Male Accessory Glands of Blister Beetles and Cantharidin Release: A Comparative Ultrastructural Analysis
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Collection of Specimens
2.2. Light Microscopy
2.3. Focused Ion Beam/Scanning Electron Microscopy (FIB/SEM)
3. Results
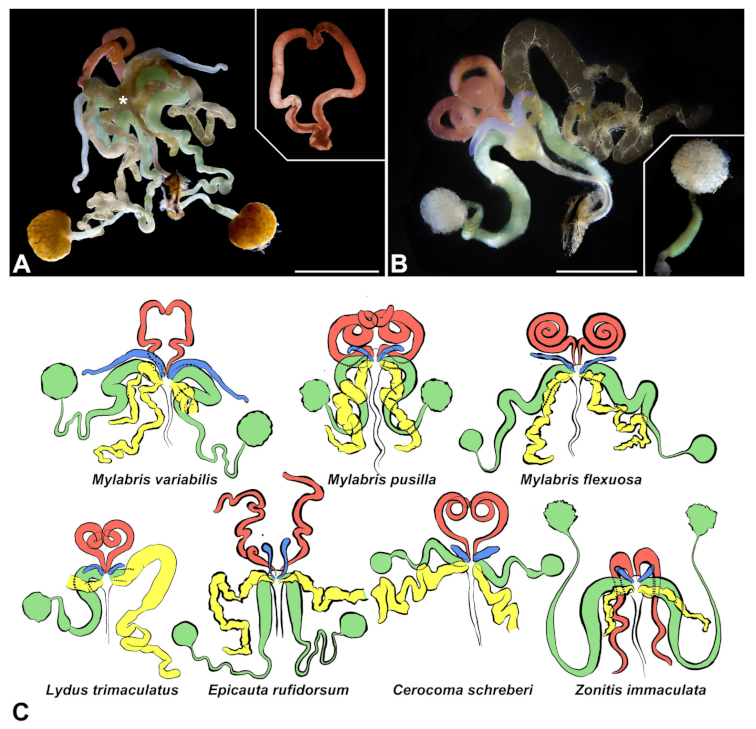
3.1. Gross Morphology of Male Accessory Glands and Vasa Deferentia
3.2. Ultrastructural Features of Accessory Glands and Vasa Deferentia in Mylabris (Meloinae, Mylabrini)
- -
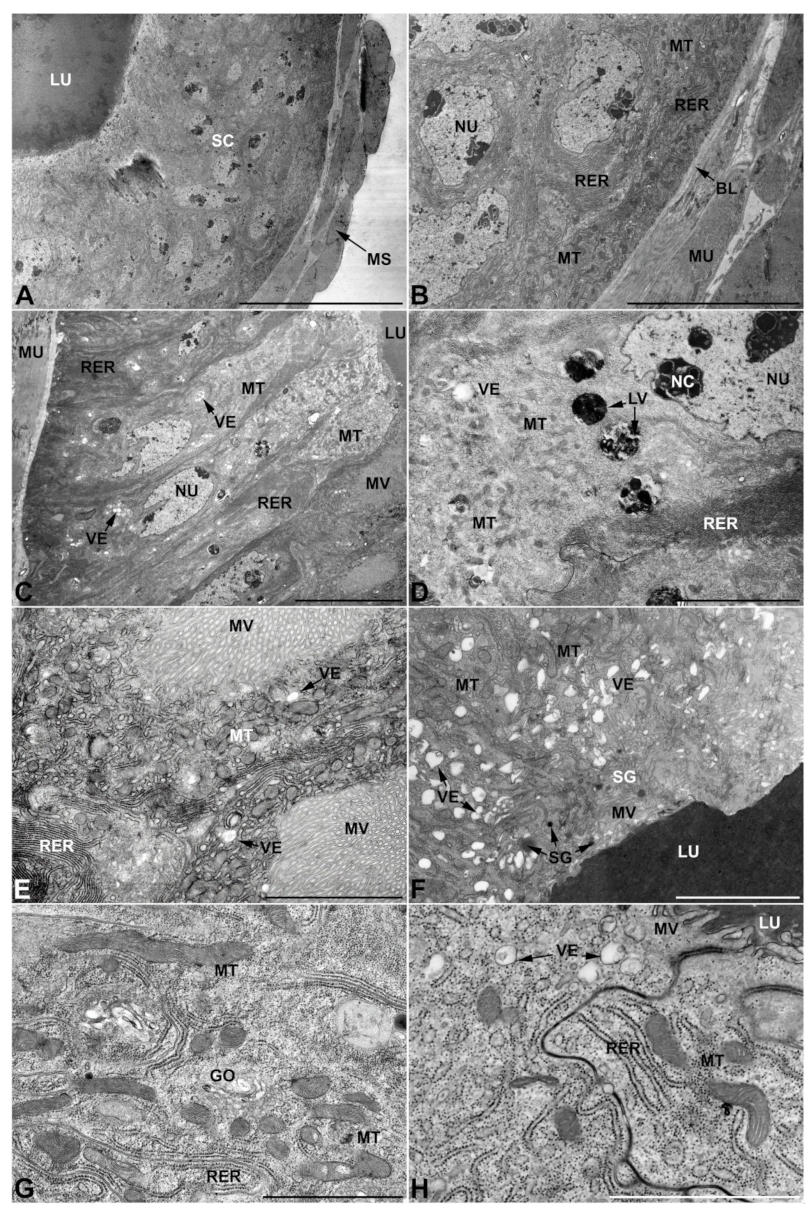
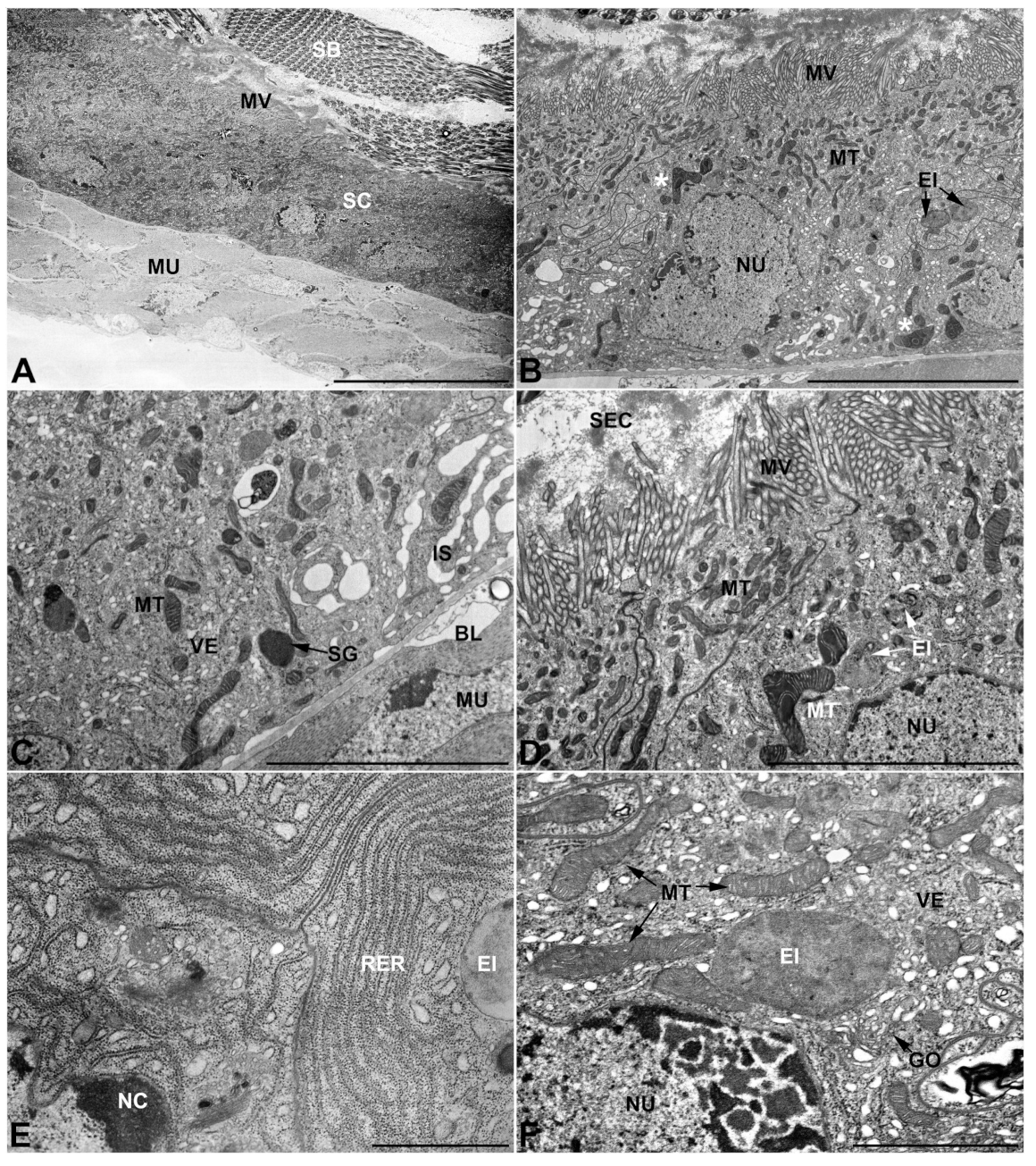
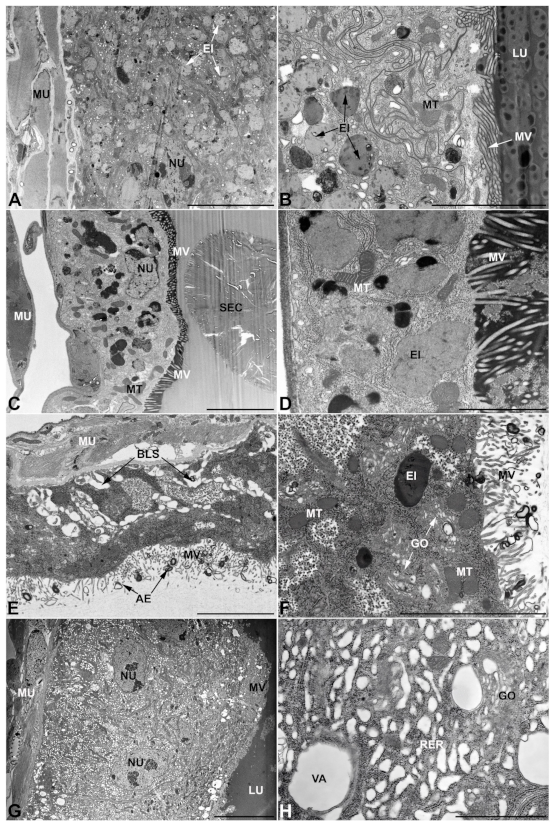
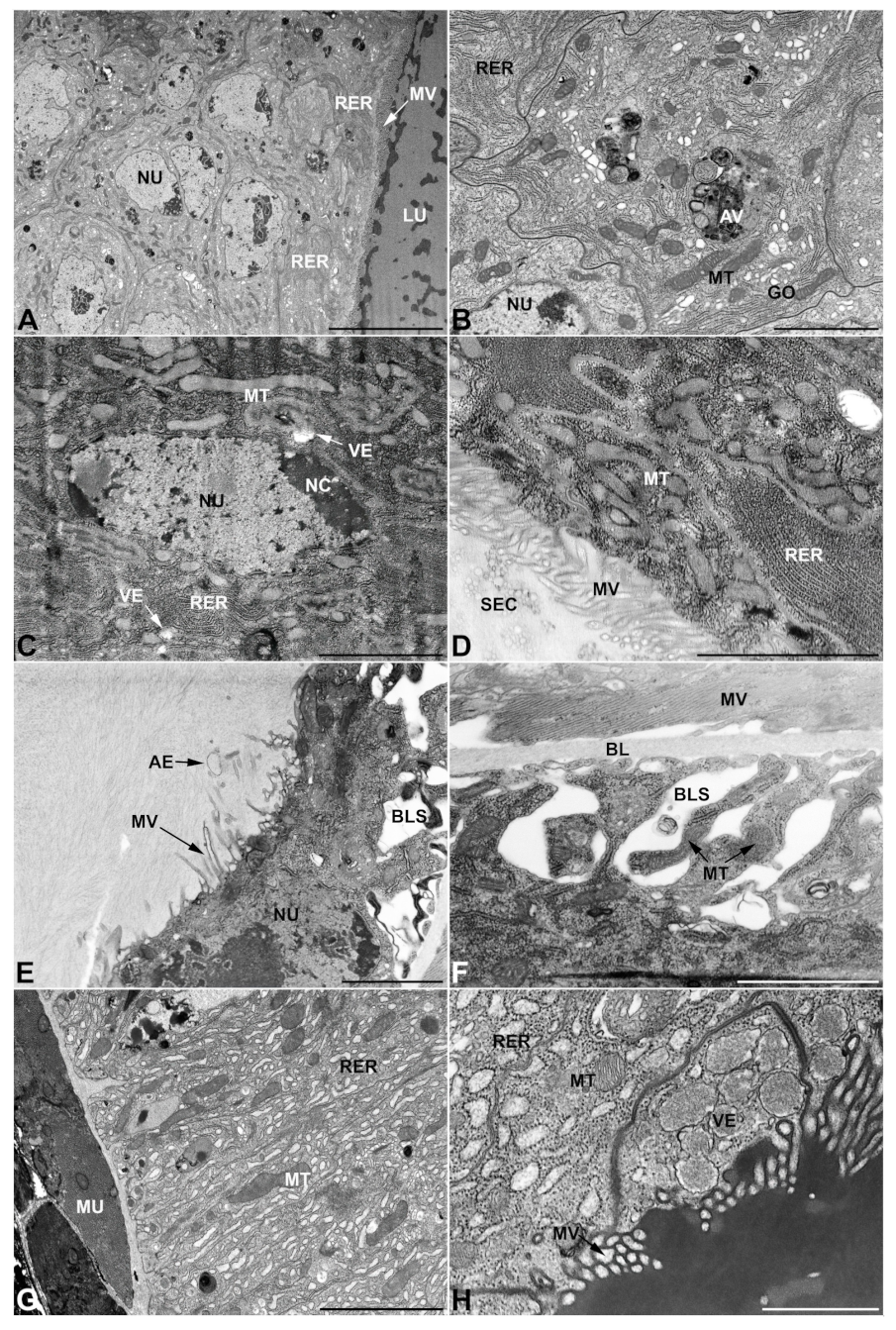
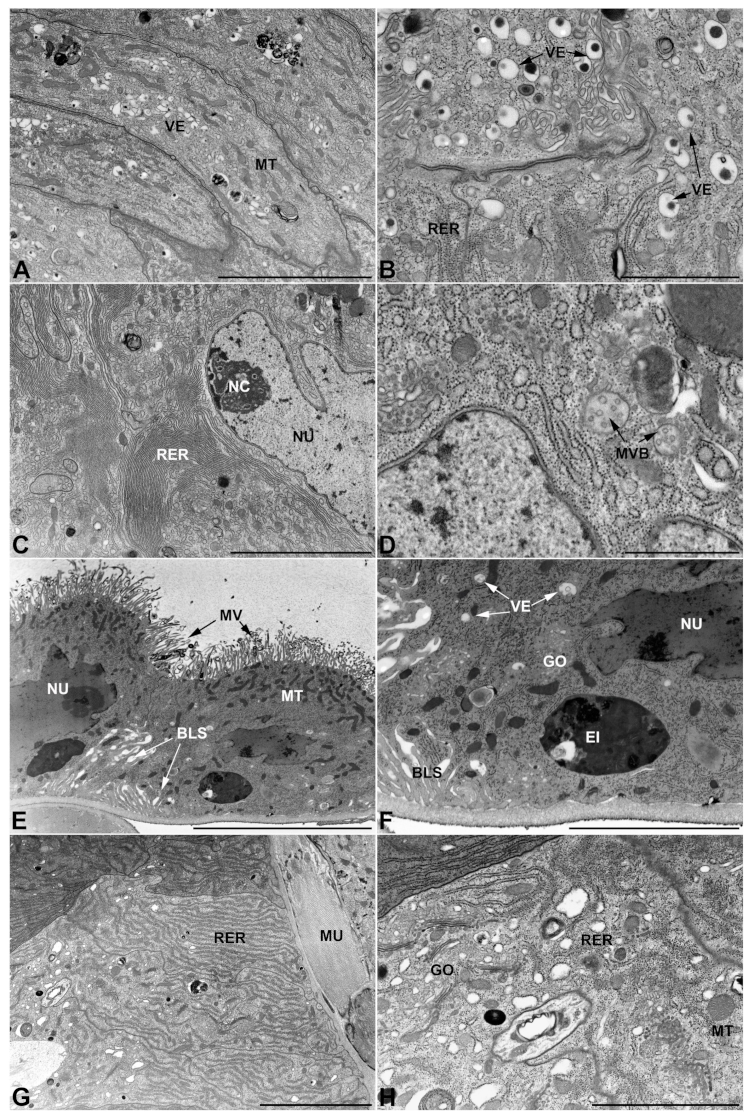
- First pair of male accessory glands in M. variabilis. The first pair of male accessory has an outer muscular layer composed of an inner circular and outer longitudinal fibers enclosing a monolayered epithelium (Figure 3A–C). This latter circumscribes a large lumen filled with a homogeneous electrondense matrix in which irregularly shaped structures of higher electron density are immersed (Figure 3A). The mononucleated epithelial cells are about 35 μm high and lie on a basal lamina 0.4–0.6 μm thick (Figure 3B,C); they are tightly appressed to each other with a straight course along their medial regions (Figure 3C) and more sinuous contours towards the apex and base (Figure 3B–D). The apical plasma membrane is always characterized by the presence of many partially overlapping and crowded microvilli that face the glandular lumen (Figure 3E,F). The ovoid nucleus, approximately 7–10 μm in size, frequently shows indentations and one or two prominent nucleoli (Figure 3B–D). It is usually located in the basal region of the cell (Figure 3A,B), however, due to the pseudostratified organization of this epithelium, it may occupy medial or more apical positions, pushing towards the glandular lumen (Figure 3A,D). The rough endoplasmic reticulum is widely disseminated throughout the cytoplasm in the form of densely packed, parallel-arranged cisternae, and is most commonly found at the base of the cell and in the perinuclear region (Figure 3B–E). Golgi systems are relatively small and uncommon while many thin and slender mitochondria are evenly distributed throughout the cytoplasm (Figure 3B–H) that is also characterized by the presence of several vesicles. Larger ones, containing irregular and electrondense material, are located medially in the cells (Figure 3D); in contrast, the smaller and more common electronlucid vesicles are mostly located in the apical region (Figure 3E,F,H), near the dense array of packed microvilli. Rare electrondense granules appear nearby and scattered between some of the microvilli (Figure 3F).
- -
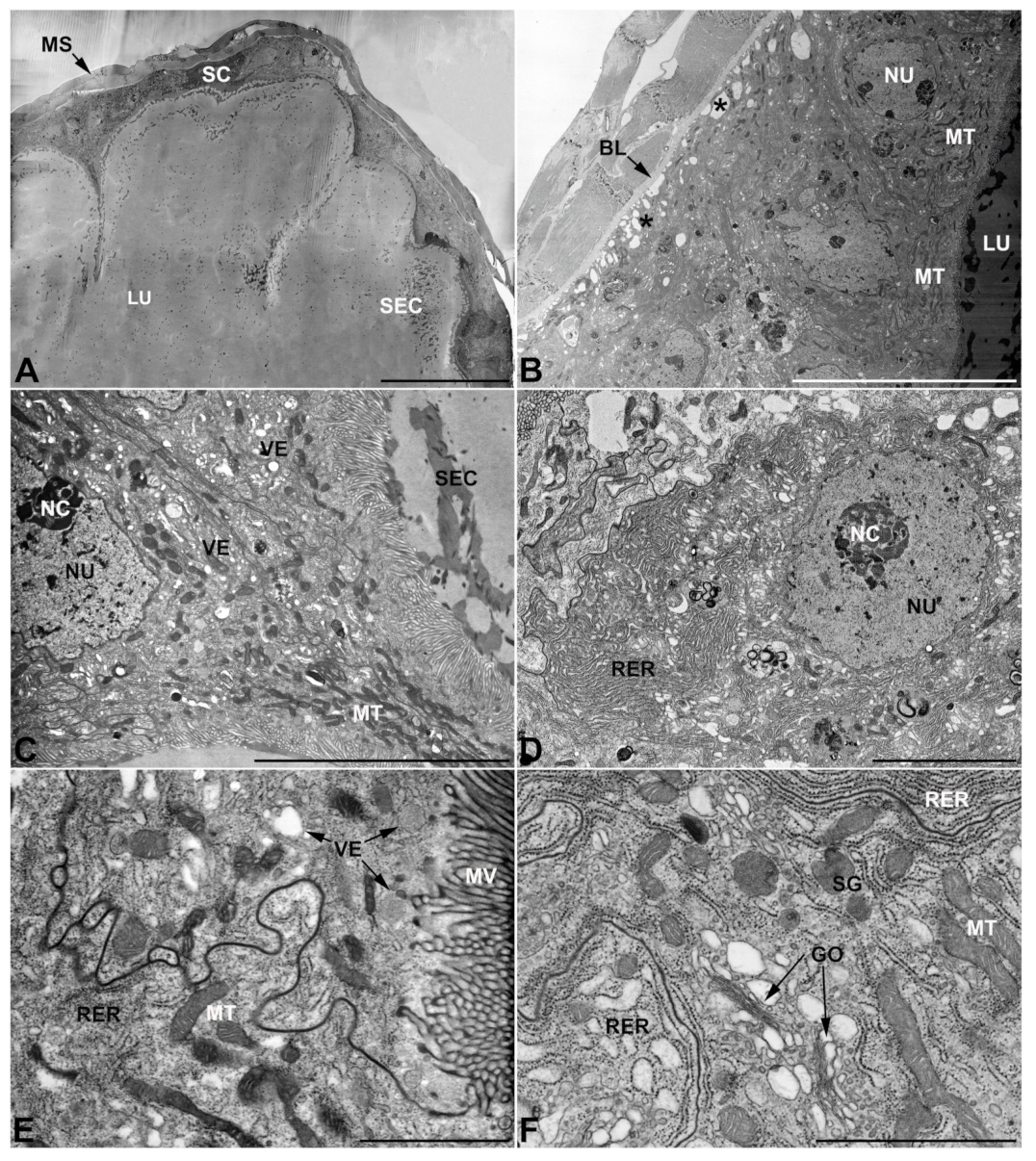
- Second pair of male accessory glands in M. variabilis. The second pair of male accessory glands has an outer muscular layer, made up of circular muscles on the inside and longitudinal outside, which encloses a glandular epithelium (Figure 4A,B). The columnar epithelial cells, roughly 25–30 μm high, are organized as a monolayer that exhibits pleats and ensuing involutions towards a large lumen (Figure 4A,C) that receives the secretory products appearing as dark amorphous bodies immersed in a lighter homogeneous matrix (Figure 4A–C). Contiguous cells are usually tightly adherent to each other and appressed to the basal lamina, however, in some regions of the epithelium they are partially detached from a 0.6–0.8 μm thick basal lamina, showing moderate folds and intercellular spaces in the baso-lateral plasma membrane (Figure 4B). A single ovoid nucleus of about 10 μm, with no indentations or lobes, is placed towards the center of the cell and is characterized by the presence of an evident central or eccentric nucleolus and sparse heterochromatin (Figure 4B–D). The rough endoplasmic reticulum is well-developed (Figure 4D–E) and located mostly near the nucleus, primarily in the form of parallel and flattened cisternae and, to a lesser extent, also in a vesiculate form with expanded cisternae (Figure 4F). Thin mitochondria (Figure 4C,E,F) are abundantly distributed throughout the cell, although they are more prevalent towards the apical region where stacks of long microvilli arise from the apical plasma membrane (Figure 4B,C,E). Several small vesicles with electronlucent contents and a few slightly electrondense secretory granules are disseminated throughout the cytoplasm (Figure 4C,E,F), while small and flattened Golgi complexes are rarely observed in these cells (Figure 4F).
- -
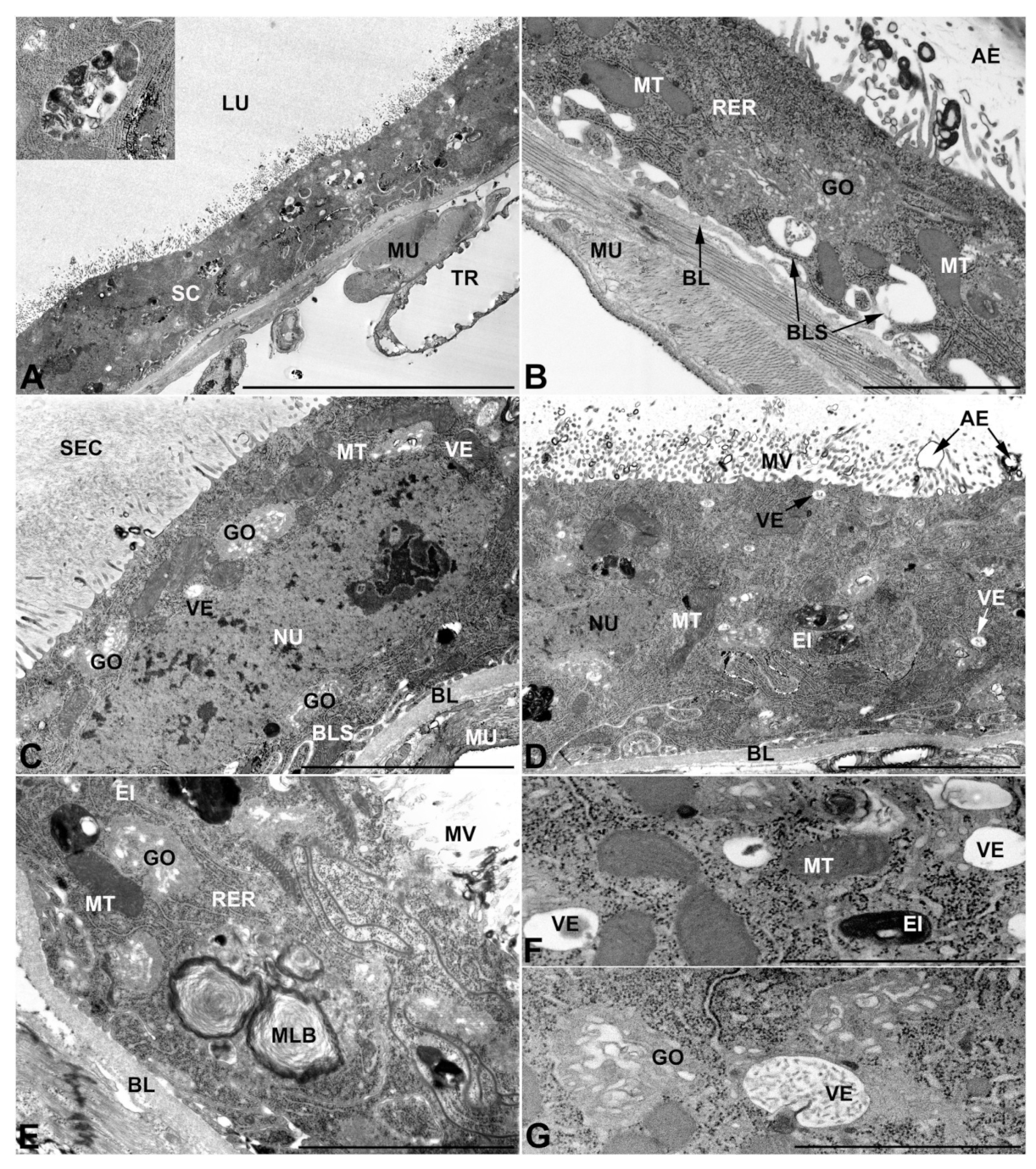
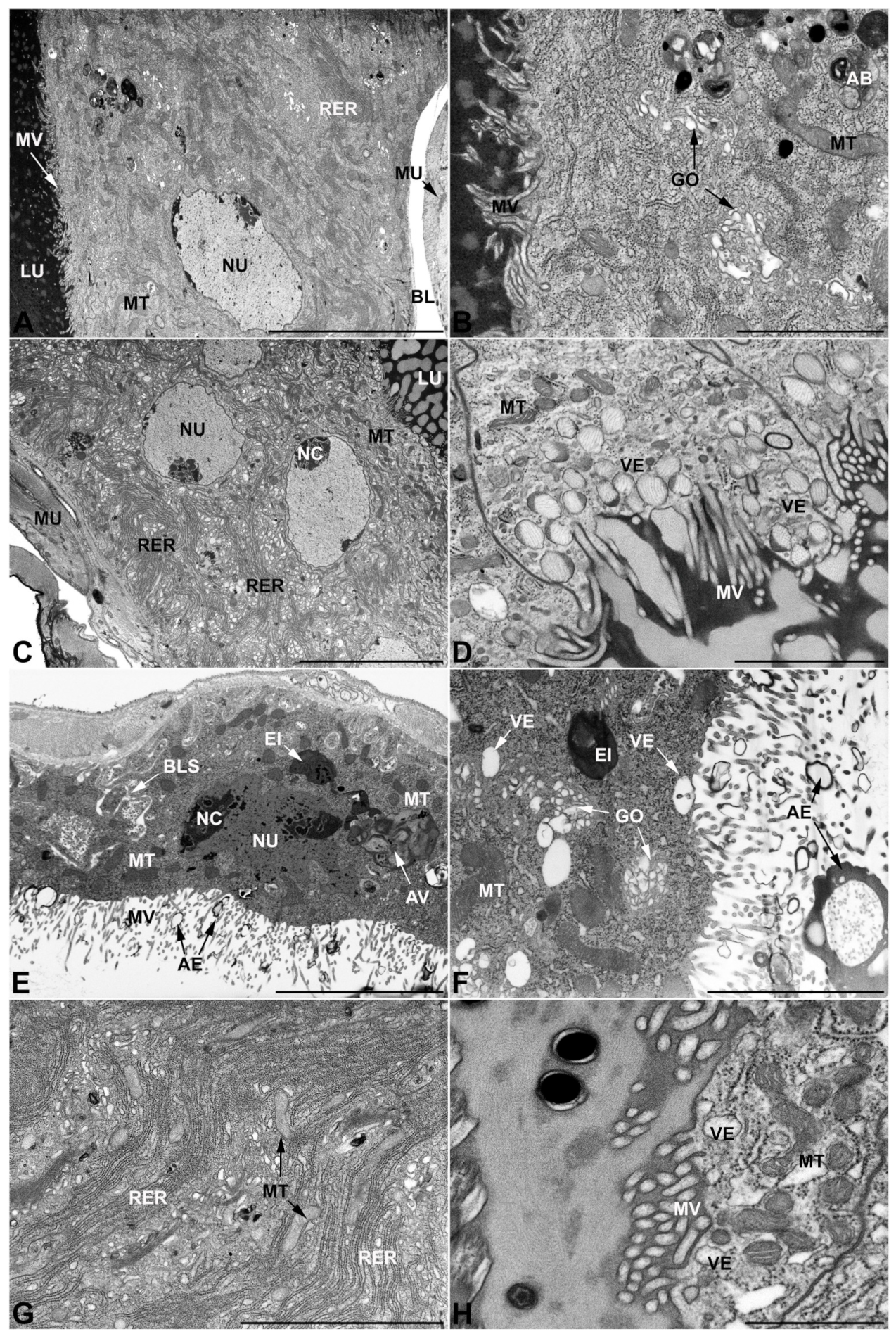
- Third pair of male accessory glands in M. variabilis. The third pair of male accessory glands is composed of a comparatively thin layer of circularly and transversely oriented muscles, gently surrounding a monolayer epithelium that delimits a large lumen filled with a homogeneous secretion that appears as slightly electrondense fibrillar structures immersed in an almost lucid matrix (Figure 5A). The glandular cells, 5–8 μm high, are flattened, squamous or barely cuboidal and lay on a basal lamina 0.4–0.6 μm thick (Figure 5A–E). Their basal membrane is usually highly folded and only partially attached to the basal lamina, creating a developed labyrinthine network of intercellular spaces (Figure 5B,C). A basal and oval nucleus, 10–12 μm long, contains a large nucleolus and heterochromatin aggregates, and is surrounded by flattened stacks of rough endoplasmic reticulum and abundant Golgi complexes featuring small cisternae that are widespread in both the basal and apical regions of the cells (Figure 5C–E). The cytoplasm abounds in mitochondria and contains several small electronlucid vesicles, some of which contain a fine flocculent particulate (Figure 5B–F). A few electrondense secretory granules and rare multilamellar bodies are found basally in the cell (Figure 5D,E). Autophagic vacuoles containing irregular electrondense inclusions are also frequently present in the cytoplasm (Figure 5A, inset). Apically, the plasma membrane develops into widely spaced, long microvilli, some of which show a characteristic irregular, ampullaceous expansion with marked dark edges (Figure 5B,D).
- -
- Vasa deferentia in M. variabilis. The glandular regions of the vasa deferentia have an outer tunica of developed striated muscle fibers that encloses a monolayered epithelium delimiting a central lumen that contains fibrillar secretions and spermatozoa bundles (Figure 6A).
- -
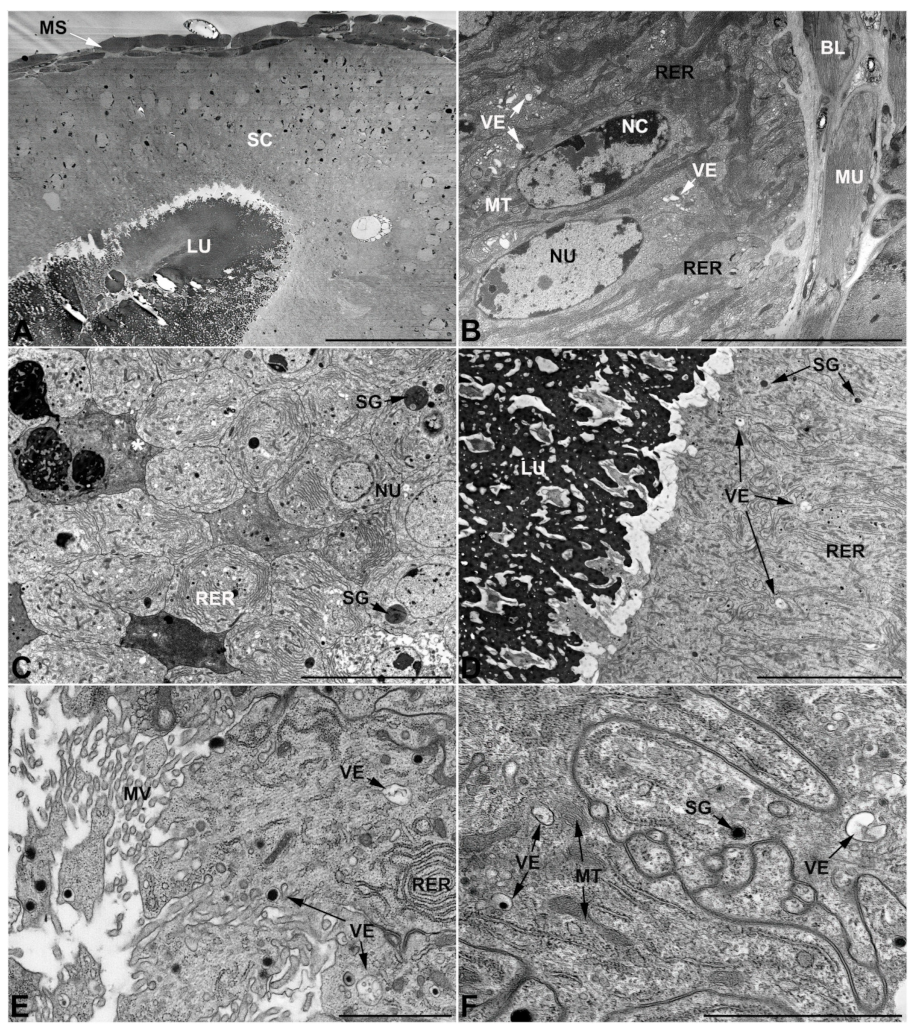
- Accessory glands and vasa deferentia in M. flexuosa and M. pusilla. In the two smaller congeneric species, M. pusilla and M. flexuosa, the cellular arrangement of the first pair of accessory glands appears similar to that observed in M. variabilis. Minor differences seem to be related to the smaller size of the gland, which present cells of diminished size (roughly 25–30 μm tall) with a reduced degree of pseudostratification (Figure 7A). The contents of the lumen are very similar in appearance (Figure 7A), as are the ultrastructural features of the cells, displaying abundant flattened endoplasmic reticulum, elongated mitochondria scattered throughout the cytoplasm, flattened Golgi complexes, minute lucent vesicles and larger vacuolar bodies containing dense structures (Figure 7A,B).
3.3. Ultrastructure of Accessory Glands and Vasa Deferentia in Lydus (Meloinae, Lyttini)
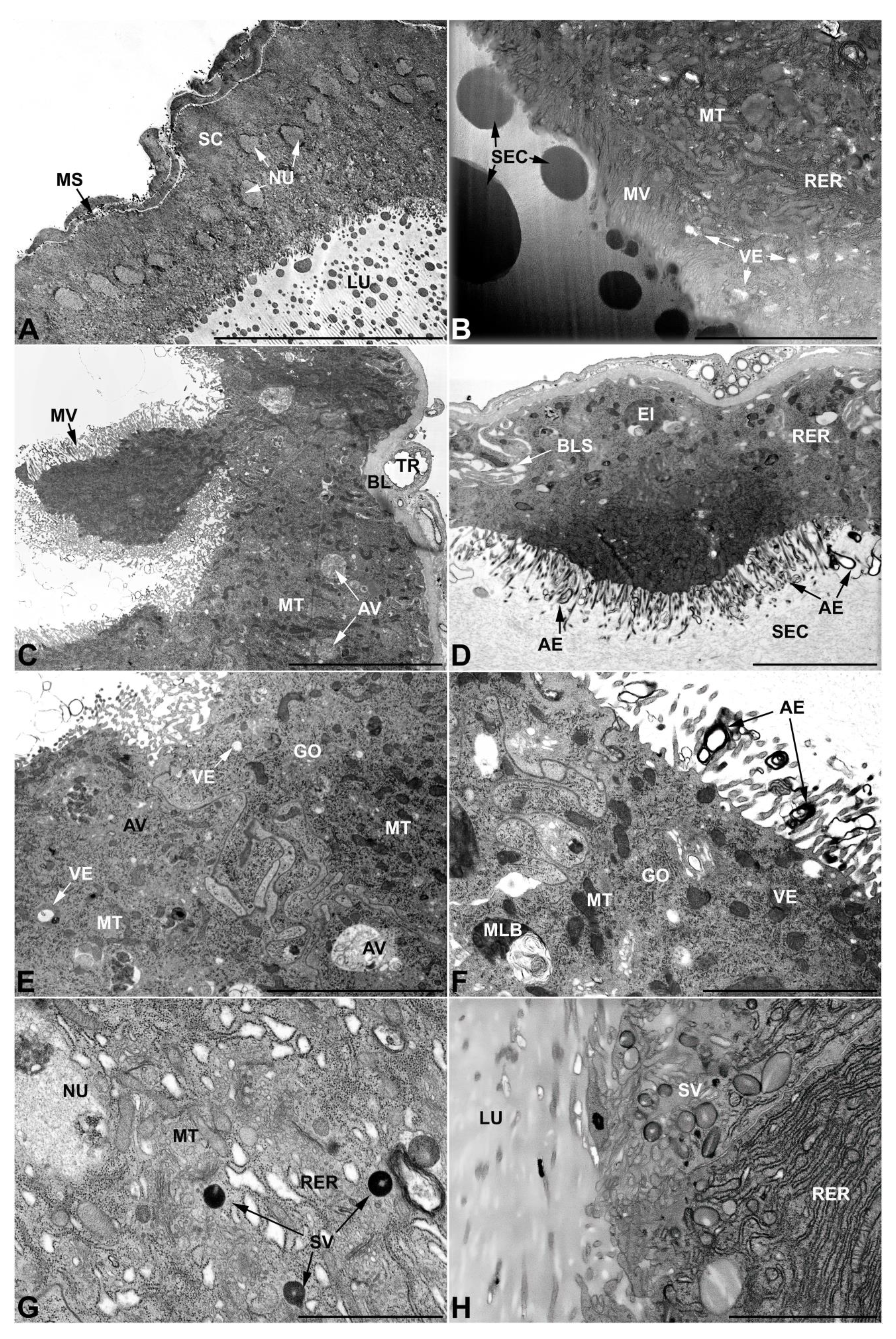
3.4. Ultrastructure of Accessory Glands and Vasa Deferentia in Epicauta (Meloinae, Epicautini)
3.5. Ultrastructure of Accessory Glands and Vasa Deferentia in Cerocoma (Meloinae, Cerocomini)
3.6. Ultrastructure of Accessory Glands and Vasa Deferentia in Zonitis (Nemognatinae, Nemognatini)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto, J.D.; Bologna, M.A. The New World Genera of Meloidae (Coleoptera): A key and Synopsis. J. Nat. Hist. 1999, 33, 569–619. [Google Scholar] [CrossRef]
- Bologna, M.A.; Pinto, J.D. The Old World Genera of Meloidae (Coleoptera): A key and Synopsis. J. Nat. Hist. 2002, 36, 2013–2102. [Google Scholar] [CrossRef]
- Bologna, M.A.; Turco, F.; Pinto, J.D. The Meloidae (Coleoptera) of Australasia: A Generic Review, Descriptions of new Taxa, and a Challenge to the Current Definition of Subfamilies Posed by Exceptional Variation in Male Genitalia. Invertebr. Syst. 2013, 27, 391–427. [Google Scholar] [CrossRef]
- Bologna, M.A.; Oliverio, M.; Pitzalis, M.; Mariottini, P. Phylogeny and Evolutionary History of the Blister Beetles (Coleoptera, Meloidae). Mol. Phylogenet. Evol. 2008, 48, 679–693. [Google Scholar] [CrossRef]
- Bologna, M.A.; D’Inzillo, B.; Cervelli, M.; Oliverio, M.; Mariottini, P. Molecular Phylogenetic Studies of the Mylabrini Blister Beetles (Coleoptera, Meloidae). Mol. Phylogenet. Evol. 2005, 37, 306–311. [Google Scholar] [CrossRef]
- Bologna, M.A.; Turco, F.; Pinto, J.D. 11.19. Meloidae Gyllenhal, 1810. In Volume 2 Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia Partim); Leschen, R.A.B., Beutel, R.G., Lawrence, J.F., Eds.; De Gruyter: Berlin, NY, USA, 2011; pp. 681–693. ISBN 3-11-091121-3. [Google Scholar]
- Bologna, M.A.; Pinto, J.D. Phylogenetic Studies of Meloidae (Coleoptera), with Emphasis on the Evolution of Phoresy. Syst. Entomol. 2001, 26, 33–72. [Google Scholar] [CrossRef]
- Bologna, M.A.; Di Giulio, A. Biological and Morphological Adaptations in the Pre-Imaginal Phases of the Beetle Family Meloidae. Atti Accad. Naz. Ital. Di Entomol. 2011, 59, 141–152. [Google Scholar]
- Selander, R.B.; Mathieu, J.M. The Ontogeny of Blister Beetles (Coleoptera, Meloidae) I. A Study of Three Species of the Genus Pyrota. Ann. Entomol. Soc. Am. 1964, 57, 711–732. [Google Scholar] [CrossRef]
- Pinto, J.D. Hypermetamorphosis. In Encyclopedia of Insects; Resh, V.H., Carde, R.T., Eds.; Academic Press: New York, NY, USA, 2009; pp. 484–486. [Google Scholar]
- Carrel, J.E.; McCairel, M.H.; Slagle, A.J.; Doom, J.P.; Brill, J.; McCormick, J.P. Cantharidin Production in a Blister Beetle. Experientia 1993, 49, 171–174. [Google Scholar] [CrossRef]
- Nakatani, T.; Konishi, T.; Miyahara, K.; Noda, N. Three Novel Cantharidin-Related Compounds from the Chinese Blister Beetle, Mylabris phalerata PALL. Chem. Pharm. Bull. 2004, 52, 807–809. [Google Scholar] [CrossRef]
- Bravo, C.; Mas-Peinado, P.; Bautista, L.M.; Blanco, G.; Alonso, J.C.; García-París, M. Cantharidin Is Conserved across Phylogeographic Lineages and Present in Both Morphs of Iberian Berberomeloe Blister Beetles (Coleoptera, Meloidae). Zool. J. Linn. Soc. 2017, 180, 790–804. [Google Scholar] [CrossRef]
- Gisondi, S.; Gasperi, T.; Roma, E.; Tomai, P.; Gentili, A.; Vignoli, L.; Bologna, M.A.; Mancini, E. Cantharidin Content in Two Mediterranean Species of Blister Beetles, Lydus trimaculatus and Mylabris variabilis (Coleoptera: Meloidae). Entomol. Sci. 2019, 22, 258–263. [Google Scholar] [CrossRef]
- Carrel, J.E.; Eisner, T. Cantharidin: Potent Feeding Deterrent to Insects. Science 1974, 183, 755–757. [Google Scholar] [CrossRef]
- Smedley, S.R.; Blankespoor, C.L.; Yuang, Y.; Carrel, J.E.; Eisner, T. Predatory Response of Spiders to Blister Beetles (Family Meloidae). Zoology 1995, 99, 211–217. [Google Scholar]
- Carrel, J.E.; Doom, J.P.; McCormick, J.P. Identification of Cantharidin in False Blister Beetles (Coleoptera, Oedemeridae) from Florida. J. Chem. Ecol. 1986, 12, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, M.; Dettner, K. Quantification of Cantharidin in Canthariphilous Ceratopogonidae (Diptera), Anthomyiidae (Diptera) and Cantharidin-Producing Oedemeridae (Coleoptera). J. Chem. Ecol. 1994, 20, 1795–1812. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-S. Medical Uses of Mylabris in Ancient China and Recent Studies. J. Ethnopharmacol. 1989, 26, 147–162. [Google Scholar] [CrossRef]
- Cutler, H.G. An Historical Perspective of Ancient Poisons. In Phytochemical Resources for Medicine and Agriculture; Nigg, H.N., Seigler, D., Eds.; Springer: Boston, MA, USA, 1992; pp. 1–13. ISBN 978-1-4899-2586-2. [Google Scholar]
- Moed, L.; Shwayder, T.A.; Chang, M.W. Cantharidin Revisited: A Blistering Defense of an Ancient Medicine. Arch. Dermatol. 2001, 137, 1357–1360. [Google Scholar] [CrossRef]
- Beauregard, H. Les Insectes Vésicants; F. Alcan: Paris, France, 1890. [Google Scholar]
- Bologna, M.A. Coleoptera Meloidae; Fauna d’Italia XXVII; Calderini: Bologna, Italy, 1991. [Google Scholar]
- Vakharia, P.P.; Chopra, R.; Silverberg, N.B.; Silverberg, J.I. Efficacy and Safety of Topical Cantharidin Treatment for Molluscum contagiosum and Warts: A Systematic Review. Am. J. Clin. Dermatol. 2018, 19, 791–803. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z. The Effects of Cantharidin and Cantharidin Derivates on Tumour Cells. Anti Cancer Agents Med. Chem. 2009, 9, 392–396. [Google Scholar] [CrossRef]
- Verma, A.K.; Prasad, S.B. Bioactive Component, Cantharidin from Mylabris cichorii and Its Antitumor Activity against Ehrlich Ascites Carcinoma. Cell Biol. Toxicol. 2012, 28, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.P.; Dong, J.; Cai, H.; Wang, W. Cantharidin as an Antitumor Agent: A Retrospective Review. Curr. Med. Chem. 2013, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Puerto Galvis, C.E.; Vargas Mendez, L.Y.; Kouznetsov, V.V. Cantharidin-based Small Molecules as Potential Therapeutic Agents. Chem. Biol. Drug Des. 2013, 82, 477–499. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.; Wu, Y.; Zhang, N.; Yang, Z.; Yu, C. Anticancer Attributes of Cantharidin: Involved Molecular Mechanisms and Pathways. Molecules 2020, 25, 3279. [Google Scholar] [CrossRef] [PubMed]
- Capinera, J.L.; Gardner, D.R.; Stermitz, F.R. Cantharidin Levels in Blister Beetles (Coleoptera: Meloidae) Associated with Alfalfa in Colorado. J. Econ. Entomol. 1985, 78, 1052–1055. [Google Scholar] [CrossRef]
- Blodgett, S.L.; Carrel, J.E.; Higgins, R.A. Cantharidin Content of Blister Beetles (Coleoptera: Meloidae) Collected from Kansas Alfalfa and Implications for Inducing Cantharidiasis. Environ. Entomol. 1991, 20, 776–780. [Google Scholar] [CrossRef]
- Nikbakhtzadeh, M.R.; Tirgari, S. Cantharidin Component of Iranian Blister Beetles (Col: Meloidae) and Their Differences between Iranian and Exotic Species. Iran. J. Public Health 2002, 31, 113–117. [Google Scholar]
- Mebs, D.; Pogoda, W.; Schneider, M.; Kauert, G. Cantharidin and Demethylcantharidin (Palasonin) Content of Blister Beetles (Coleoptera: Meloidae) from Southern Africa. Toxicon 2009, 53, 466–468. [Google Scholar] [CrossRef]
- Sierra, J.R.; Woggon, W.D.; Schmid, H. Transfer of Cantharidin (1) during Copulation from the Adult Male to the Female Lytta vesicatoria (‘Spanish Flies’). Experientia 1976, 32, 142–144. [Google Scholar] [CrossRef]
- Carrel, J.E.; Doom, J.P.; McCormick, J.P. Cantharidin Biosynthesis in a Blister Beetle: Inhibition by 6-Fluoromevalonate Causes Chemical Disarmament. Experientia 1986, 42, 853–854. [Google Scholar] [CrossRef]
- Nikbakhtzadeh, M.R.; Dettner, K.; Boland, W.; Gäde, G.; Dötterl, S. Intraspecific Transfer of Cantharidin within Selected Members of the Family Meloidae (Insecta: Coleoptera). J. Insect Physiol. 2007, 53, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Selander, R.B. Sexual Behavior in Blister Beetles (Coleoptera: Meloidae): I. The Genus Pyrota. Can. Entomol. 1964, 96, 1037–1082. [Google Scholar] [CrossRef]
- Dettner, K. Inter and Intraspecific Transfer of Toxic Insect Compound Cantharidin. In Vertical Food Web Interactions; Dettner, K., Bauer, G., Völkl, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 115–145. [Google Scholar]
- Nikbakhtzadeh, M.R.; Vahedi, M.; Vatandoost, H.; Mehdinia, A. Origin, Transfer and Distribution of Cantharidin-Related Compounds in the Blister Beetle Hycleus scabiosae. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 88–96. [Google Scholar] [CrossRef]
- Jiang, M.; Lü, S.; Zhang, Y. The Potential Organ Involved in Cantharidin Biosynthesis in Epicauta chinensis Laporte (Coleoptera: Meloidae). J. Insect Sci. 2017, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Leidy, J. ART. IV.--On the Seat of the Vesicating Principle of Lytta vittata. Am. J. Med. Sci. (1827–1924) 1860, 39, 60. [Google Scholar] [CrossRef]
- Meyer, D.; Schlatter, C.; Schlatter-Lanz, I.; Schmid, H.; Bovey, P. Die Zucht von Lytta vesicatoria im Laboratorium und Nachweis der Cantharidinsynthese in Larven. Experientia 1968, 24, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, C.; Waldner, E.E.; Schmid, H. Zur Biosynthese Des Cantharidins. I. Experientia 1968, 24, 994–995. [Google Scholar] [CrossRef]
- Zha, S.; Yin, Y.; Wang, Y.; Huang, Y.; Li, Y.; Wang, Z. Cloning and Functional Analysis of Farnesyl Pyrophosphate Synthase (FPPS) Gene from Mylabris cichorii. Biotechnol. Appl. Biochem. 2017, 64, 667–676. [Google Scholar] [CrossRef]
- Jiang, M.; Lü, S.-M.; Qi, Z.-Y.; Zhang, Y.-L. Characterized Cantharidin Distribution and Related Gene Expression Patterns in Tissues of Blister Beetles, Epicauta chinensis. Insect Sci. 2019, 26, 240–250. [Google Scholar] [CrossRef]
- McCormick, J.P.; Carrel, J.E. Cantharidin Biosynthesis and Function in Meloid Beetles. In Pheromone Biochemistry; Prestwich, G.D., Blomquist, G.J., Eds.; Academic Press: Orlando, FL, USA, 1987; pp. 307–350. [Google Scholar]
- Gupta, A.P. The Digestive and Reproductive Systems of the Meloidae (Coleoptera) and their Significance in the Classification of the Family. Ann. Entomol. Soc. Am. 1965, 58, 442–474. [Google Scholar] [CrossRef]
- Gupta, A.P. Further Studies on the Internal Anatomy of the Meloidae (Coleoptera). I. The Digestive and Reproductive Systems of Rusadiria, Oenas, Lagorina, Sitaris, and Zonitis. Ann. Entomol. Soc. Am. 1966, 59, 751–758. [Google Scholar] [CrossRef]
- Gupta, A.P. Further Studies on the Internal Anatomy of the Meloidae (Coleoptera). II. The Digestive and Reproductive Systems of the S.A. Blister Beetle, Picnoseus nitidipennis Fairmaire and Germain (Coleoptera: Meloidae). J. N. Y. Entomol. Soc. 1966, 74, 80–83. [Google Scholar]
- Gupta, A.P. Further Studies on the Internal Anatomy of the Meloidae. III. The Digestive and Reproductive Systems as Bases for Tribal Designation of Pseudomeloe miniaceomaculata (Blanchard)(Coleoptera: Meloidae). J. N. Y. Entomol. Soc. 1967, 75, 93–99. [Google Scholar]
- Gerber, G.H.; Church, N.S.; Rempel, J.G. The Anatomy, Histology, and Physiology of the Reproductive Systems of Lytta nuttalli Say (Coleoptera: Meloidae). I. The Internal Genitalia. Can. J. Zool. 1971, 49, 523–533. [Google Scholar] [CrossRef]
- Gerber, G.H.; Church, N.S.; Rempel, J.G. The Structure, Formation, Histochemistry, Fate, and Functions of the Spermatophore of Lytta nuttalli Say (Coleoptera: Meloidae). Can. J. Zool. 1971, 49, 1595–1610. [Google Scholar] [CrossRef]
- Muzzi, M.; Di Giulio, A.; Mancini, E.; Fratini, E.; Cervelli, M.; Gasperi, T.; Mariottini, P.; Persichini, T.; Bologna, M.A. The Male Reproductive Accessory Glands of the Blister Beetle Meloe proscarabaeus Linnaeus, 1758 (Coleoptera: Meloidae): Anatomy and Ultrastructure of the Cantharidin-Storing Organs. Arthropod Struct. Dev. 2020, 59, 100980. [Google Scholar] [CrossRef]
- Di Giulio, A.; Muzzi, M. Two Novel Approaches to Study Arthropod Anatomy by Using Dualbeam FIB/SEM. Micron 2018, 106, 21–26. [Google Scholar] [CrossRef]
- Bugnion, E. Le “Cissites testaceus” Fabr. des Indes et de Ceylan: Métamorphoses, appareil génital. Bull. De La Société Entomol. D’Egypte 1910, 2, 182–200, 3 pls. [Google Scholar]
- Li, C. External Morphology and Internal Anatomy of the Blister Beetle Mylabris phalerata Pall. In Essays and Papers in Memory of Late President Fu Ssu-Nien; National Taiwan University: Taipei, Taiwan, 1952; pp. 505–554. [Google Scholar]
- De Loof, A.; Lagasse, A. The Ultrastructure of the Male Accessory Reproductive Glands of the Colorado Beetle. Z. Für Zellforsch. Und Mikrosk. Anat. 1972, 130, 545–552. [Google Scholar] [CrossRef]
- Gadzama, N.M.; Happ, C.M.; Happ, G.M. Cytodifferentiation in the Accessory Glands of Tenebrio molitor. I. Ultrastructure of the Tubular Gland in the Post-ecdysial Adult Male. J. Exp. Zool. 1977, 200, 211–221. [Google Scholar] [CrossRef]
- Cassier, P.; Huignard, J. Étude ultrastructurale des glandes annexes de l’appareil génitale male chez Acanthoscelides obtectus Say (Coleoptera: Bruchidae). Int. J. Insect Morphol. Embryol. 1979, 8, 183–201. [Google Scholar] [CrossRef]
- Davey, K.G. The Male Reproductive Tract. In Comprehensive Insect Physiology Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 1–14. [Google Scholar]
- Gillott, C. Male Accessory Gland Secretions: Modulators of Female Reproductive Physiology and Behavior. Annu. Rev. Entomol. 2003, 48, 163–184. [Google Scholar] [CrossRef]
- Tongu, Y.; Suguri, S.; Sakumoto, D.; Itano, K.; Inatomi, S. The Ultrastructure of Mosquitoes: 6. Male Accessory Gland of Culex pipiens pallens. Med. Entomol. Zool. 1972, 23, 129–139. [Google Scholar] [CrossRef]
- Ramalingam, S.; Craig, G.B., Jr. Fine Structure of the Male Accessory Glands in Aedes triseriatus. J. Insect Physiol. 1978, 24, 251–259. [Google Scholar] [CrossRef]
- Dailey, P.J.; Gadzama, N.M.; Happ, G.M. Cytodifferentiation in the Accessory Glands of Tenebrio molitor. VI. A Congruent Map of Cells and Their Secretions in the Layered Elastic Product of the Male Bean-shaped Gland. J. Morphol. 1980, 166, 289–322. [Google Scholar] [CrossRef]
- Chen, P.S. The Functional Morphology and Biochemistry of Insect Male Accessory Glands and Their Secretions. Annu. Rev. Entomol. 1984, 29, 233–255. [Google Scholar] [CrossRef]
- Krueger, S.; Ferenz, H.-J.; Randall, M.; Hodgson, A.N. Structure of the Male Reproductive Accessory Glands of Pterostichus nigrita (Coleoptera: Carabidae), Their Role in Spermatophore Formation. Invertebr. Reprod. Dev. 2014, 58, 75–88. [Google Scholar] [CrossRef]
- Fratini, E.; Salvemini, M.; Lombardo, F.; Muzzi, M.; Molfini, M.; Gisondi, S.; Roma, E.; D’Ezio, V.; Persichini, T.; Gasperi, T.; et al. Unraveling the Role of Male Reproductive Tract and Haemolymph in Cantharidin-Exuding Lydus trimaculatus and Mylabris variabilis (Coleoptera: Meloidae): A Comparative Transcriptomics Approach. BMC Genom. 2021, 22, 808. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzzi, M.; Mancini, E.; Fratini, E.; Cervelli, M.; Gasperi, T.; Mariottini, P.; Persichini, T.; Bologna, M.A.; Di Giulio, A. Male Accessory Glands of Blister Beetles and Cantharidin Release: A Comparative Ultrastructural Analysis. Insects 2022, 13, 132. https://doi.org/10.3390/insects13020132
Muzzi M, Mancini E, Fratini E, Cervelli M, Gasperi T, Mariottini P, Persichini T, Bologna MA, Di Giulio A. Male Accessory Glands of Blister Beetles and Cantharidin Release: A Comparative Ultrastructural Analysis. Insects. 2022; 13(2):132. https://doi.org/10.3390/insects13020132
Chicago/Turabian StyleMuzzi, Maurizio, Emiliano Mancini, Emiliano Fratini, Manuela Cervelli, Tecla Gasperi, Paolo Mariottini, Tiziana Persichini, Marco Alberto Bologna, and Andrea Di Giulio. 2022. "Male Accessory Glands of Blister Beetles and Cantharidin Release: A Comparative Ultrastructural Analysis" Insects 13, no. 2: 132. https://doi.org/10.3390/insects13020132
APA StyleMuzzi, M., Mancini, E., Fratini, E., Cervelli, M., Gasperi, T., Mariottini, P., Persichini, T., Bologna, M. A., & Di Giulio, A. (2022). Male Accessory Glands of Blister Beetles and Cantharidin Release: A Comparative Ultrastructural Analysis. Insects, 13(2), 132. https://doi.org/10.3390/insects13020132









