The Native Bees of Lolland (Denmark) Revisited after 100 Years: The Demise of the Specialists
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Collections
2.3. Feeding Specialization and Historic Pollen Samples
2.4. Status
3. Results
3.1. Collections
3.2. Feeding Specialization and Historic Pollen Samples
3.3. Status
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasmussen, C.; Engel, M.S.; Vereecken, N.J. A primer of host-plant specialization in bees. Emerg. Top. Life Sci. 2020, 4, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.H.; Sipes, S.D. Characterizing floral specialization by bees: Analytical methods and a revised lexicon for oligolecty. In Plant-Pollinator Interactions: From Specialization to Generalization; Waser, N.M., Ollerton, J., Eds.; University of Chicago Press: Chicago, IL, USA, 2006; pp. 99–122. [Google Scholar]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, A.L.; Youngsteadt, E.; Frank, S.D. Wild bee abundance declines with urban warming, regardless of floral density. Urban Ecosyst. 2018, 21, 419–428. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarli, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef]
- Zattara, E.E.; Aizen, M.A. Global bee decline. bioRxiv 2020, 869784. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [Green Version]
- Ollerton, J.; Erenler, H.; Edwards, M.; Crockett, R. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 2014, 346, 1360–1362. [Google Scholar] [CrossRef] [Green Version]
- Colla, S.R.; Gadallah, F.; Richardson, L.; Wagner, D.; Gall, L. Assessing declines of North American bumble bees (Bombus spp.) using museum specimens. Biodivers. Conserv. 2012, 21, 3585–3595. [Google Scholar] [CrossRef]
- Scheper, J.; Reemer, M.; van Kats, R.; Ozing, W.A.; van der Linden, G.T.J.; Schaminée, J.H.J.; Siepel, H.; Kleijn, D. Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in the Netherlands. Proc. Natl. Acad. Sci. USA 2014, 111, 17552–17557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathiasson, M.E.; Rehan, S.M. Wild bee declines linked to plant-pollinator network changes and plant species introductions. Insect Conserv. Divers. 2020, 13, 595–605. [Google Scholar] [CrossRef]
- Robertson, C. Flowers and Insects, Lists of Visitors of Four Hundred and Fifty-Three Flowers; Science Press Printing Company: Lancaster, PA, USA, 1929; p. 221. [Google Scholar]
- Marlin, J.C.; LaBerge, W.E. The native bee fauna of Carlinville, Illinois, revisited after 75 years: A case for persistence. Conserv. Ecol. 2001, 5, 9. Available online: http://www.consecol.org/vol5/iss1/art9 (accessed on 1 December 2021). [CrossRef] [Green Version]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef] [Green Version]
- Soroye, P.; Newbold, T.; Kerr, J. Climate change contributes to widespread declines among bumble bees across continents. Science 2020, 367, 685–688. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.M.J.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Didham, R.K.; Barbero, F.; Collins, C.M.; Forister, M.L.; Hassall, C.; Leather, S.R.; Packer, L.; Saunders, M.E.; Stewart, A.J.A. Spotlight on insects: Trends, threats and conservation challenges. Insect Conserv. Divers. 2020, 13, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Madsen, H.B. Bier. In Den Danske Rødliste 2019; Moeslund, J.E., Nygaard, B., Erjnæs, R., Bell, N., Bruun, L.D., Bygebjerg, R., Carl, H., Damgaard, J., Dylmer, E., Elmeros, M., et al., Eds.; Aarhus Universitet, DCE—Nationalt Center for Miljø og Energi: Aarhus, Denmark, 2019. [Google Scholar]
- Jørgensen, L. Bier. In Danmarks Fauna; GEC Gad: København, Denmark, 1921; Volume 25, p. 264. [Google Scholar]
- Jørgensen, L. Fortegnelse over Lolland-Falsters storsommerfugle (Macrolepidoptera). Flora Og Fauna 1913, 19, 87–96, 105–112, 136–144. [Google Scholar]
- Jørgensen, L. Fluer. Flora Og Fauna 1917, 23, 19–20. [Google Scholar]
- Jørgensen, L. Fortegnelse over Lolland-Falsters planvinger (Neuroptera-Planipennia). Flora Og Fauna 1914, 20, 113–114. [Google Scholar]
- Henriksen, K.L. Oversigt over dansk entomologis historie. Entomol. Medd. 1921–1937, 15, 1–578. [Google Scholar]
- Jørgensen, L. Biernes “trompeter”. Flora Og Fauna 1913, 19, 33–36. [Google Scholar]
- Jørgensen, L. Et par genfundne bier. Flora Og Fauna 1915, 21, 74–75. [Google Scholar]
- Jørgensen, L. Danske bier. Flora Og Fauna 1916, 22, 78–90, 129–144. [Google Scholar]
- Jørgensen, L. Humlebierne (Bombus). Flora Og Fauna 1917, 23, 30–32. [Google Scholar]
- Jørgensen, L. Danske bier. Flora Og Fauna 1917, 23, 94–96. [Google Scholar]
- Jørgensen, L. Danske bier. Flora Og Fauna 1918, 24, 117–123. [Google Scholar]
- Jørgensen, L. Danske bier. Flora Og Fauna 1919, 25, 24–28, 44–48. [Google Scholar]
- Jørgensen, L. De danske arter af slægten Nomada Scop. Entomol. Medd. 1919, 13, 40–72. [Google Scholar]
- Jørgensen, L. Smaa iagttagelser af nogle danske biers liv. Entomol. Medd. 1920, 13, 153–159. [Google Scholar]
- Jørgensen, P. Beobachtungen über Blumenbesuch, Biologie, Verbreitung usw. Der Bienen von Mendoza. (Hym.). Teil I. Dtsch. Entomol. Z. 1909, 1909, 53–65. [Google Scholar]
- Jørgensen, P. Beobachtungen über Blumenbesuch, Biologie, Verbreitung usw. Der Bienen von Mendoza. (Hym.). Teil II. Dtsch. Entomol. Z. 1909, 1909, 211–227. [Google Scholar]
- Jørgensen, P. Revision der Apiden der Provinz Mendoza, Republica Argentina (Hym.). Zool. Jahrbücher Abt. Für Syst. Geogr. Und Biol. Der Tiere 1912, 32, 89–162. [Google Scholar]
- Rasmussen, C.; Garcete-Barrett, B.R.; Gonçalves, R.B. Curt Schrottky (1874–1937): South American entomology at the beginning of the 20th century (Hymenoptera, Lepidoptera, Diptera). Zootaxa 2009, 2282, 1–50. [Google Scholar] [CrossRef]
- Scharling, M.; Cappelen, J. (Eds.) Klimadata Danmark Ver. 4 (Inkl. Landstal). Kommunale Og Landets Referenceværdier 2006–2015. Måneds-Og Årsværdier for Temperatur, Nedbør Og Solskin. Kommunernes Og Landets Generelle Vejr Og Klima. Klimadata Anvendt i ”Trap Danmark 6. Udgave”; Danish Meteorological Institute: Copenhagen, Denmark, 2017; p. 214. [Google Scholar]
- Enghoff, H.; Pedersen, J.; Thomsen, P.F.; Iversen, L. Tusindben, skolopendre og mejere fra Rødbyhavn og omegn—Med fem nye arter for den danske fauna (Diplopoda, Chilopoda, Opiliones). Entomol. Medd. 2011, 79, 3–12. [Google Scholar]
- Cappelen, J. (Ed.) Denmark—DMI Historical Climate Data Collection 1768–2019; Danish Meteorological Institute: Copenhagen, Denmark, 2020; p. 112. [Google Scholar]
- Hansen, K. Det Tabte Land. Den Store Fortælling om Magten over det Danske Landskab; GAD: Copenhagen, Denmark, 2008; p. 847. [Google Scholar]
- Jørgensen, L. Fortegnelse over de i Danmark Hidtil Fundne Apidae—Strandby Skole. 1921; unpublished note-book. [Google Scholar]
- Madsen, H.B.; Calabuig, I. Kommenteret checkliste over Danmarks bier—Del 1: Colletidae (Hymenoptera, Apoidea). Entomol. Medd. 2008, 76, 145–163. [Google Scholar]
- Calabuig, I.; Madsen, H.B. Kommenteret checkliste over Danmarks bier—Del 2: Andrenidae (Hymenoptera, Apoidea). Entomol. Medd. 2009, 77, 83–113. [Google Scholar]
- Madsen, H.B.; Calabuig, I. Kommenteret checkliste over Danmarks bier—Del 3: Melittidae & Megachilidae (Hymenoptera, Apoidea). Entomol. Medd. 2010, 78, 73–99. [Google Scholar]
- Madsen, H.B.; Calabuig, I. Kommenteret checkliste over Danmarks bier—Del 4: Halictidae (Hymenoptera, Apoidea). Entomol. Medd. 2011, 79, 85–115. [Google Scholar]
- Madsen, H.B.; Calabuig, I. Kommenteret checkliste over Danmarks bier—Del 5: Apidae (Hymenoptera, Apoidea). Entomol. Medd. 2012, 80, 7–52. [Google Scholar]
- Madsen, H.B.; Schmidt, H.T.; Rasmussen, C. Distriktskatalog over Danmarks bier (Hymenoptera, Apoidea). Entomol. Medd. 2016, 83, 43–70. [Google Scholar]
- Rasmussen, C.; Schmidt, H.T.; Madsen, H.B. Distribution, phenology and host plants of Danish bees (Hymenoptera, Apoidea). Zootaxa 2016, 4212, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.B.; Poulsen, K.R.; Rasmussen, C.; Schmidt, H.T. En ny dansk hvepsebi Nomada bifasciata Olivier, 1811 og andre bemærkelsesværdige bier fundet på Ærø (Hymenoptera, Apoidea, Apiformes). Entomol. Medd. 2021, 88, 23–29. [Google Scholar]
- Madsen, H.B.; Schmidt, H.T.; Rasmussen, C. Opdateret distriktskatalog over Danmarks bier (Hymenoptera, Apoidea, Apiformes. Entomol. Tidskr. 2021, 88, 1–22. [Google Scholar]
- Madsen, H.B. Anvendelse af digitalt landkort og GPS i forbindelse med UTM-angivelser ved etikettering af indsamlede insekter i Danmark. Entomol. Medd. 1999, 67, 65–69. [Google Scholar]
- Enghoff, H.; Nielsen, E.S. Et nyt grundkort til brug for faunistiske undersøgelser i Danmark, baseret på UTM-koordinatsystemet. Entomol. Medd. 1977, 45, 65–74. [Google Scholar]
- Westrich, P. Die Wildbienen Baden-Württembergs. Teil 1: Lebensräume, Verhalten, Ökologie und Schutz; Ulmer: Stuttgart, Germany, 1990; p. 972. [Google Scholar]
- Westrich, P. Die Wildbienen Baden-Württembergs. Teil 2: Die Gattungen und Arten; Ulmer: Stuttgart, Germany, 1990; p. 972. [Google Scholar]
- Westrich, P. Die Wildbienen Deutschlands; Ulmer: Stuttgart, Germany, 2018. [Google Scholar]
- Scheuchl, E.; Willner, W. Taschenlexikon der Wildbienen Mitteleuropas. Alle Arten im Porträt; Quelle & Meyer: Wiebelsheim, Germany, 2016; p. 917. [Google Scholar]
- Peeters, T.M.J.; Nieuwenhuijsen, H.; Smit, J.; van der Meer, F.; Raemakers, I.P.; Heitmans, W.R.B.; Achterberg, C.v.; Kwak, M.; Loonstra, A.J.; de Rond, J.; et al. De Nederlandse Bijen (Hymenoptera: Apidae s.l.); Naturalis Biodiversity Center & European Invertebrate Survey: Leiden, The Netherlands, 2012; Volume 11, p. 560. [Google Scholar]
- Falk, S.; Lewington, R. Field Guide to the Bees of Great Britain and Ireland; British Wildlife Publishing: London, UK, 2015; p. 336. [Google Scholar]
- Kendall, L.K.; Rader, R.; Gagic, V.; Cariveau, D.P.; Albrecht, M.; Baldock, K.C.R.; Freitas, B.M.; Hall, M.; Holzschuh, A.; Molina, F.P.; et al. Pollinator size and its consequences: Robust estimates of body size in pollinating insects. Ecol. Evol. 2019, 9, 1702–1714. [Google Scholar] [CrossRef] [Green Version]
- Kearns, C.A.; Inouye, D.W. Techniques for Pollination Biologists; University of Colorado: Denver, CO, USA, 1993; p. 583. [Google Scholar]
- Steffan-Dewenter, I.; Munzenberg, U.; Burger, C.; Thies, C.; Tscharntke, T. Scale-dependent effects of landscape context on three pollinator guilds. Ecology 2002, 83, 1421–1432. [Google Scholar] [CrossRef]
- Dupont, Y.L.; Madsen, H.B. Humlebier. Nat. Og Mus. 2010, 49, 1–36. [Google Scholar]
- von Hagen, E. Hummeln Bestimmen—Ansiedeln—Vermehren—Schützen; Natur: Augsburg, Germany, 1990. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O′Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 December 2021).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.4. 2021. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 1 December 2021).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publishing: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Fox, J. Effect displays in R for generalised linear models. J. Stat. Softw. 2003, 8, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Madsen, H.B. Humlebier. In Den Danske Rødliste; Wind, P., Ed.; Fagdatacenter for Biodiversitet og Terrestrisk Natur (B-FDC)—Danmarks Miljøundersøgelser, Aarhus Universitet: Aarhus, Denmark, 2009. [Google Scholar]
- Michener, C.D. The Social Behavior of the Bees; Harvard University Press: Cambridge, MA, USA, 1974; pp. xii+404. [Google Scholar]
- Heinrich, B. Bumblebee Economics; Harvard University Press: Cambridge, MA, USA, 1979. [Google Scholar]
- Madsen, H.B.; Poulsen, K.R.; Rasmussen, C.; Calabuig, I.; Schmidt, H.T. Fire bier nye for den danske fauna (Hymenoptera, Apoidea, Apiformes). Entomol. Medd. 2018, 86, 39–50. [Google Scholar]
- Schmidt, H.T.; Poulsen, K.R.; Madsen, H.B. Fem nye arter af bier for den danske fauna (Hymenoptera, Apoidea). Entomol. Medd. 2013, 81, 62–71. [Google Scholar]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; Criado, M.G.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; Rúa, P.D.l.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2015; p. 98. [Google Scholar]
- Fontaine, C.; Dajoz, I.; Meriguet, J.; Loreau, M. Functional diversity of plant-pollinator interaction webs enhances the persistence of plant communities. PLoS Biol. 2006, 4, e1. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Hartvig, P.; Vestergaard, P. Atlas Flora Danica; Gyldendal: Copenhagen, Denmark, 2015; Volume 1–3, p. 1230. [Google Scholar]
- Dupont, Y.L.; Damgaard, C.; Simonsen, V. Quantitative historical change in bumblebee (Bombus spp.) assemblages of red clover fields. PLoS ONE 2011, 6, e25172. [Google Scholar] [CrossRef] [Green Version]
- Goulson, D.; Hanley, M.E.; Darvill, B.; Ellis, J.S.; Knight, M.E. Causes of rarity in bumblebees. Biol. Conserv. 2005, 122, 1–8. [Google Scholar] [CrossRef]
- Stapel, C. Undersøgelser over humlebier (Bombus Latr.), deres udbredelse, trækplanter og betydning for bestøvning af rødkløver (Trifolium pratense L.). Tidsskr. Landbr. Planteavl 1933, 39, 193–294. [Google Scholar]
- Skovgaard, O.S. Rødkløverens bestøvning, humlebier og humleboer. Undersøgelser over nogle i Danmark forekommende arter af slægten Bombus Latr., deres trækplanter, boer og bopladser, samt deres betydning for bestøvningen af rødkløver (Trifolium pratense). Det K. Dan. Vidensk. Selsk. Skr. (Nat. Og Math. Afd.) 1936, 9, 1–140. [Google Scholar]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- van der Vecht, J. Über eine neue holländische Andrena-art aus der A.ovatula K.-Gruppe, Andrena gelriae n. sp. Zool. Meded. 1927, 10, 87–89. [Google Scholar]
- Smith, F. Descriptions of the British wasp-bees, (Nomada of authors). Zoologist 1844, 2, 587–606. [Google Scholar]
- Herrich-Schäffer, G.A.W. Auseinandersetzung der europäischen Arten einiger Bienengattungen. Gattung Nomada. Zeitschrift für Entomologie (Leipzig) 1839, 1, 267–288. [Google Scholar]
- Didham, R.K.; Basset, Y.; Collins, C.M.; Leather, S.R.; Littlewood, N.A.; Menz, M.H.M.; Müller, J.; Packer, L.; Saunders, M.E.; Schönrogge, K.; et al. Interpreting insect declines: Seven challenges and a way forward. Insect Conserv. Divers. 2020, 13, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Isaac, N.J.B.; Pocock, M.J.O. Bias and information in biological records. Biol. J. Linn. Soc. 2015, 115, 522–531. [Google Scholar] [CrossRef] [Green Version]


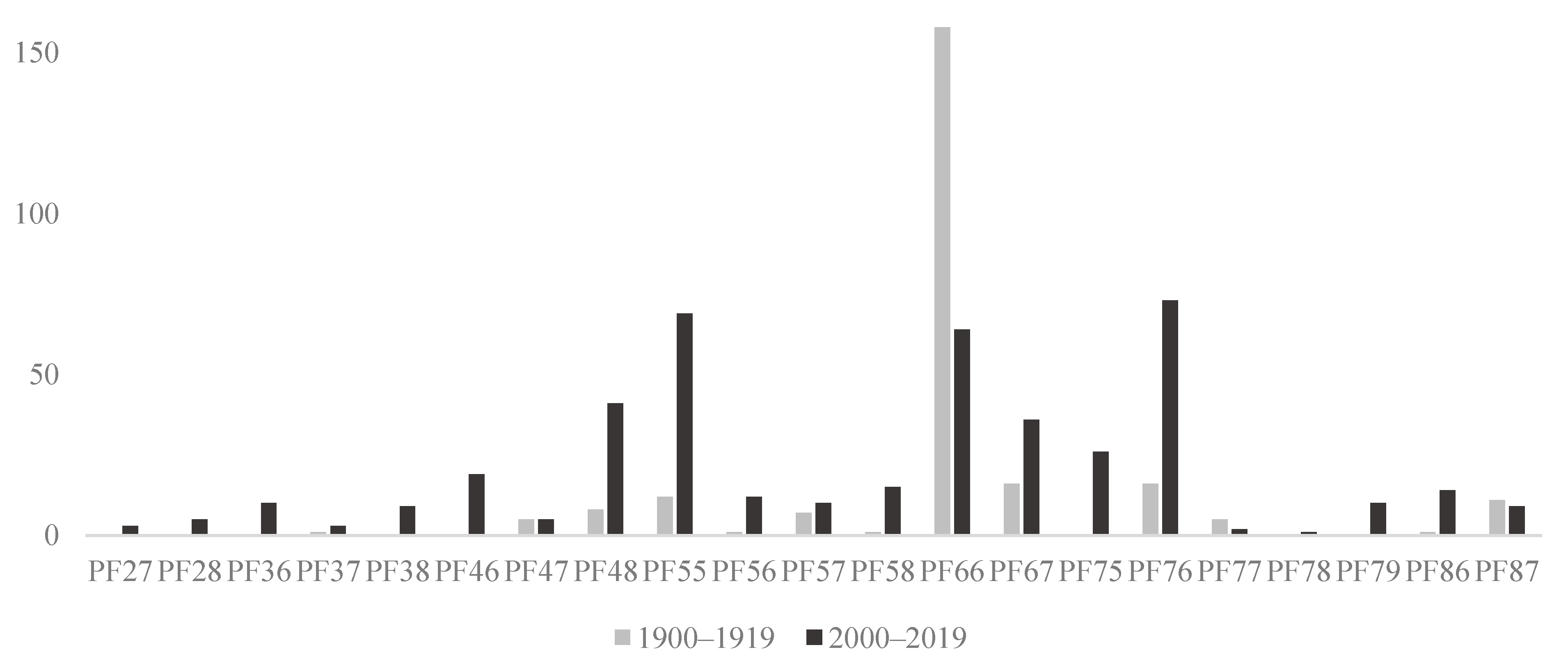



| Danish Category [21] | English [21] | IUCN Interpretation | IUCN |
|---|---|---|---|
| “forsvundet” | “disappeared” | RE | Regionally Extinct |
| meget sjælden | very rare | CR | Critically Endangered |
| Sjælden | rare | EN | Endangered |
| temmelig sjælden | rather rare | EN | Endangered |
| noget sjælden | somewhat rare | VU | Vulnerable |
| ikke sjælden | not rare | NT | Near Threatened |
| ikke almindelig | not common | NT | Near Threatened |
| temmelig almindelig | rather common | LC | Least Concern |
| almindelig | common | LC | Least Concern |
| meget almindelig | very common | LC | Least Concern |
| Bee Species Collected | |
|---|---|
| Total no. spp. in both studies | 203 |
| No. spp. 1900–1919 | 174 |
| No. spp. 2000–2019 | 121 |
| No. spp. unique to 1900–1919 | 82 |
| No. spp. unique to 2000–2019 | 29 |
| No. spp. common to both periods | 92 |
| Jaccard similarity index for two periods | 0.453 |
| Sørensen similarity index for two periods | 0.624 |
| Total no. spp. in both studies, excluding Apis, Bombus and parasite species | 138 |
| No. spp. unique to 1900–1919, excluding Apis, Bombus and parasitic species | 64 |
| No. spp. unique to 2000–2019, excluding Apis, Bombus and parasitic species | 16 |
| Both Periods | 1900–1919 | 2000–2019 | Unique to 1900–1919 | Unique to 2000–2019 | Common for Both Periods | ||
|---|---|---|---|---|---|---|---|
| Parasitic | 24.6 | 21.8 | 29.8 | 17.1 | 41.4 | 26.1 | |
| Feeding preference | |||||||
| Oligolectic | 24.6 | 26.4 | 15.7 | 37.8 | 13.8 | 16.3 | |
| Polylectic | 50.7 | 51.7 | 54.5 | 45.1 | 44.8 | 57.6 | |
| Excluding ‘Not applicable’ (NA) | |||||||
| Oligolectic | 21.2 | 22.4 | 16.1 | 31.1 | 14.8 | 16.5 | |
| Polylectic | 52.0 | 53.3 | 54.2 | 47.5 | 44.4 | 57.1 | |
| Excluding Bombus, Apis and parasitic bees: | |||||||
| Oligolectic | 36.2 | 37.7 | 25.7 | 48.4 | 25.0 | 25.9 | |
| Polylectic | 63.8 | 62.3 | 74.3 | 51.6 | 75.0 | 74.1 | |
| Excluding ‘Not applicable’ (NA), Bombus, Apis and parasitic bees: | |||||||
| Oligolectic | 29.0 | 29.6 | 22.9 | 39.6 | 25.0 | 22.4 | |
| Polylectic | 71.0 | 70.4 | 77.1 | 60.4 | 75.0 | 77.6 | |
| Voltinism | |||||||
| Univoltine | 89.7 | 89.7 | 86.0 | 95.1 | 89.7 | 84.8 | |
| Bi- or multivoltine | 10.3 | 10.3 | 14.0 | 4.9 | 10.3 | 15.2 | |
| Excluding ‘Not applicable’ (NA), Bombus, Apis and parasitic bees: | |||||||
| Univoltine | 93.9 | 93.0 | 94.0 | 93.8 | 100.0 | 92.5 | |
| Bi- or multivoltine | 6.1 | 7.0 | 6.0 | 6.3 | 0.0 | 7.5 | |
| Average body length (mm) | 10.6 | 10.7 | 10.6 | 10.6 | 10.0 | 10.8 | |
| Excluding Bombus, Apis, and parasitic bees: | 9.7 | 9.9 | 9.3 | 10.2 | 8.4 | 9.6 | |
| Both Periods | Only 1900–1919 | Only 2000–2019 | ||||
|---|---|---|---|---|---|---|
| Jørgensen | Madsen | Jørgensen | Madsen | Jørgensen | Madsen | |
| RE | - | 10 (5%) | - | 10 (12%) | - | (0%) |
| CR | 29 (14%) | 8 (4%) | 20 (24%) | 6 (7%) | 4 (14%) | (0%) |
| EN | 27 (13%) | 10 (5%) | 17 (21%) | 9 (11%) | 1 (3%) | (0%) |
| VU | 13 (6%) | 12 (6%) | 6 (7%) | 10 (12%) | (0%) | 1 (3%) |
| NT | 48 (24%) | 14 (7%) | 22 (27%) | 6 (7%) | 3 (10%) | 2 (7%) |
| Redlisted | 117 (58%) | 54 (27%) | 65 (79%) | 41 (50%) | 8 (28%) | 3 (10%) |
| LC | 57 (28%) | 125 (62%) | 10 (12%) | 20 (24%) | 5 (17%) | 24 (83%) |
| NA | - | 24 (12%) | - | 21 (26%) | - | 2 (7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, C.; Sydenham, M.A.K.; Schmidt, H.T.; Madsen, H.B. The Native Bees of Lolland (Denmark) Revisited after 100 Years: The Demise of the Specialists. Insects 2022, 13, 153. https://doi.org/10.3390/insects13020153
Rasmussen C, Sydenham MAK, Schmidt HT, Madsen HB. The Native Bees of Lolland (Denmark) Revisited after 100 Years: The Demise of the Specialists. Insects. 2022; 13(2):153. https://doi.org/10.3390/insects13020153
Chicago/Turabian StyleRasmussen, Claus, Markus Arne Kjær Sydenham, Hans Thomsen Schmidt, and Henning Bang Madsen. 2022. "The Native Bees of Lolland (Denmark) Revisited after 100 Years: The Demise of the Specialists" Insects 13, no. 2: 153. https://doi.org/10.3390/insects13020153






