Do It by Yourself: Larval Locomotion in the Black Soldier Fly Hermetia illucens, with a Novel “Self-Harvesting” Method to Separate Prepupae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Housing
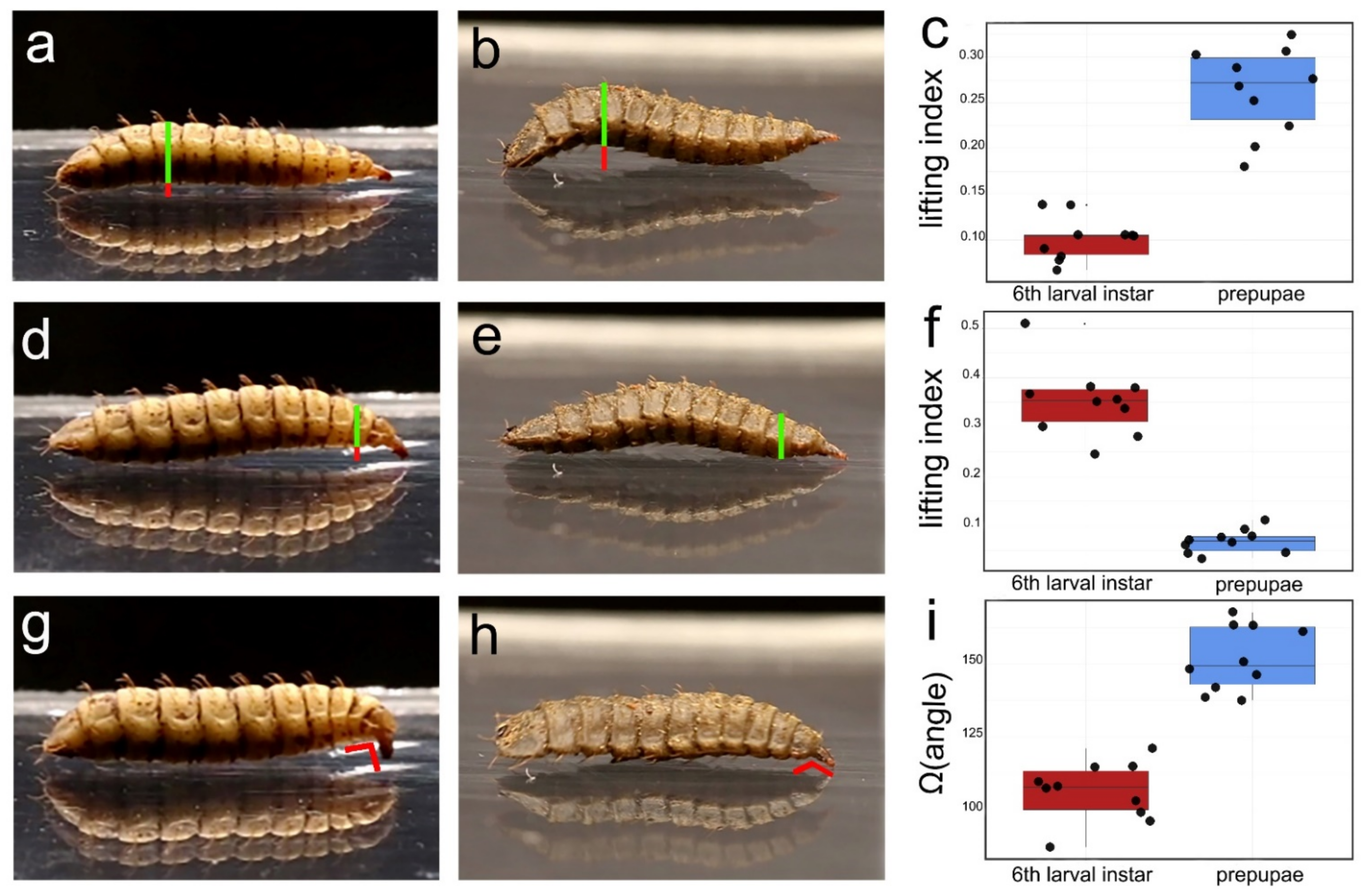
2.2. Larval Instar Classification
2.3. Laboratory Conditions
2.4. Larval Response upon Disturbance
2.5. Locomotory Pattern
2.6. Locomotory Speed under Different Stress Conditions
- No light (24 °C)
- No light + heated mat (32 °C)
- Ambient light (24 °C)
- Ambient light + heated mat (32 °C)
- Cold white light (24 °C)
- Warm white light (32 °C)
- Infrared light (33 °C)
2.7. Separation of Prepupae from the Younger Larvae
2.8. Statistical Analyses
3. Results
3.1. Larval Response upon Disturbance
3.2. Locomotory Pattern
3.3. Locomotory Speed under Different Stress Conditions
3.4. Separation of Prepupae from the Younger Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leclerq, M. Dispersion et transport des insectes nuisibles: Herrnetia illucens L. (Diptera Stratiomyidae). Bull. Rech. Agron. Gem-bloux N.S. 1969, 4, 139–143. [Google Scholar]
- McCallan, E. Hermetia illucens (L.) (Dipt., Stratiomyidae), a cosmpolitan American species long established in Australia and New Zealand. Entomol. Mo. Mag. 1974, 109, 232–234. [Google Scholar]
- Gujarathi, G.; Pejaver, M. Occurrence of black soldier fly Hermetia illucens (Diptera: Stratiomyidae) in biocompost. Res. J. Recent Sci. 2013, 2, 65–66. [Google Scholar]
- Roháček, J.; Hora, M. A northernmost European record of the alien black soldier fly Hermetia illucens (Linnaeus, 1758) (Dip-tera: Stratiomyidae)/Nejsevernější evropský výskyt nepůvodní bráněnky Hermetia illucens (Linnaeus, 1758) (Diptera: Strati-omyidae). Cas. Slez. Zemskeho Muz. A 2013, 62, 101–106. [Google Scholar]
- Marshall, S.A.; Woodley, N.E.; Hauser, M. The historical spread of the Black Soldier Fly, Hermetia illucens (L.) (Diptera, Stra-tiomyidae, Hermetiinae), and its establishment in Canada. J. Entomol. Soc. Ont. 2015, 146, 51–54. [Google Scholar]
- Castracani, C.; Bulgarini, G.; Giannetti, D.; Spotti, F.A.; Maistrello, L.; Mori, A.; Grasso, D.A. Predatory ability of the ant Crematogaster scutellaris on the brown marmorated stink bug Halyomorpha halys. J. Pest Sci. 2017, 90, 1181–1190. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Menalled, F.D. Supporting beneficial insects for agricultural sustainability: The role of livestock-integrated or-ganic and cover cropping to enhance ground beetle (Carabidae) communities. Agronomy 2020, 10, 1210. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tomberlin, J.K.; VanLaerhoven, S.L. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Èntomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Da Silva, G.D.P.; Hesselberg, T. A Review of the Use of Black Soldier Fly Larvae, Hermetia illucens (Diptera: Stratiomyidae), to Compost Organic Waste in Tropical Regions. Neotrop. Èntomol. 2020, 49, 151–162. [Google Scholar] [CrossRef]
- Surendra, K.; Tomberlin, J.K.; van Huis, A.; Cammack, J.A.; Heckmann, L.-H.L.; Khanal, S.K. Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manag. 2020, 117, 58–80. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Fonseca, K.B.; Dicke, M.; Van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste sub-strates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Saadoun, J.H.; Montevecchi, G.; Zanasi, L.; Bortolini, S.; Macavei, L.I.; Masino, F.; Maistrello, L.; Antonelli, A. Lipid profile and growth of black soldier flies (Hermetia illucens, Stratiomyidae) reared on by-products from different food chains. J. Sci. Food Agric. 2020, 100, 3648–3657. [Google Scholar] [CrossRef]
- Barbi, S.; Messori, M.; Manfredini, T.; Pini, M.; Montorsi, M. Rational design and characterization of bioplastics from Hermetia illucens prepupae proteins. Biopolymers 2019, 110, e23250. [Google Scholar] [CrossRef]
- Barbi, S.; Macavei, L.I.; Caligiani, A.; Maistrello, L.; Montorsi, M. From Food Processing Leftovers to Bioplastic: A Design of Experiments Approach in a Circular Economy Perspective. Waste Biomass Valorization 2021, 12, 5121–5130. [Google Scholar] [CrossRef]
- Setti, L.; Francia, E.; Pulvirenti, A.; De Leo, R.; Martinelli, S.; Maistrello, L.; Macavei, L.I.; Montorsi, M.; Barbi, S.; Ronga, D. Bioplastic Film From Black Soldier Fly Prepupae Proteins Used As Mulch: Preliminary Results. Agronomy 2020, 10, 933. [Google Scholar] [CrossRef]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens—a promising source for sustainable production of proteins, lipids and bioactive substances. Z. Für Nat. C 2017, 72, 351–363. [Google Scholar] [CrossRef]
- Smetana, S.; Schmitt, E.; Mathys, A. Sustainable use of Hermetia illucens insect biomass for feed and food: Attribtional and consequential life cycle assessment. Resour. Conserv. Recycl. 2019, 144, 285–296. [Google Scholar] [CrossRef]
- Setti, L.; Francia, E.; Pulvirenti, A.; Gigliano, S.; Zaccardelli, M.; Pane, C.; Caradonia, F.; Bortolini, S.; Maistrello, L.; Ronga, D. Use of black soldier fly (Hermetia illucens (L.), Diptera: Stratiomyidae) larvae processing residue in peat-based growing media. Waste Manag. 2019, 95, 278–288. [Google Scholar] [CrossRef]
- Schmitt, E.; Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green. Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- Castracani, C.; Maienza, A.; Grasso, D.; Genesio, L.; Malcevschi, A.; Miglietta, F.; Vaccari, F.; Mori, A. Biochar–macrofauna interplay: Searching for new bioindicators. Sci. Total. Environ. 2015, 536, 449–456. [Google Scholar] [CrossRef]
- Kim, J.-G.; Choi, Y.-C.; Choi, J.-Y.; Kim, W.-T.; Jeong, G.-S.; Park, K.-H.; Hwang, S.-J. Ecology of the Black Soldier Fly, Hermetia illucens (Diptera: Stratmyidae) in Korea. Korean J. Appl. Èntomol. 2008, 47, 337–343. [Google Scholar] [CrossRef]
- Kim, W.T.; Bae, S.W.; Park, H.C.; Park, K.H.; Lee, S.B.; Choi, Y.C.; Han, S.M.; Koh, Y.H. The larval age and mouth morphology of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2010, 21, 185–187. [Google Scholar]
- Barros, L.M.; Gutjahr, A.L.N.; Keppler, R.L.F.; Martins, R.T. Morphological description of the immature stages of Hermetia illucens (Linnaeus, 1758) (Diptera: Stratiomyidae). Microsc. Res. Tech. 2019, 82, 178–189. [Google Scholar] [CrossRef]
- Gligorescu, A.; Toft, S.; Hauggaard-Nielsen, H.; Axelsen, J.A.; Nielsen, S.A. Development, growth and metabolic rate of Hermetia illucens larvae. J. Appl. Èntomol. 2019, 143, 875–881. [Google Scholar] [CrossRef]
- Holmes, L.A.; VanLaerhoven, S.L.; Tomberlin, J.K. Substrate Effects on Pupation and Adult Emergence of Hermetia illucens (Diptera: Stratiomyidae): Table 1. Environ. Èntomol. 2013, 42, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Pastor, B.; Velásquez, Y.; Gobbi, P.; Rojo, S. Conversion of organic wastes into fly larval biomass: Bottlenecks and challenges. J. Insects Food Feed 2015, 1, 179–193. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Volk, N.; Diehl, J.J.E.; van Loon, J.J.A.; Belušič, G. Photoreceptor spectral sensitivity of the compound eyes of black soldier fly (Hermetia illucens) informing the design of LED-based illumination to enhance indoor reproduction. J. Insect Physiol. 2016, 95, 133–139. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS ONE 2018, 13, e0206097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heussler, C.D.; Walter, A.; Oberkofler, H.; Insam, H.; Arthofer, W.; Schlick-Steiner, B.; Steiner, F.M. Influence of three artificial light sources on oviposition and half-life of the Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae): Improving small-scale indoor rearing. PLoS ONE 2018, 13, e0197896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupi, D.; Savoldelli, S.; Leonardi, M.G.; Jucker, C. Feeding in the adult of Hermetia illucens (Diptera Stratiomyidae): Reality or fic-tion? J. Entomol. Acarol. Res. 2019, 51, 8046. [Google Scholar] [CrossRef]
- Hoc, B.; Noël, G.; Carpentier, J.; Francis, F.; Megido, R.C. Optimization of black soldier fly (Hermetia illucens) artificial repro-duction. PLoS ONE 2019, 30, e0216160. [Google Scholar]
- Bertinett, C.; Samayoa, A.C.; Hwang, S.-Y. Effects of Feeding Adults of Hermetia illucens (Diptera: Stratiomyidae) on Longevity, Oviposition, and Egg Hatchability: Insights into Optimizing Egg Production. J. Insect Sci. 2019, 19, 2411–2502. [Google Scholar]
- Liu, Z.; Najar-Rodriguez, A.J.; Minor, M.A.; Hedderley, D.I.; Morel, P.C. Mating success of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), under four artificial light sources. J. Photochem. Photobiol. B Biol. 2020, 205, 111815. [Google Scholar] [CrossRef]
- Gold, M.; Binggeli, M.; Kurt, F.; De Wouters, T.; Reichlin, M.; Zurbrügg, C.; Mathys, A.; Kreuzer, M. Novel Experimental Methods for the Investigation of Hermetia illucens (Diptera: Stratiomyidae) Larvae. J. Insect Sci. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Macavei, L.I.; Benassi, G.; Stoian, V.; Maistrello, L. Optimization of Hermetia illucens (L.) egg laying under different nutrition and light conditions. PLoS ONE 2020, 15, e0232144. [Google Scholar] [CrossRef]
- Raimondi, S.; Spampinato, G.; Macavei, L.I.; Lugli, L.; Candeliere, F.; Rossi, M.; Maistrello, L.; Amaretti, A. Effect of Rearing Temperature on Growth and Microbiota Composition of Hermetia illucens. Microorganisms 2020, 8, 902. [Google Scholar] [CrossRef]
- Reguzzi, M.; Cominelli, F.; Bardone, M.; Aldini, R.N.; Chiesa, O.; Panini, M.; Casu, G.; Mazzoni, E. Unwelcome guests at farms breeding the black soldier fly, Hermetia illucens (L.) (Diptera Stratiomyidae). J. Insects Food Feed. 2021, 7, 1177–1181. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Randazzo, B.; Messina, M.; Zarantoniello, M.; Giorgini, E.; Zimbelli, A.; Bruni, L.; Parisi, G.; Olivotto, I.; Tulli, F. Effects of graded dietary inclusion level of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (On-corhynchus mykiss). Animals 2019, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Giannetto, A.; Oliva, S.; Lanes, C.F.C.; de Araújo Pedron, F.; Savastano, D.; Baviera, C.; Parrino, V.; Lo Paro, G.; Spanò, N.C.; Cappello, T.; et al. Hermetia illucens (Diptera: Stratiomydae) larvae and prepupae: Bio-mass production, fatty acid profile and expression of key genes involved in lipid metabolism. J. Biotechnol. 2020, 307, 44–54. [Google Scholar] [CrossRef]
- Hoc, B.; Genva, M.; Fauconnier, M.-L.; Lognay, G.; Francis, F.; Megido, R.C. About lipid metabolism in Hermetia illucens (L. 1758): On the origin of fatty acids in prepupae. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Newton, G.L.; Thompson, S.A.; Savage, S. A value added manure management system using the black soldier fly. Bioresour. Technol. 1994, 50, 275–279. [Google Scholar] [CrossRef]
- Roberts, M.J. On the locomotion of cyclorrhaphan maggots (Diptera). J. Nat. Hist. 1971, 5, 583–590. [Google Scholar] [CrossRef]
- Berrigan, D.; Pepin, D.J. How maggots move: Allometry and kinematics of crawling in larval Diptera. J. Insect Physiol. 1995, 41, 329–337. [Google Scholar] [CrossRef]
- Rotheray, G.; Lyszkowski, R. Diverse mechanisms of feeding and movement in Cyclorrhaphan larvae (Diptera). J. Nat. Hist. 2015, 49, 2139–2211. [Google Scholar] [CrossRef]
- Puig-Diví, A.; Escalona-Marfil, C.; Padullés-Riu, J.M.; Busquets, A.; Padullés-Chando, X.; Marcos-Ruiz, D. Validity and reliability of the Kinovea program in obtaining angles and distances using coordinates in 4 perspectives. PLoS ONE 2019, 14, e0216448. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 13 December 2021).
- Chelini, M.-C.; Willemart, R.; Hebets, E.A. Costs and benefits of freezing behaviour in the harvestman Eumesosoma roeweri (Arachnida, Opiliones). Behav. Process. 2009, 82, 153–159. [Google Scholar] [CrossRef]
- Humphreys, R.K.; Ruxton, G.D. A review of thanatosis (death feigning) as an anti-predator behaviour. Behav. Ecol. Sociobiol. 2018, 72, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.F.; Morgan, E.D. Chemical communication in insect communities: A guide to insect pheromones with special emphasis on social insects. Biol. Rev. 1990, 65, 227–247. [Google Scholar] [CrossRef]
- Grasso, D.A.; Mori, A.; Le Moli, F. Chemical communication during foraging in the harvesting ant Messor capitatus (Hymenoptera, Formicidae). Insectes Soc. 1998, 45, 85–96. [Google Scholar] [CrossRef]
- Grasso, D.A.; Mori, A.; Le Moli, F. Recruitment and trail communication in two species of messor ants (Hymenoptera, Formicidae). Ital. J. Zool. 1999, 66, 373–378. [Google Scholar] [CrossRef]
- Grasso, D.A.; Sledge, M.F.; Le Moli, F.; Mori, A.; Turillazzi, S. Nest-area marking with faeces: A chemical signature that al-lows colony-level recognition in seed harvesting ants (Hymenoptera, Formicidae). Insectes Soc. 2005, 52, 36–44. [Google Scholar] [CrossRef]
- Scheidler, N.H.; Liu, C.; Hamby, K.A.; Zalom, F.G.; Syed, Z. Volatile codes: Correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci. Rep. 2015, 5, srep14059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán-Valdivia, C.H.; Blanco-Ortega, A.; Oliver-Salazar, M.Y.; Carrera-Escobedo, J.L. Therapeutic motion analysis of lower limbs using Kinovea. Int. J. Soft Comput. Eng. 2013, 3, 2231–2307. [Google Scholar]
- Shishov, N.; Elabd, K.; Komisar, V.; Chong, H.; Robinovitch, S.N. Accuracy of Kinovea software in estimating body segment movements during falls captured on standard video: Effects of fall direction, camera perspective and video calibration technique. PLoS ONE 2021, 16, e0258923. [Google Scholar] [CrossRef] [PubMed]
- Beulens, A.J.; Namba, H.F.; Brinkman, W.M.; Meijer, R.P.; Koldewijn, E.L.; Hendrikx, A.J.; van Basten, J.P.; van Mer-riënboer, J.J.G.; Van der Poel, H.G.; Bangma, C.; et al. Analysis of the video motion tracking system “Kinovea” to assess surgical movements during robot-assisted radical prostatectomy. Int. J. Med. Robot 2020, 16, e2090. [Google Scholar] [CrossRef]
- Fernández-González, P.; Koutsou, A.; Cuesta-Gómez, A.; Carratalá-Tejada, M.; Miangolarra-Page, J.C.; Molina-Rueda, F. Reliability of Kinovea ® Software and Agreement with a Three-Dimensional Motion System for Gait Analysis in Healthy Subjects. Sensors 2020, 20, 3154. [Google Scholar] [CrossRef]
- Klinhom, S.; Chaichit, T.; Nganvongpanit, K. A comparative study of range of motion of forelimb and hindlimb in walk pattern and trot pattern of Chihuahua dogs affected and non-affected with patellar luxation. Asian J. Anim. Vet. Adv. 2015, 10, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Grasso, D.A.; Giannetti, D.; Castracani, C.; Spotti, F.A.; Mori, A. Rolling away: A novel context-dependent escape behaviour dis-covered in ants. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Hong, Y.R.; Moon, E. Reliability and validity of free software for the analysis of locomotor activity in mice. Yeungnam Univ. J. Med. 2018, 35, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Souza, J.G.D.S.; Libeck, L.T.; Virote, B.D.C.R.; Egger, R.C.; De Sá, G.C.R.; Machado, G.J.; Murgas, L.D.S. A method to analyze the relationship between locomotor activity and feeding behaviour in larvae of Betta splendens. Aquac. Int. 2020, 28, 1141–1152. [Google Scholar] [CrossRef]
- Shi, Q.; Gao, Z.; Jia, G.; Li, C.; Huang, Q.; Ishii, H.; Takanishi, A.; Fukuda, T. Implementing Rat-Like Motion for a Small-Sized Biomimetic Robot Based on Extraction of Key Movement Joints. IEEE Trans. Robot. 2020, 37, 747–762. [Google Scholar] [CrossRef]
- Charabidze, D.; Bourel, B.; Leblanc, H.; Hedouin, V.; Gosset, D. Effect of body length and temperature on the crawling speed of Protophormia terraenovae larvae (Robineau-Desvoidy) (Diptera Calliphoridae). J. Insect Physiol. 2008, 54, 529–533. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetti, D.; Schifani, E.; Reggiani, R.; Mazzoni, E.; Reguzzi, M.C.; Castracani, C.; Spotti, F.A.; Giardina, B.; Mori, A.; Grasso, D.A. Do It by Yourself: Larval Locomotion in the Black Soldier Fly Hermetia illucens, with a Novel “Self-Harvesting” Method to Separate Prepupae. Insects 2022, 13, 127. https://doi.org/10.3390/insects13020127
Giannetti D, Schifani E, Reggiani R, Mazzoni E, Reguzzi MC, Castracani C, Spotti FA, Giardina B, Mori A, Grasso DA. Do It by Yourself: Larval Locomotion in the Black Soldier Fly Hermetia illucens, with a Novel “Self-Harvesting” Method to Separate Prepupae. Insects. 2022; 13(2):127. https://doi.org/10.3390/insects13020127
Chicago/Turabian StyleGiannetti, Daniele, Enrico Schifani, Roberto Reggiani, Emanuele Mazzoni, Maria Cristina Reguzzi, Cristina Castracani, Fiorenza A. Spotti, Beatrice Giardina, Alessandra Mori, and Donato A. Grasso. 2022. "Do It by Yourself: Larval Locomotion in the Black Soldier Fly Hermetia illucens, with a Novel “Self-Harvesting” Method to Separate Prepupae" Insects 13, no. 2: 127. https://doi.org/10.3390/insects13020127
APA StyleGiannetti, D., Schifani, E., Reggiani, R., Mazzoni, E., Reguzzi, M. C., Castracani, C., Spotti, F. A., Giardina, B., Mori, A., & Grasso, D. A. (2022). Do It by Yourself: Larval Locomotion in the Black Soldier Fly Hermetia illucens, with a Novel “Self-Harvesting” Method to Separate Prepupae. Insects, 13(2), 127. https://doi.org/10.3390/insects13020127








