Demographic Evaluation of the Control Potential of Orius minutus (Hemiptera: Anthocoridae) Preying on Dendrothrips minowai Priesner (Thysanoptera: Thripidae) at Different Temperatures
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing of Insects
2.2. Life Table and Predation Rate Studies at Different Temperatures
2.2.1. Life Table and Predation Rate Study of O. minutus
2.2.2. Life Table Study of D. minowai
2.3. Data Analysis
2.3.1. Two-Sex Life Table Analysis
2.3.2. Predation Rate Analysis
2.3.3. Population and Predation Projections
3. Results
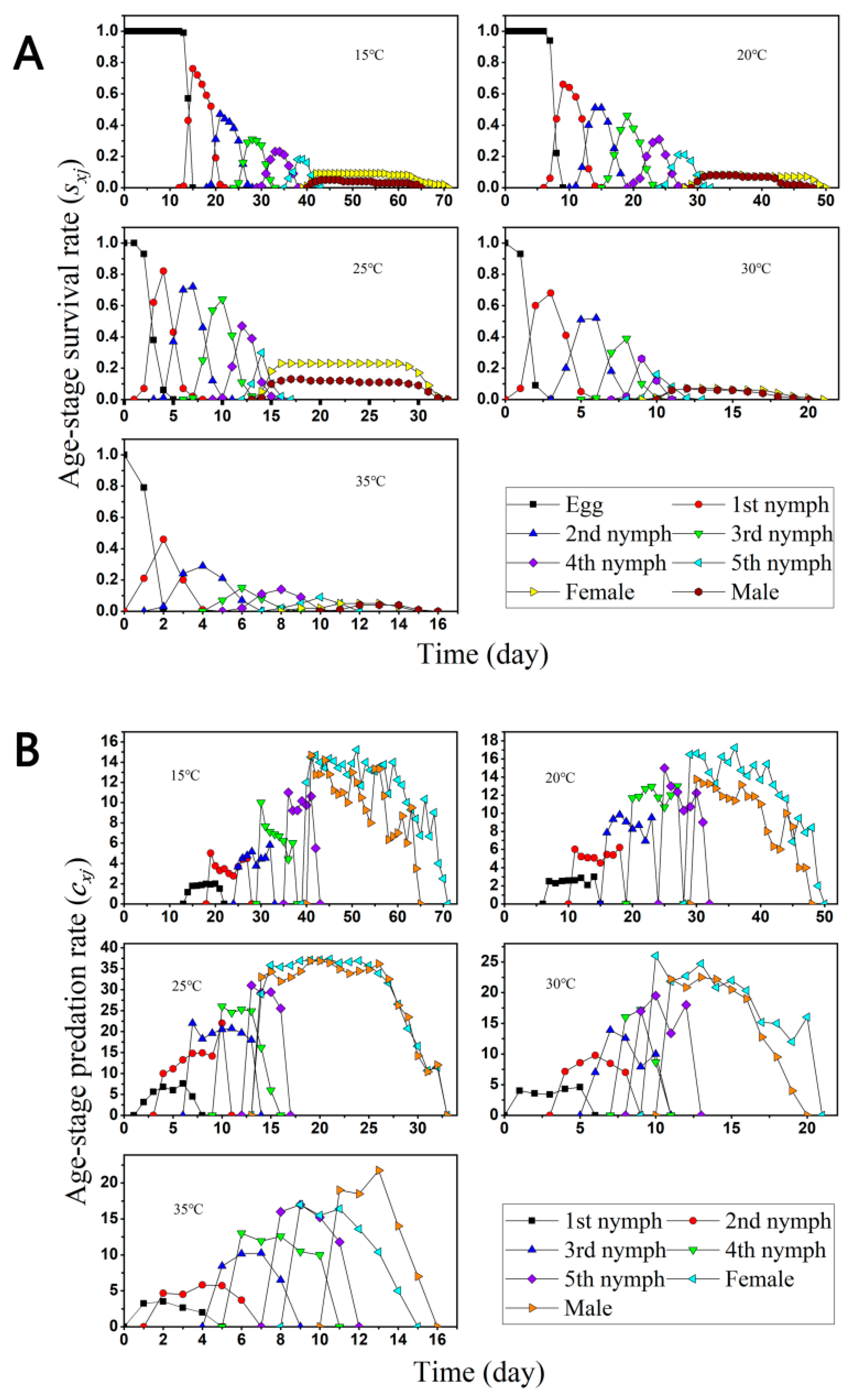
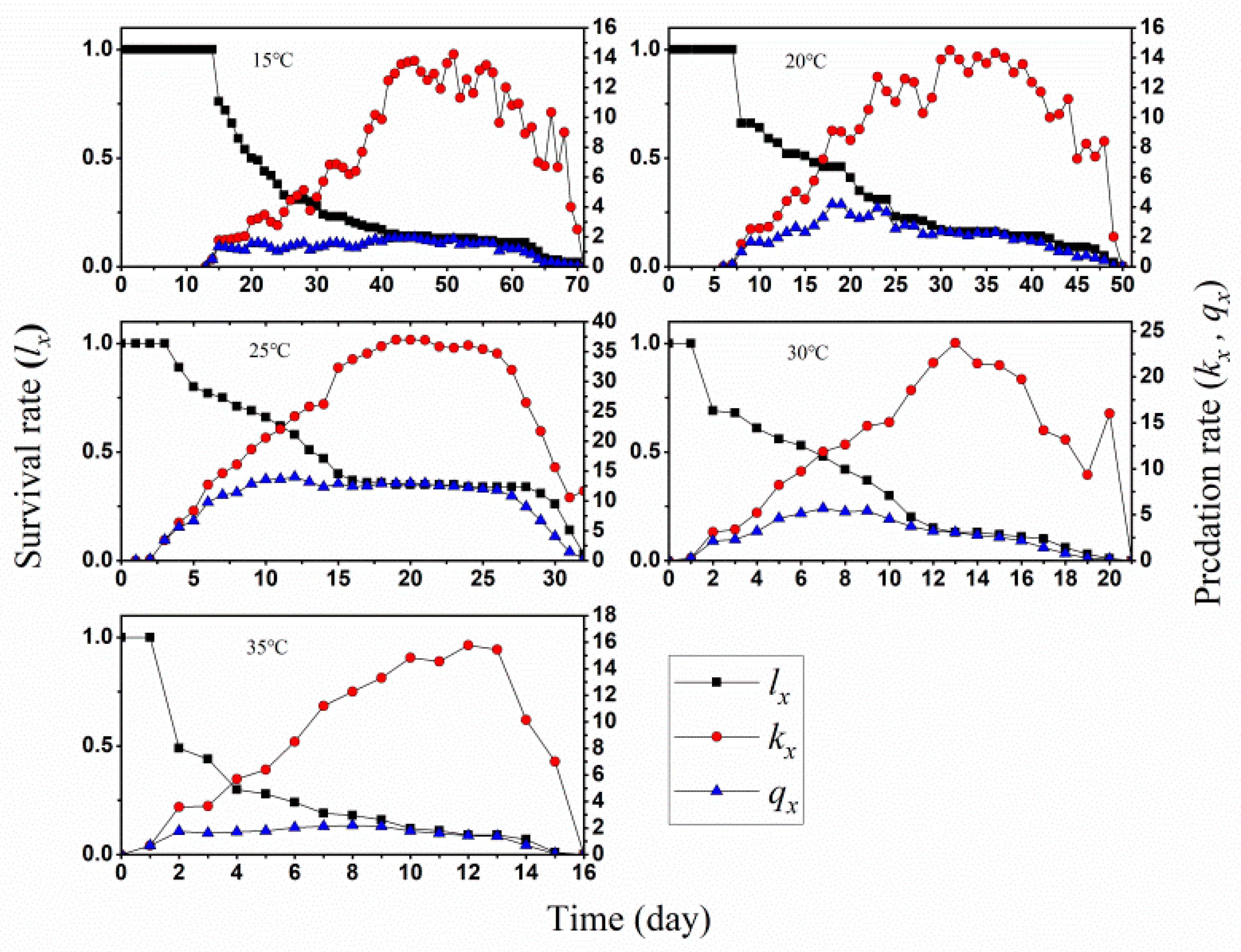
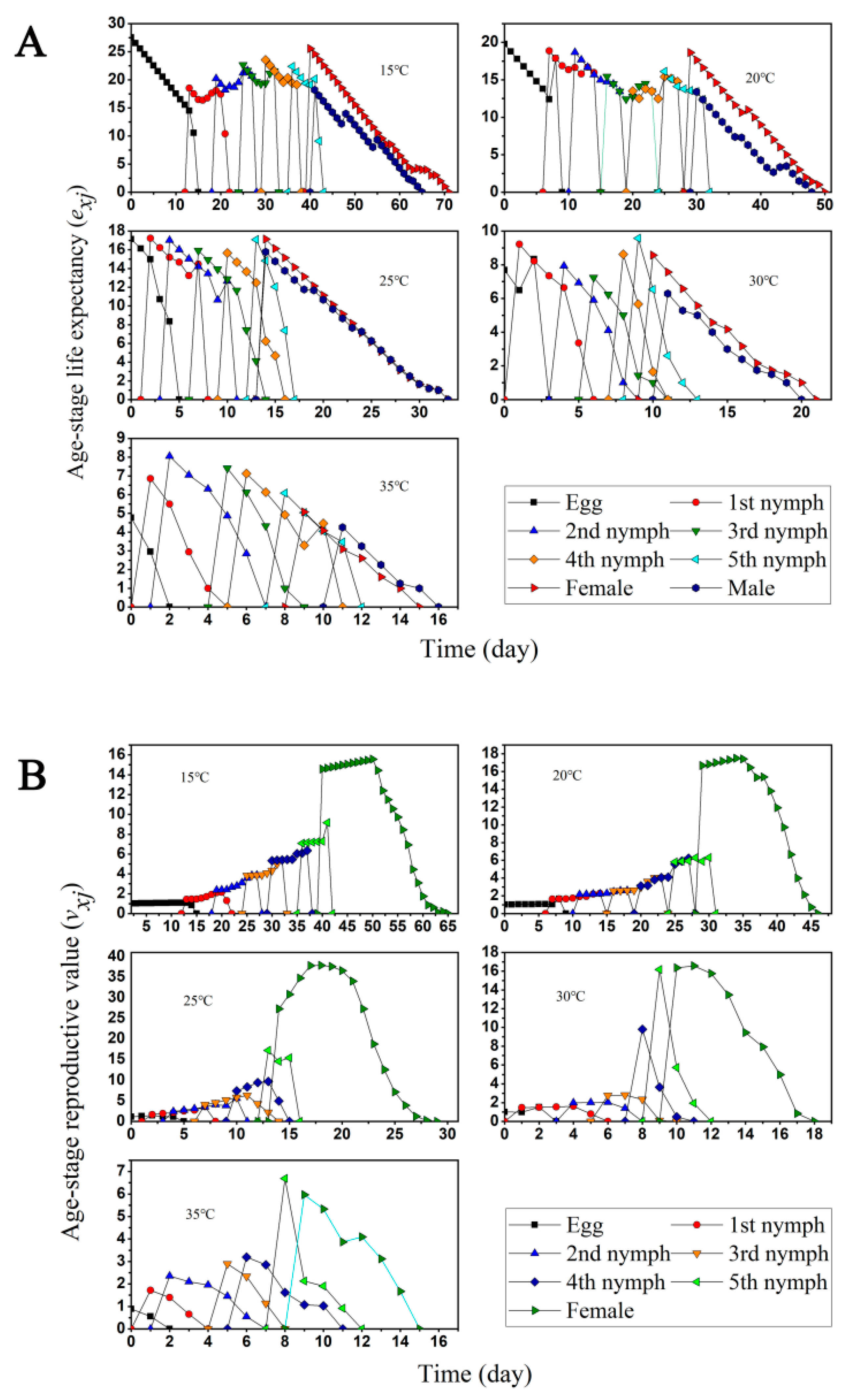
3.1. Two-Sex Life Table and Predation Rate
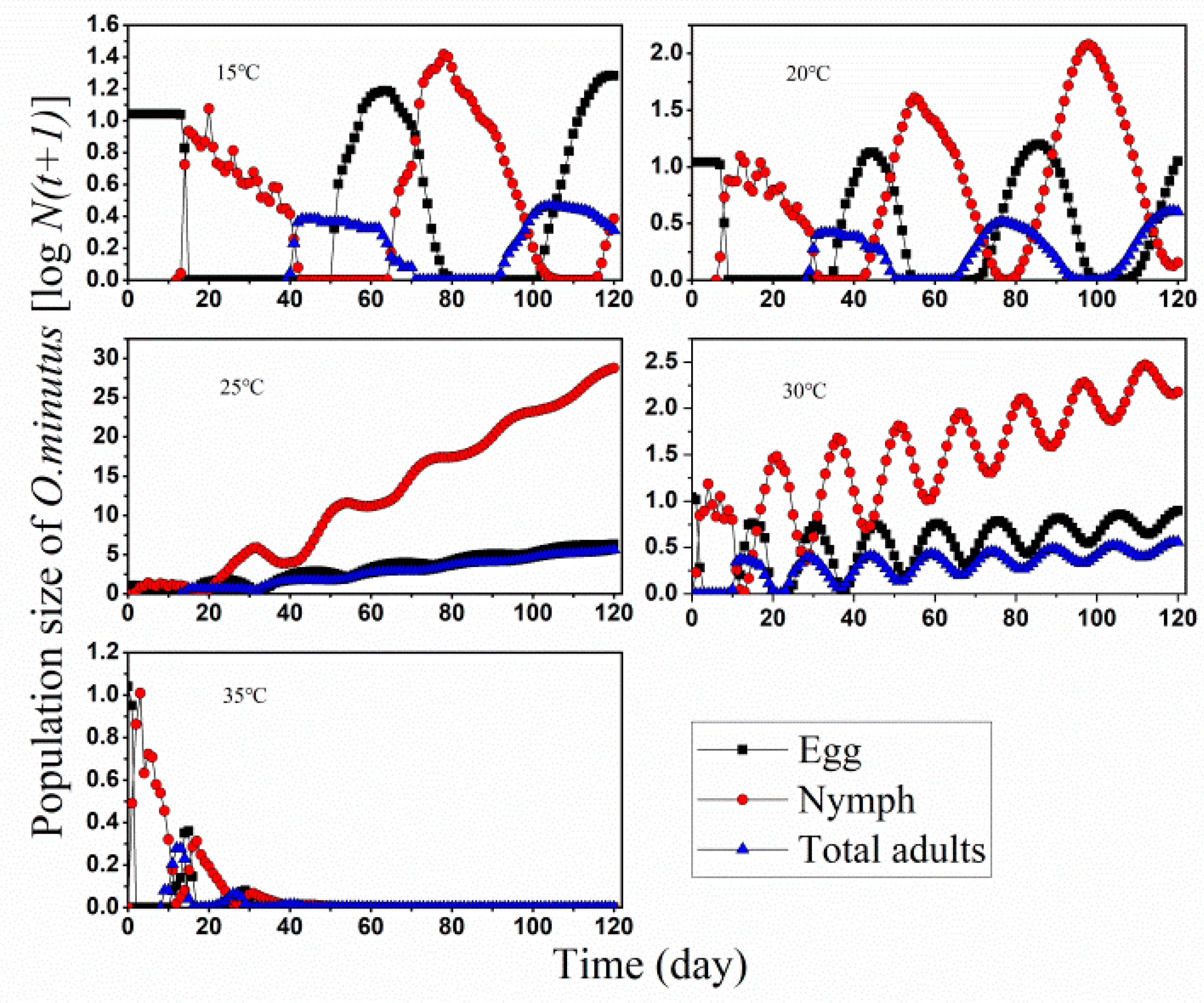
3.2. Population Projection
3.3. Population Growth and Predation Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, R.; Ji, D.; Zhang, Q.; Jin, L. Evaluation of eleven plant species as potential banker plants to support predatory orius sauteri in tea plant systems. Insects 2021, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, R.; Zhang, Q.; Ji, D.; Zhou, X.; Jin, L. Functional response and control potential of Orius sauteri (Hemiptera: Anthocoridae) on tea thrips (Dendrothrips minowai Priesner). Insects 2021, 12, 1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, M.; Ban, F.; Shang, X.; Liu, S.; Mao, T.; Zhang, X.; Zhi, J. Establishment of a Faba Bean Banker Plant System with Predator Orius strigicollis for the Control of Thrips Dendrothrips minowai on Tea Plants under Laboratory Conditions. Insects 2021, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Mccaffrey, J.P.; Horsburgh, R.L. Biology of Orius insidiosus (Heteroptera: Anthocoridae): A predator in virginia apple orchards. Environ. Entomol. 1986, 15, 984–988. [Google Scholar] [CrossRef]
- Baez, I.; Reitz, S.R.; Funderburk, J.E.; Olson, S.M. Predation of Frankliniella occidentalis (Thysanoptera: Thripidae) in field peppers by Orius insidiosus (Hemiptera: Anthocoridae). Environ. Entomol. 2000, 29, 376–382. [Google Scholar] [CrossRef]
- Lenteren, J.C.v.; Roskam, M.M.; Timmer, R. Commercial mass production and pricing of organisms for biological control of pests in Europe. Biol. Control 1997, 10, 143–149. [Google Scholar] [CrossRef]
- Zhang, A.; Yu, Y.; Li, L.; Zhang, S.; Men, X. Funcyional response and searching rate of Orius sauteri adults on Frankliniella occidentalis nymphs. Chin. J. Ecol. 2007, 8, 1233–1237. [Google Scholar]
- Viswanathan, T.R.; Ananthakrishnan, T.N. Population fluctuations of 3 species of anthophilous thysanoptera in relation to the numerical response of their predator, Orius minutus L. (Anthocoridae: Hemiptera). Curr. Sci. India 1974, 43, 19–20. [Google Scholar]
- Rahman, M.A.; Sarker, S.; Ham, E.; Lee, J.S.; Lim, U.T. Development and fecundity of Orius minutus (Hemiptera: Anthocoridae) and O. laevigatus reared on Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 2020, 113, 1735–1740. [Google Scholar] [CrossRef]
- Jun, H.; Kim, K.; Ham, E.-H. Basic studies aiming at Orius minutus (Hemiptera: Anthocoridae) mass-rearing. Insects 2022, 13, 77. [Google Scholar] [CrossRef]
- Maria, V.A.; Ioana, G.; Ramona, S.; Alin, C.; Levente, M.; Veaceslav, M. Biological control of Odontothrips loti (Hal.) with anthocorid predators, Orius minutus (L.) and Orius niger (Wolf.). J. Biotechnol. 2016, 231, S88. [Google Scholar] [CrossRef]
- RanjitSingh, H. PranitaHazarika. In Biotechnological Approaches for Tea Improvement; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: New York, NY, USA, 2020; pp. 111–148. [Google Scholar] [CrossRef]
- Yu, X.; Meng, Z.; Yang, M.; Zhou, Y.; Han, C.; Zou, X. Investigation and taxonomic revision of green tea leafhopper species of Guizhou and other provinces in south China. Chin. J. Appl. Entomol. 2015, 52, 11. [Google Scholar]
- Chown, S.L.; Chown, S.; Nicolson, S. Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Angilletta, M.J., Jr. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Doucet, D.; Walker, V.K.; Qin, W. The bugs that came in from the cold: Molecular adaptations to low temperatures in insects. Cell. Mol. Life Sci. 2009, 66, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Zhu, Q.; Jaleel, W.; Rehman, S.U.; Rasheed, M.A.; Khan, M.M.; Islam, Y.; Hafeez, M.; Zhou, X. Determination of fitness traits of Orius strigicollis Poppius (Hemiptera: Anthocoridae) on Pectinophora gossypiella (Lepidoptera: Gelechiidae) using two-sex life table analysis. PeerJ 2020, 8, e9594. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, H.; Aragón-Sánchez, M.; Sáenz-Romo, M.G.; Román-Fernández, L.R.; Veas-Bernal, A.; Marco-Mancebón, V.S.; Pérez-Moreno, I. Mathematical Models for Predicting Development of Orius majusculus (Heteroptera: Anthocoridae) and Its Applicability to Biological Control. J. Econ. Entomol. 2018, 111, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): With additional comments on life table research criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef]
- Rehman, S.U.; Zhou, X.; Ali, S.; Rasheed, M.A.; Islam, Y.; Hafeez, M.; Sohail, M.A.; Khurram, H. Predatory functional response and fitness parameters of Orius strigicollis (Poppius) when fed Bemisia tabaci and Trialeurodes vaporariorum as determined by age-stage, two-sex life table. PeerJ 2020, 8, e9540. [Google Scholar] [CrossRef]
- Bussaman, P.; Uth, C.S.; Chandrapatya, A.; Atlihan, R.; Gökçe, A.; Saska, P.; Chi, H. Fast population growth in physogastry reproduction of Luciaphorus perniciosus (Acari: Pygmephoridae) at different temperatures. J. Econ. Entomol. 2017, 110, 1397–1403. [Google Scholar] [CrossRef]
- Birch, L.C. The intrinsic rate of natural increase an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Applied Demography for Biologists, with Special Emphasis on Insects; Oxford University Press: New York, NY, USA, 1993; Volume 118, p. 243. [Google Scholar] [CrossRef]
- Lewis, E.G. On the generation and growth of a population. Sankhyā Indian J. Stat. (1933–1960) 1942, 6, 93–96. [Google Scholar]
- Ding, H.; Lin, Y.; Tuan, S.; Tang, L.; Chi, H.; Remzi, A.; Salih, Ö.M.; Al, G. Integrating demography, predation rate, and computer simulation for evaluation of Orius strigicollis as biological control agent against Frankliniella intonsa. Entomol. Gen. 2021, 41, 179–196. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, D.; Yang, M.; Liu, J. The effect of temperatures and hosts on the life cycle of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, C.; Yang, X.; Chi, H.; Dai, P.; Desneux, N.; Benelli, G.; Zang, L. Yacon as an alternative host plant for encarsia formosa mass-rearing: Validating a multinomial theorem for bootstrap technique in life table research. Pest Manag. Sci. 2021, 77, 2324–2336. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Yu, J.Z.; Chi, H.; Chen, B. Comparison of the life tables and predation rates of Harmonia dimidiata (F.) (Coleoptera: Coccinellidae) fed on Aphis gossypii Glover (Hemiptera: Aphididae) at different temperatures. Biol. Control 2013, 64, 1–9. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Twosex Life Table Analysis. Available online: http://140.120.197.173/Ecology/ (accessed on 20 November 2021).
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Yuan, S. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Tuan, S.; Lee, C.; Chi, H. Population and damage projection of Spodopteralitura (F.) on peanuts (Arachishypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef]
- Chi, H.; Yang, T. Two-sex life table and predation rate of propylaea japonicathunberg (Coleoptera: Coccinellidae) fed on Myzus persicae(Sulzer) (Homoptera: Aphididae). Environ. Entomol. 2003, 32, 327–333. [Google Scholar] [CrossRef]
- Chi, H. CONSUME-MSChart: A Computer Program for Consumption Rate Analysis Based on the Age Stage, Two-Sex Life Table. Available online: http://140.120.197.173/Ecology/ (accessed on 20 November 2021).
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table. Available online: http://140.120.197.173/Ecology (accessed on 20 November 2021).
- Liu, Y.; Li, G.; Yang, L.; Chi, H.; Chen, X. Demography and mass rearing of the medicinal Blister Beetle Epicauta impressicornis (Pic) (Coleoptera: Meloidae) at different temperatures. J. Econ. Entomol. 2018, 111, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Hassell, M.P. The dynamics of arthropod predator-prey systems. Biometrics 1979, 13, 1–237. [Google Scholar]
- Mou, D.F.; Lee, C.C.; Smith, C.L.; Chi, H. Using viable eggs to accurately determine the demographic andpredation potential of Harmonia dimidiata (Coleoptera: Coccinellidae). J. Appl. Entomol. 2015, 139, 579–591. [Google Scholar] [CrossRef]
- Chase, J.M.; Leibold, M.A. Spatial scale dictates the productivity-biodiversity relationship. Nature 2002, 416, 427–430. [Google Scholar] [CrossRef]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoddle, M.S. The Influence of Temperature Variation on Life History Parameters and Thermal Performance Curves of Tamarixia radiata (Hymenoptera: Eulophidae), a Parasitoid of the Asian Citrus Psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef]
- Milosavljević, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of Constant and Fluctuating Temperatures on Development Rates and Longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Laws, A.N. Climate change effects on predator-prey interactions. Curr. Opin. Insect Sci. 2017, 23, 28–34. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]

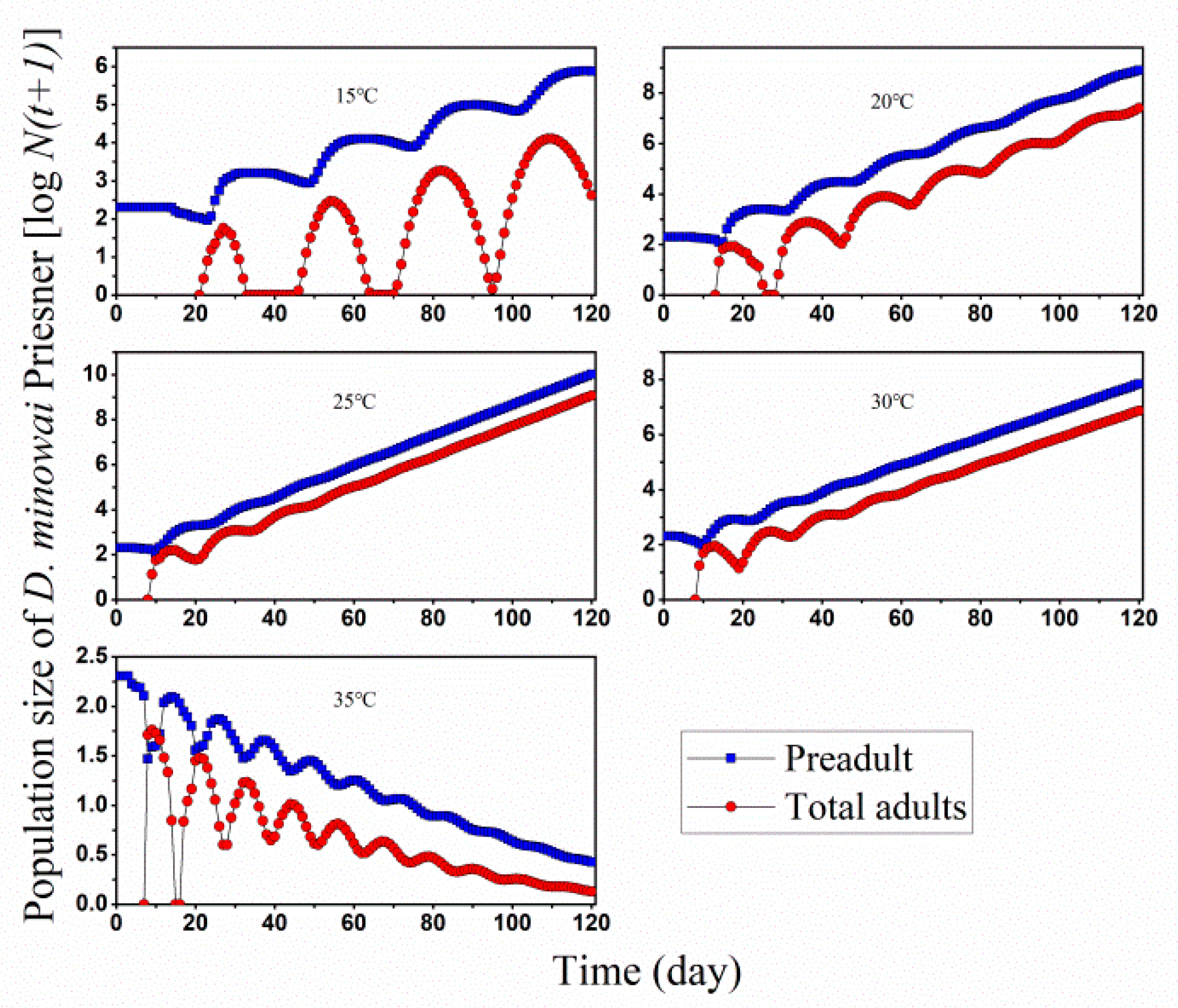
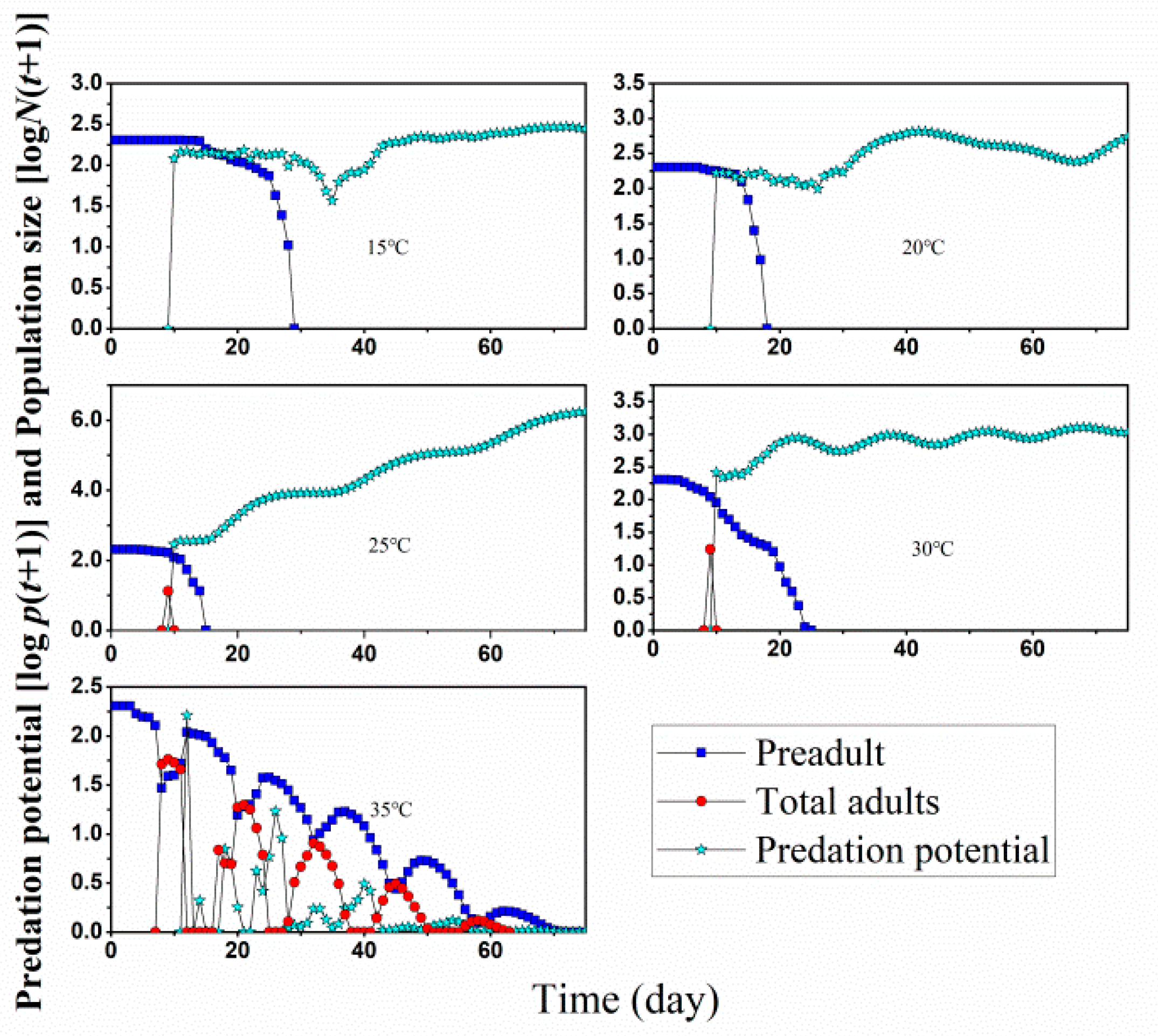
| Stage | 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | |
| Egg | 76 | 14.42 ± 0.06 a | 66 | 8.24 ± 0.07 b | 86 | 3.23 ± 0.07 c | 69 | 2.03 ± 0.06 d | 49 | 1.57 ± 0.07 e |
| 1st nymphs | 48 | 5.92 ± 0.09 a | 52 | 4.67 ± 0.09 b | 76 | 2.33 ± 0.06 d | 53 | 2.58 ± 0.07 c | 30 | 1.50 ± 0.09 e |
| 2nd nymphs | 31 | 6.13 ± 0.10 a | 46 | 4.65 ± 0.08 b | 66 | 3.12 ± 0.05 c | 41 | 2.59 ± 0.08 c | 20 | 2.70 ± 0.11 d |
| 3rd nymphs | 23 | 5.43 ± 0.11 a | 33 | 4.52 ± 0.09 b | 53 | 2.96 ± 0.05 c | 29 | 1.79 ± 0.09 d | 16 | 1.44 ± 0.13 e |
| 4th nymphs | 18 | 5.39 ± 0.16 a | 22 | 4.00 ± 0.15 b | 41 | 2.20 ± 0.09 c | 20 | 1.10 ± 0.07 d | 12 | 2.25 ± 0.18 c |
| 5th nymphs | 14 | 4.21 ± 0.11 a | 16 | 4.06 ± 0.11 a | 36 | 1.17 ± 0.06 c | 14 | 1.00 ± 0 d | 10 | 1.70 ± 0.15 b |
| Preadult | 14 | 41.50 ± 0.20 a | 16 | 30.38 ± 0.20 b | 36 | 15.08 ± 0.12 c | 14 | 11.07 ± 0.13 d | 10 | 11.16 ± 0.37 d |
| Female adult longevity | 9 | 24.11 ± 1.29 a | 8 | 17.50 ± 1.39 b | 23 | 16.13 ± 0.26 b | 7 | 7.57 ± 0.66 c | 6 | 3.67 ± 0.34 d |
| Male adult longevity | 5 | 17.60 ± 3.26 a | 8 | 12.75 ± 1.15 a | 13 | 14.54 ± 1.14 a | 7 | 6.14 ± 0.81 b | 6 | 3.50 ± 0.29 c |
| Total longevity of females | 9 | 65.56 ± 1.37 a | 8 | 47.62 ± 1.40 b | 23 | 31.13 ± 0.23 c | 7 | 18.57 ± 0.72 d | 6 | 14.17 ± 0.48 e |
| Total Longevity of males | 5 | 59.20 ± 3.35 a | 8 | 43.38 ± 1.18 b | 13 | 29.77 ± 1.09 c | 7 | 17.29 ± 0.87 d | 4 | 15.25 ± 0.25 e |
| Adult preoviposition period (APOP) | 9 | 9.67 ± 0.47 a | 8 | 5.75 ± 0.53 b | 23 | 1.87 ± 0.16 c | 7 | 1.57 ± 0.43 c | 6 | 2.00 ± 0.45 c |
| Total preoviposition period (TPOP) | 9 | 51.11 ± 0.54 a | 8 | 35.88 ± 0.48 b | 23 | 16.87 ± 0.81 c | 7 | 12.57 ± 0.48 d | 6 | 12.5 ± 0.56 d |
| Oviposition days (Od) | 9 | 6.22 ± 0.62 b | 8 | 6.75 ± 0.65 b | 23 | 10.00 ± 0.30 a | 7 | 4.00 ± 0.49 c | 6 | 2.00 ± 0.45 d |
| Statistics Parameter | Mean ± SE | ||||
|---|---|---|---|---|---|
| 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | |
| Intrinsic rate of increase, r (d−1) | 0.0066 ± 0.0065 b | 0.0096 ± 0.0098 b | 0.1224 ± 0.0089 a | 0.0116 ± 0.0294 b | −0.1132 ± 0.00386 c |
| Finite rate of increase, λ (d−1) | 1.0066 ± 0.0065 c | 1.0096 ± 0.0098 c | 1.1302 ± 0.0100 b | 1.0117 ± 0.0291 c | 0.8930 ± 0.0334 a |
| Net reproductive rate, R0 (offspring/individual) | 1.45 ± 0.48 b | 1.48 ± 0.52 b | 14.76 ± 2.73 a | 1.19 ± 0.45 c | 0.21 ± 0.09 b |
| Mean generation time, T (d) | 56.27 ± 1.16 a | 40.83 ± 0.74 b | 22.00 ± 0.14 c | 15.00 ± 0.65 d | 13.79 ± 0.52 d |
| Net predation rate, c0 (prey/individual) | 74.54 ± 13.83 c | 90.23 ± 13.27 b | 310.92 ± 31.39 a | 58.79 ± 8.01 d | 17.29 ± 4.31 e |
| Finite predation rate, ω (d−1) | 2.35 ± 0.28 d | 4.11 ± 0.33 c | 9.85 ± 0.48 a | 7.41 ± 0.67 b | 6.44 ± 0.76 b |
| Transformation rate, Qp (c0/R0) | 55.50 ± 17.12 b | 60.99 ± 31.05 b | 21.07 ± 3.04 d | 49.40 ± 33.42 c | 82.33 ± 87.56 a |
| The gross reproduction rate (GRR) | 12.05 ± 2.40 b | 11.38 ± 2.76 b | 42.23 ± 5.26 a | 9.4 ± 2.68 c | 2.42 ± 0.87 d |
| Fecundity (no. of eggs per female) | 16.11 ± 1.53 b | 18.50 ± 1.84 b | 64.17 ± 1.53 a | 17.00 ± 2.12 b | 3.50 ± 0.67 c |
| F:M:N | 9:5:86 | 8:8:84 | 23:13:64 | 7:7:86 | 6:4:90 |
| Stage | 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | |
| 1st nymphs | 48 | 11.23 ± 0.44 h | 52 | 12.48 ± 0.43 g | 76 | 14.56 ± 0.57 f | 53 | 10.39 ± 0.47 d | 30 | 5.34 ± 0.45 e |
| 2nd nymphs | 31 | 22.55 ± 0.71 g | 46 | 24.11 ± 1.10 f | 66 | 44.64 ± 0.93 d | 41 | 23.96 ± 1.10 b | 20 | 14.75 ± 1.07 d |
| 3rd nymphs | 23 | 25.57 ± 1.27 f | 33 | 41.48 ± 1.83 e | 53 | 60.77 ± 1.84 c | 29 | 24.55 ± 1.24 b | 16 | 14.55 ± 1.42 d |
| 4th nymphs | 18 | 36.19 ± 2.05 e | 22 | 50.50 ± 1.66 d | 41 | 55.74 ± 2.45 c | 20 | 18.65 ± 1.40 c | 12 | 28.52 ± 2.40 c |
| 5th nymphs | 14 | 43.69 ± 2.23 d | 16 | 49.00 ± 1.83 d | 36 | 36.34 ± 2.21 e | 14 | 19.79 ± 0.69 c | 10 | 26.30 ± 2.32 c |
| Preadult | 14 | 140.31 ± 3.06 c | 16 | 183.68 ± 5.15 b | 36 | 212.14 ± 3.94 b | 14 | 97.86 ± 4.60 a | 10 | 85.79 ± 5.15 a |
| Adult | 14 | 262.07 ± 22.08 b | 16 | 186.98 ± 15.11 b | 36 | 503.71 ± 15.92 a | 14 | 137.06 ± 11.03 a | 10 | 52.58 ± 5.15 b |
| Female adult | 9 | 303.12 ± 19.37 a | 8 | 232.63 ± 14.97 a | 22 | 527.60 ± 11.95 a | 7 | 154.25 ± 13.13 a | 6 | 47.01 ± 5.66 b |
| Male adult | 5 | 188.25 ± 33.26 c | 8 | 141.33 ± 13.18 c | 14 | 466.11 ± 35.02 a | 7 | 119.87 ± 16.53 a | 4 | 60.76 ± 9.38 b |
| Stage | 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mj | Mean ± SE | mj | Mean ± SE | mj | Mean ± SE | mj | Mean ± SE | mj | Mean ± SE | |
| 1st nymphs | 76 | 9.12 ± 0.47 h | 66 | 11.38 ± 0.48 h | 86 | 14.42 ± 0.55 f | 69 | 9.74 ± 0.47 d | 49 | 5.82 ± 0.39 d |
| 2nd nymphs | 48 | 17.73 ± 1.11 g | 52 | 22.85 ± 1.10 g | 76 | 43.29 ± 1.09 d | 53 | 23.53 ± 1.04 c | 30 | 14.57 ± 0.90 c |
| 3rd nymphs | 31 | 22.9 ± 1.37 f | 46 | 36.69 ± 1.86 f | 66 | 60.75 ± 2.08 c | 41 | 24.49 ± 1.17 c | 20 | 15.29 ± 1.26 c |
| 4th nymphs | 23 | 33.22 ± 2.27 e | 33 | 43.75 ± 2.33 e | 53 | 54.47 ± 2.23 c | 29 | 20.11 ± 1.11 c | 16 | 27.34 ± 2.02 c |
| 5th nymphs | 18 | 42.16 ± 1.92 d | 22 | 43.76 ± 2.46 e | 41 | 37.35 ± 2.01 e | 20 | 22.70 ± 1.39 c | 12 | 26.09 ± 1.99 b |
| Preadult | 100 | 37.79 ± 5.15 d | 100 | 60.33 ± 7.07 d | 100 | 129.59 ± 8.83 b | 100 | 39.61 ± 4.12 b | 100 | 17.83 ± 2.99 b |
| Adult | 14 | 261.85 ± 22.08 b | 16 | 186.98 ± 15.11 b | 36 | 503.71 ± 15.92 a | 14 | 137.06 ± 11.03 a | 10 | 52.58 ± 5.15 a |
| Female adult | 9 | 303.12 ± 19.37 a | 8 | 232.63 ± 14.97 a | 22 | 527.60 ± 11.95 a | 7 | 154.25 ± 13.13 a | 6 | 47.01 ± 5.66 a |
| Male adult | 5 | 188.2 ± 2.24 c | 8 | 141.33 ± 13.18 c | 14 | 466.11 ± 35.02 a | 7 | 119.87 ± 16.53 a | 4 | 60.76 ± 9.38 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, R.; Ren, X.; Cao, K.; Zhou, X.; Jin, L. Demographic Evaluation of the Control Potential of Orius minutus (Hemiptera: Anthocoridae) Preying on Dendrothrips minowai Priesner (Thysanoptera: Thripidae) at Different Temperatures. Insects 2022, 13, 1158. https://doi.org/10.3390/insects13121158
Lan R, Ren X, Cao K, Zhou X, Jin L. Demographic Evaluation of the Control Potential of Orius minutus (Hemiptera: Anthocoridae) Preying on Dendrothrips minowai Priesner (Thysanoptera: Thripidae) at Different Temperatures. Insects. 2022; 13(12):1158. https://doi.org/10.3390/insects13121158
Chicago/Turabian StyleLan, Rongmeng, Xiaoli Ren, Kunqian Cao, Xia Zhou, and Linhong Jin. 2022. "Demographic Evaluation of the Control Potential of Orius minutus (Hemiptera: Anthocoridae) Preying on Dendrothrips minowai Priesner (Thysanoptera: Thripidae) at Different Temperatures" Insects 13, no. 12: 1158. https://doi.org/10.3390/insects13121158
APA StyleLan, R., Ren, X., Cao, K., Zhou, X., & Jin, L. (2022). Demographic Evaluation of the Control Potential of Orius minutus (Hemiptera: Anthocoridae) Preying on Dendrothrips minowai Priesner (Thysanoptera: Thripidae) at Different Temperatures. Insects, 13(12), 1158. https://doi.org/10.3390/insects13121158





