11.52 × 106 | 0.488 |
| G2 | 30 | 6.25 × 106 a | 3.00 × 106 | 1.60 × 106 | 9.5 × 106 | ||
| Total motility (%) | G1 | 30 | 88.00 a | 8.13 | 70.00 | 98.00 | 0.481 |
| G2 | 30 | 91.00 a | 8.30 | 70.00 | 98.00 | ||
| Morphology (% of normal spermatozoa) | G1 | 30 | 85.30 a | 8.38 | 69.00 | 95.00 | 0.001 |
| G2 | 30 | 75.70 b | 11.84 | 60.00 | 93.00 | ||
| Membrane integrity (%) | G1 | 30 | 89.07 a | 8.16 | 70.00 | 99.00 | 0.787 |
| G2 | 30 | 89.60 a | 7.00 | 74.00 | 98.00 | ||
| Sperm Parameters | Group | n | Mean | Std. Deviation | Values | p Values | |
|---|---|---|---|---|---|---|---|
| Min | Max | ||||||
| Acrosome length (μm) | G1 1 | 50 | 4.02 a | 0.39 | 3.16 | 4.74 | 0.111 |
| G2 2 | 50 | 4.12 a | 0.21 | 3.37 | 4.71 | ||
| Nucleus length (μm) | G1 | 50 | 4.72 a | 0.52 | 3.79 | 5.68 | 0.104 |
| G2 | 50 | 4.84 a | 0.17 | 4.44 | 5.31 | ||
| Head length (μm) | G1 | 50 | 8.74 a | 0.64 | 7.40 | 10.42 | 0.023 |
| G2 | 50 | 8.97 b | 0.27 | 8.00 | 9.75 | ||
| Tail length (μm) | G1 | 50 | 221.36 a | 25.62 | 147.73 | 250.90 | 0.068 |
| G2 | 50 | 229.13 a | 15.15 | 148.87 | 254.31 | ||
| Spermatozoa length (μm) | G1 | 50 | 230.10 a | 25.68 | 156.71 | 260.21 | 0.061 |
| G2 | 50 | 238.10 a | 15.17 | 158.19 | 263.54 | ||
| Morphometric Traits | Nucleus Length | Head Length | Tail Length | Sperm Length |
|---|---|---|---|---|
| Acrosome length | −0.020 (p > 0.05) | 0.591 (p < 0.001) | −0.028 (p > 0.05) | −0.013 (p > 0.05) |
| Nucleus length | - | 0.795 (p < 0.001) | 0.131 (p > 0.05) | 0.150 (p > 0.05) |
| Head length | - | - | 0.089 (p > 0.05) | 0.113 (p > 0.05) |
| Tail length | - | - | - | 1.00 (p < 0.001) |
| Morphometric Traits | Nucleus Length | Head Length | Tail Length | Sperm Length |
|---|---|---|---|---|
| Acrosome length | −0.012 (p > 0.05) | 0.773 (p < 0.001) | −0.122 (p > 0.05) | −0.108 (p > 0.05) |
| Nucleus length | - | 0.625 (p < 0.001) | 0.250 (p > 0.05) | 0.260 (p > 0.05) |
| Head length | - | - | 0.063 (p > 0.05) | 0.081 (p > 0.05) |
| Tail length | - | - | - | 1.00 (p < 0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bratu, I.C.; Igna, V.; Simiz, E.; Dunea, I.B.; Pătruică, S. The Influence of Body Weight on Semen Parameters in Apis mellifera Drones. Insects 2022, 13, 1141. https://doi.org/10.3390/insects13121141
Bratu IC, Igna V, Simiz E, Dunea IB, Pătruică S. The Influence of Body Weight on Semen Parameters in Apis mellifera Drones. Insects. 2022; 13(12):1141. https://doi.org/10.3390/insects13121141
Chicago/Turabian StyleBratu, Ioan Cristian, Violeta Igna, Eliza Simiz, Ioan Bănățean Dunea, and Silvia Pătruică. 2022. "The Influence of Body Weight on Semen Parameters in Apis mellifera Drones" Insects 13, no. 12: 1141. https://doi.org/10.3390/insects13121141
APA StyleBratu, I. C., Igna, V., Simiz, E., Dunea, I. B., & Pătruică, S. (2022). The Influence of Body Weight on Semen Parameters in Apis mellifera Drones. Insects, 13(12), 1141. https://doi.org/10.3390/insects13121141






