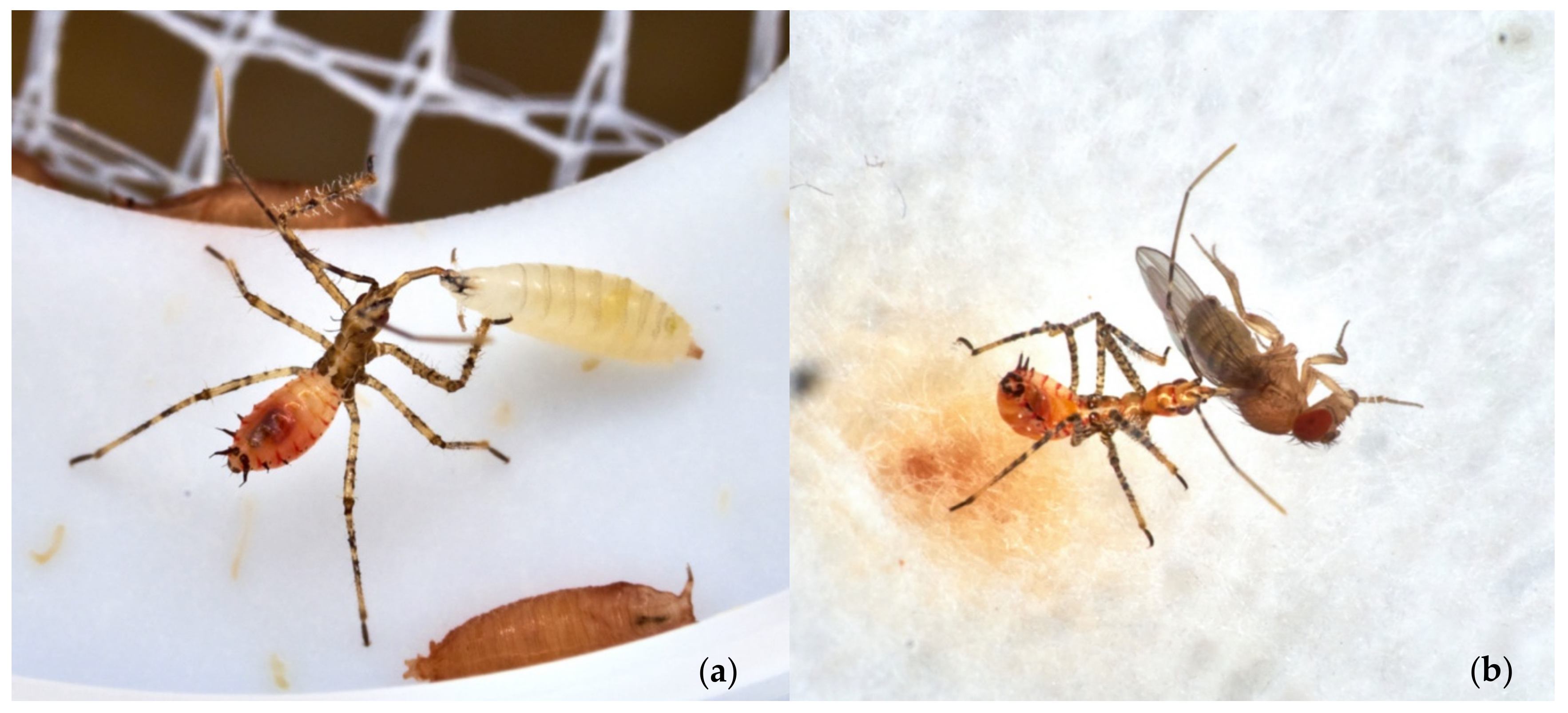
Zelus renardii Roaming in Southern Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. LAB References Retrieval
2.2. LAB Field Collection
2.3. Laboratory Work
2.4. LAB Rearing
2.5. LAB Rearing on Drosophila melanogaster, Drosophila suzukii, and Megaselia scalaris
2.6. LAB Rearing on Macrohomotoma gladiata
2.7. LAB Test on Pseudococcidae
2.8. LAB Test on Aleyrodidae
2.9. LAB Test on Coccomorpha
2.10. LAB Test on Xylella vectors, Miridae and Issidae
2.11. LAB Test on Harmonia axyridis
2.12. LAB Test on Bactrocera oleae, the Olive Fly
2.13. LAB Test on Apis mellifera
2.14. LAB and Humans
2.15. LAB’s Tests Interpretation Issues
3. Results and Discussion
3.1. References Retrieval
3.2. Field Observations and Tests
3.3. LAB Rearing on Drosophila melanogaster, Drosophila suzukii and Megaselia scalaris
3.4. LAB Rearing on Macrohomotoma gladiata
3.5. LAB Tests on Pseudococcidae
3.6. LAB Tests on Aleyrodidae
3.7. LAB Tests on Coccomorpha
3.8. LAB Tests on Xylella vectors, Issidae and Miridae
3.9. LAB Tests on Harmonia axyridis
3.10. LAB Tests on Bactrocera oleae (BO, the Olive Fly)
3.11. LAB Tests on Apis mellifera (AM)
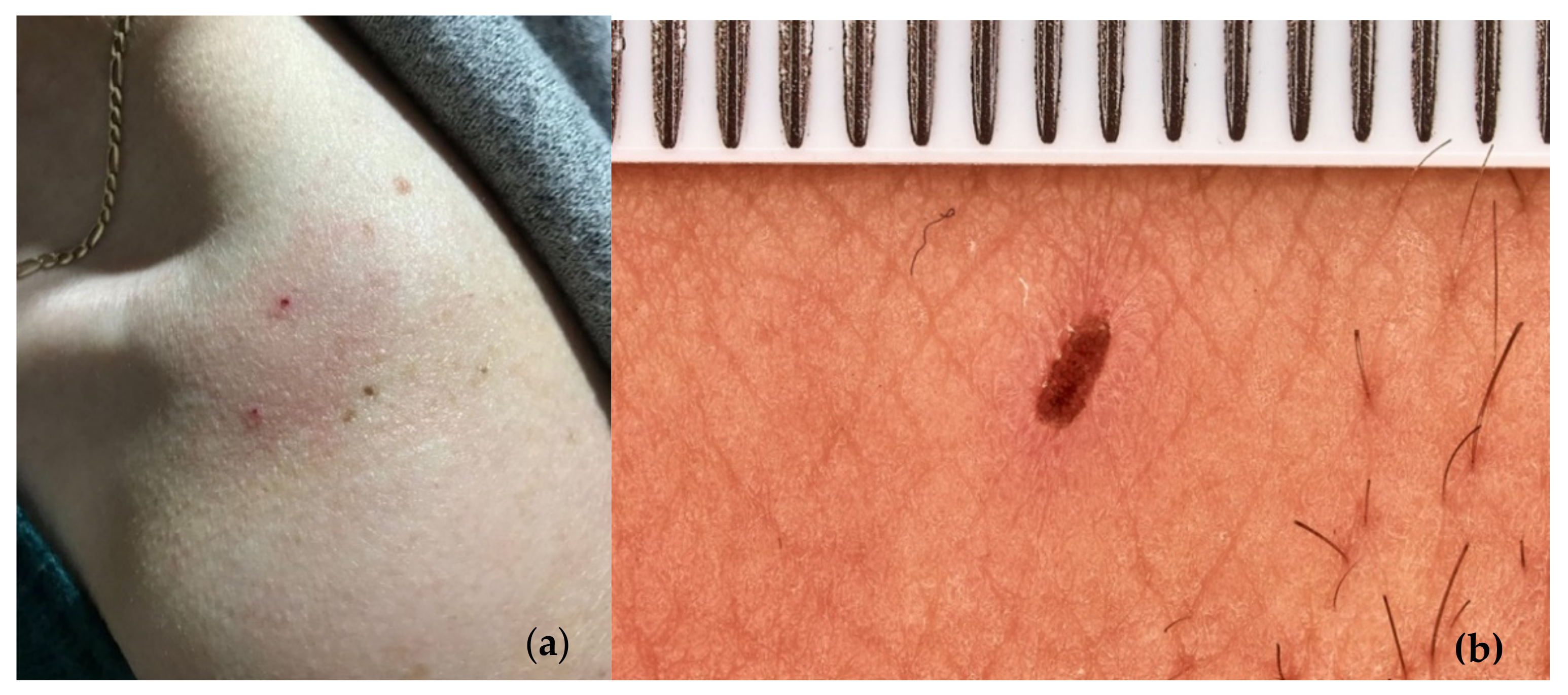
3.12. LAB and Humans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornara, D.; Nocera, A.; Corrado, I.; Verrastro, V.; Lamaj, F.; El Kenawy, A.; Russo, V.; Porcelli, F. Lo Zelus renardii (Kolenati, 1857) (Heteroptera Reduviidae): Un promettente predatore della Macrohomotoma gladiata (Kuwayama, 1908) (Psylloidea Homotomidae) sui Ficus microcarpa Hort. Berol. ex Walp. (Moraceae) ornamentali del verde urbano a Bari. In Proceedings of the XXV Congresso Nazionale Entomologia, Padova, Italy, 20–24 June 2016. [Google Scholar]
- Cioffi, M.; Cornara, D.; Corrado, I.; Jansen, M.G.M.; Porcelli, F. The status of Aleurocanthus spiniferus from its unwanted introduction in Italy to date. Bull. Insectol. 2013, 66, 273–281. [Google Scholar]
- Cornara, D.; Morente, M.; Markheiser, A.; Bodino, N.; Tsai, C.W.; Fereres, A.; Redak, R.A.; Perring, T.M.; Lopes, J.R.S. An overview on the worldwide vectors of Xylella fastidiosa. Entomol. Gen. 2019, 39, 157–181. [Google Scholar] [CrossRef]
- Picciotti, U.; Lahbib, N.; Sefa, V.; Porcelli, F.; Garganese, F. Aphrophoridae role in Xylella fastidiosa subsp. pauca ST53 invasion in southern Italy. Pathogens 2021, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Acquasanta, F.; Bacci, L.; Baser, N.; Carmignano, P.M.; Cavalieri, V.; Cioffi, M.; Convertini, S.; D’Accolti, A.; Dal Maso, E.; Diana, F.; et al. Tradizione e innovazione nel controllo del Philaenus spumarius Linnaeus, 1758 (Hemiptera Aphrophoridae). In Proceedings of the Giornate Fitopatologiche 2018 Protezione delle Piante, Qualità, Ambiente, Chianciano Terme, Italy, 6–9 March 2018; pp. 181–190. [Google Scholar]
- Di Serio, F.; Bodino, N.; Cavalieri, V.; Demichelis, S.; Di Carolo, M.; Dongiovanni, C.; Fumarola, G.; Gilioli, G.; Guerrieri, E.; Picciotti, U.; et al. Collection of data and information on biology and control of vectors of Xylella fastidiosa. EFSA Support Publ. 2019, 16, 1628E. [Google Scholar] [CrossRef]
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. J. Appl. Entomol. 2017, 141, 80–87. [Google Scholar] [CrossRef]
- Cunty, A.; Legendre, B.; de Jerphanion, P.; Juteau, V.; Forveille, A.; Germain, J.F.; Ramel, J.M.; Reynaud, P.; Olivier, V.; Poliakoff, F. Xylella fastidiosa subspecies and sequence types detected in Philaenus spumarius and in infected plants in France share the same locations. Plant Pathol. 2020, 69, 1798–1811. [Google Scholar] [CrossRef]
- Scortichini, M. Predisposing Factors for “Olive Quick Decline Syndrome” in Salento (Apulia, Italy). Agronomy 2020, 10, 1445. [Google Scholar] [CrossRef]
- Fierro, A.; Liccardo, A.; Porcelli, F. A lattice model to manage the vector and the infection of the Xylella fastidiosa on olive trees. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Liccardo, A.; Fierro, A.; Garganese, F.; Picciotti, U.; Porcelli, F. A biological control model to manage the vector and the infection of Xylella fastidiosa on olive trees. PLoS ONE 2020, 15, e0232363. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Wilhoit, L.R.; Armer, C.A. Influence of intraguild predation among generalist insect predators on the suppression of an herbivore population. Oecologia 1993, 96, 439–449. [Google Scholar] [CrossRef]
- Cisneros, J.J.; Rosenheim, J.A. Ontogenetic change of prey preference in the generalist predator Zelus renardii and its influence on predator–predator interactions. Ecol. Entomol. 1997, 22, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Cisneros, J.J.; Rosenheim, J.A. Changes in the foraging behavior, within-plant vertical distribution, and microhabitat selection of a generalist insect predator: An age analysis. Environ. Entomol. 1998, 27, 949–957. [Google Scholar] [CrossRef]
- Law, Y.H.; Rosenheim, J.A. Effects of combining an intraguild predator with a cannibalistic intermediate predator on a species-level trophic cascade. Ecology 2011, 92, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, D.P. Biocontrol potential of assassin bugs (Hemiptera: Reduviidae). J. Exp. Zool. 2003, 6, 1–44. [Google Scholar]
- Miller, N.C. The Biology of the Heteroptera; Leonard Hill Ltd.: London, UK, 1956. [Google Scholar]
- Loomans, A.J. Every generalist biological control agent requires a special risk assessment. BioControl 2021, 66, 23–35. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [Green Version]
- Hurd, L.E. Predation: The role of generalist predators in biodiversity and biological control. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 3038–3042. [Google Scholar]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batáry, P.; Bergtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.F.; et al. Landscape moderation of biodiversity patterns and processes-eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef]
- Piper, M.D. Using artificial diets to understand the nutritional physiology of Drosophila melanogaster. Curr. Opin. Insect Sci. 2017, 23, 104–111. [Google Scholar] [CrossRef]
- Grassi, A.; Giongo, L.; Palmieri, L. Drosophila (Sophophora) suzukii (Matsumura), new pest of soft fruits in Trentino (North-Italy) and in Europe. IOBC/WPRS Bull. 2011, 70, 121–128. [Google Scholar]
- Baser, N.; Broutou, O.; Lamaj, F.; Verrastro, V.; Porcelli, F. First finding of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in Apulia, Italy, and its population dynamics throughout the year. Fruits 2015, 70, 225–230. [Google Scholar] [CrossRef]
- Baser, N.; Broutou, O.; Verrastro, V.; Porcelli, F.; Ioriatti, C.; Anfora, G.; Mazzoni, V.; Rossi Stacconi, M.V. Susceptibility of table grape varieties grown in south-eastern Italy to Drosophila suzukii. J. Appl. Entomol. 2018, 142, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Mifsud, D.; Porcelli, F. The psyllid Macrohomotoma gladiata Kuwayama, 1908 (Hemiptera: Psylloidea: Homotomidae): A Ficus pest recently introduced in the EPPO region. EPPO Bull. 2012, 42, 161–164. [Google Scholar] [CrossRef]
- Pedata, P.A.; Burckhardt, D.; Mancini, D. Severe infestations of the jumping plant-louse Macrohomotoma gladiata, a new species for Italy in urban Ficus plantations. Bull. Insectol. 2012, 65, 95–98. [Google Scholar]
- Taskin, E. Efficacy of Several Natural Substances on Planococcus ficus (Signoret) and Their Impact on Its Two Natural Enemies Anagyrus sp. Near pseudococci (Girault) and Cryptolaemus montrouzieri (Mulsant). Master’s Thesis, CIHEAM—Mediterranean Agronomic Institute of Bari, Valenzano, Italy, 2014. [Google Scholar]
- Taskin, E.; Lamaj, F.; Verrastro, V.; Baldacchino, F. Laboratory efficacy of natural substances on Planococcus ficus (Sign.) and their impact on its two natural enemies. In Proceedings of the Fifth International Scientific Agricultural Symposium Agrosym (Agrosym 2014), Jahorina, Bosnia and Herzegovina, 23–26 October 2014; pp. 483–490. [Google Scholar]
- Hanife, G. Modified agar-based diet for small scale laboratory rearing of olive fruit fly, Bactrocera oleae (Diptera: Tephritidae). Fla. Entomol. 2008, 91, 651–656. [Google Scholar]
- Smout, S.; Asseburg, C.; Matthiopoulos, J.; Fernández, C.; Redpath, S.; Thirgood, S.; Harwood, J. The functional response of a generalist predator. PLoS ONE 2010, 5, e10761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahzoum, A.M.; Villa, M.; Benhadi-Marín, J.; Pereira, J.A. Functional response of Chrysoperla carnea (Neuroptera: Chrysopidae) larvae on Saissetia oleae (Olivier)(Hemiptera: Coccidae): Implications for biological control. Agronomy 2020, 10, 1511. [Google Scholar] [CrossRef]
- Davranoglou, L.R. Zelus renardii (Kolenati, 1856), a new world reduviid discovered in Europe (Hemiptera: Reduviidae: Harpactorinae). Entomol. Mon. Mag. 2011, 147, 157–162. [Google Scholar]
- Horton, J.R. The citrus thrips. USDA Bull. 1918, 616, 1–42. [Google Scholar]
- Horton, J.R. Argentine ant in relation to citrus groves. USDA Bull. 1918, 647, 1–30. [Google Scholar]
- Miranda-Salcedo, M.A.; Loera-Alvarado, E. Fluctuación poblacional de enemigos naturales de trips (Thysanoptera: Thripidae) asociados a limón mexicano (Citrus aurantifolia Swingle) en Michoacán. Entomol. Mex. 2019, 6, 151–155. [Google Scholar]
- Haviland, D.R.; Beede, R.H.; Daane, K.M. Crop loss relationships and economic injury levels for Ferrisia gilli (Hemiptera: Pseudococcidae) infesting pistachio in California. J. Econ. Entomol. 2015, 108, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Hicks, V.; Gilder, K.A.; Heinz, K.M. Active predators of crapemyrtle bark scale. In Proceedings of the 63rd Southern Nursery Association Research Conference, Acworth, GA, USA, 12 June 2019; pp. 198–201. [Google Scholar]
- Garrison, W. New Agricultural Pest for Southern California: Spotted Gum Lerp Psyllid, Eucalyptolyma Maideni; San Diego County Agricultural Commissioner’s Office: San Diego, CA, USA, 2001; pp. 1–4.
- Pereira, J.M. Resistência de Genótipos de Eucalipto ao Psilídeo-de-Concha Glycaspis brimblecombei Moore (Hemiptera: Psyllidae). Ph.D. Thesis, Universidade Estadual Paulista “Julio de Mesquita Filho”—UNESP, São Paulo, Brazil, 22 March 2011. [Google Scholar]
- Alonso, O. Aspectos fitosanitarios acerca de las plagas insectiles de las arbóreas forrajeras. Past. Forr. 2001, 24, 1–18. [Google Scholar]
- D’Hervé, F.E.; Olave, A.; Dapoto, G.L. Zelus renardii (Hemiptera: Reduviidae: Harpactorinae: Harpactorini): First record from Argentina. Rev. Soc. Entomol. Arg. 2018, 77, 32–35. [Google Scholar] [CrossRef] [Green Version]
- Barrera, J.F.; Gómez-Ruiz, J.; Herrera-Muñoz, J. Biología y Método de Cría de Zelus renardii (Hemiptera: Reduviidae), enemigo natural de Diaphorina citri (Hemiptera: Psyllidae). In Proceedings of the 1er Simposio Nacional Sobre Investigación Para el Manejo del Psílido Asiático de los Críticos y el Huanglongbing en México, Monterrey, Nuevo León, Mexico, 8–9 December 2010; pp. 277–291. [Google Scholar]
- Miranda-Salcedo, M.A.; López-Arroyo, J.I.; Velazquez-Monroy, J. Manejo regional de Diaphorina citri Kuwayama (Hemiptera: Psyllidae) en Michoacan. In Proceedings of the XLVII Congreso Nacional de Entomología, Benemérita Universidad Autónoma de Puebla Complejo Cultural Universitario, Puebla, México, 21–24 May 2012; pp. 777–782. [Google Scholar]
- Albores-Flores, C.I. Detección del ADN de Diaphorina citri (Hemiptera: Liviidae) en el Contenido Intestinal de Zelus Renardii (Hemiptera: Reduviidae). Master’s Thesis, El Colegio de la Frontera Sur, Lerma, Campeche, Mexico, 2016. [Google Scholar]
- Miranda-Salcedo, M.A.; Pardo-Melgarejo, S. Manejo del psillido asiatico de los citricos Diaphorina citri Kuwayama (Hemiptera: Psyllidae) en Michoacan. In Proceedings of the XXXVIII Congreso Nacional de Control Biológico, Sociedad Mexicana de Control Biológico, León de los Aldama, Guanajuato, Mexico, 5–6 November 2015; pp. 203–208. [Google Scholar]
- Miranda-Salcedo, M.A. Manejo en areas regionales del control de Diaphorina citri Kuwayama, 1908 (Hemiptera: Liviidae) en Michoacan. Entomol. Mex. 2018, 5, 342–347. [Google Scholar]
- Hagler, J.R.; Naranjo, S.E. Determining the frequency of heteropteran predation on sweetpotato whitefly and pink bollworm using multiple ELISAs. Entomol. Exp. Appl. 1994, 72, 59–66. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Chu, C.C.; Henneberry, T.J. Conservation of predatory arthropods in cotton: Role of action thresholds for Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 2002, 95, 682–691. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Hagler, J.R. Conservation of natural enemies in cotton: Role of insect growth regulators in management of Bemisia tabaci. Biol. Control 2004, 30, 52–72. [Google Scholar] [CrossRef] [Green Version]
- Hagler, J.R.; Blackmer, F. Identifying inter-and intra-guild feeding activity of an arthropod predator assemblage. Ecol. Entomol. 2013, 38, 258–271. [Google Scholar] [CrossRef]
- Nielson, M.W.; Henderson, J.A. Biology of Collops vittatus (Say) in Arizona, and feeding habits of seven predators of the spotted alfalfa aphid. J. Econ. Entomol. 1959, 52, 159–162. [Google Scholar] [CrossRef]
- Fye, R.E. Cotton insect populations: Development and impact of predators and other mortality factors [Arizona]. USDA Tech. Bull. 1979, 1592, 1–65. [Google Scholar]
- Rosenheim, J.A.; Kaya, H.K.; Ehler, L.E.; Marois, J.J.; Jaffee, B.A. Intraguild predation among biological-control agents: Theory and evidence. Biol. Control 1995, 5, 303–335. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Limburg, D.D.; Colfer, R.G. Impact of generalist predators on a biological control agent, Chrysoperla carnea: Direct observations. Ecol. Appl. 1999, 9, 409–417. [Google Scholar] [CrossRef]
- Nelson, E.H.; Rosenheim, J.A. Encounters between aphids and their predators: The relative frequencies of disturbance and consumption. Entomol. Exp. Appl. 2006, 118, 211–219. [Google Scholar] [CrossRef]
- Potin Téllez, C.A. Tabla de Vida Del Depredador Zelus Renardii (Kolenati) (Hemíptera: Heteróptera: Reduviidae) en Laboratorio. Master’s Thesis, Universidad de Chile, Santiago, Chile, 2008. [Google Scholar]
- El-Tom, H.A. The Biology of Zelus renardii Kolenati and Zelus socius Uhler (Hemiptera: Reduviidae). Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 15 January 1965. [Google Scholar]
- Hagler, J.; Groves, R.; Johnson, M.W.; Morgan, D. An immunological approach for quantifying predation rates on the glassy-winged sharpshooter. In Proceedings of the Pierce’s Disease Research Symposium, San Diego, CA, USA, 27–29 November 2006; pp. 70–72. [Google Scholar]
- Fournier, V.; Hagler, J.R.; Daane, K.; De León, J.; Groves, R. Identifying the predator complex of Homalodisca vitripennis (Hemiptera: Cicadellidae): A comparative study of the efficacy of an ELISA and PCR gut content assay. Oecologia 2008, 157, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Hagler, J.; Groves, R.; Johnson, M.W.; Morgan, D. Pinpointing predation events: A different molecular approach. In Proceedings of the Third International Symposium on Biological Control of Arthropods (ISBCA 3), Christchurch, New Zealand, 8–13 February 2009; pp. 461–467. [Google Scholar]
- Salerno, M.; Russo, V.; Sefa, V.; Lamaj, F.; Basher, N.; Verrastro, V.; Porcelli, F. Zelus renardii an assassin bug candidate for Philaenus spumarius biocontrol. In Proceedings of the European Conference on Xylella Finding Answer to a Global Problem, Palma de Mallorca, Spain, 13–15 November 2017; pp. 22–23. [Google Scholar]
- Kron, C.R.; Sisterson, M.S. Spissistilus festinus (Hemiptera: Membracidae) susceptibility to six generalist predators. PLoS ONE 2020, 15, e0242775. [Google Scholar] [CrossRef] [PubMed]
- Muir, F. The sugar cane leafhopper and its parasites in Hawaii. Hawaiian Planters’ Record 1921, 25, 108–123. [Google Scholar]
- Nishida, T. Natural enemies of the melon fly, Dacus cucurbitae Coq. in Hawaii. Ann. Entomol. Soc. Am. 1955, 48, 171–178. [Google Scholar] [CrossRef]
- Risco, S.H. Notas adicionales sobre el "saltahoja" de la caña de azúcar Perkinsiella saccharicida K. Rev. Peru. Entomol. 1969, 9, 181–187. [Google Scholar]
- Ali, A.S.A. Predator-Prey Relationship of Zelus renardii (Kolenati) and Bucculatrix thurberiella (Busck). Master’s Thesis, University of Arizona, Tucson, AZ, USA, 1978; p. 98. [Google Scholar]
- Weirauch, C. Observations on the sticky trap predator Zelus luridus Stål (Heteroptera, Reduviidae, Harpactorinae), with the description of a novel gland associated with the female genitalia. Denisia 2006, 19, 1169–1180. [Google Scholar]
- Fullaway, D.T. The corn leaf hopper (Peregrinus maidis, Ashm.). Bull. Hawaii Entomol. 1918, 4, 1–16. [Google Scholar]
- Swezey, O.H. Biological control of the sugar cane leafhopper in Hawaii. Hawaiian Sugar Planters’ Assoc. Exp. Sta. Bull. Entomol. Ser. 1936, 21, 57–101. [Google Scholar]
- Zimmerman, E.C. Insects of Hawaii, vol. 3. Heteroptera. Bernice, P. Bishop Museum; Experiment Station, Hawaiian Sugar Planters’ Association: Honolulu, HI, USA, 1948. [Google Scholar]
- Napompeth, B. Ecology and Population Dynamics of the Corn Planthopper, Peregrinus maidis (Ashmead) (Homoptera: Delphaddae), in Hawaii. Ph.D. Thesis, University of Hawaii, Honolulu, HI, USA, 1973. [Google Scholar]
- Holdaway, F.G.; Look, W.C. Insects of the garden bean in Hawaii. Proc. Hawaii Entomol. Soc. 1942, 11, 249–260. [Google Scholar]
- Hagler, J.R.; Allen, C.C.; Bradley-Dunlop, D.; Enriquez, F.J. Field evaluation of predation on Lygus hesperus (Hemiptera: Miridae) using a species-and stage-specific monoclonal antibody. Environ. Entomol. 1992, 21, 896–900. [Google Scholar] [CrossRef]
- Hagler, J.R. Development of an immunological technique for identifying multiple predator–prey interactions in a complex arthropod assemblage. Ann. Appl. Biol. 2006, 149, 153–165. [Google Scholar] [CrossRef]
- Zink, A.G.; Rosenheim, J.A. Stage-specific predation on Lygus hesperus affects its population stage structure. Entomol. Exp. Appl. 2008, 126, 61–66. [Google Scholar] [CrossRef]
- Asiimwe, P.; Naranjo, S.E.; Ellsworth, P.C. Effects of irrigation levels on interactions among Lygus hesperus (Hemiptera: Miridae), insecticides, and predators in cotton. Environ. Entomol. 2014, 43, 263–273. [Google Scholar] [CrossRef]
- Rosenheim, J.A. Source–sink dynamics for a generalist insect predator in habitats with strong higher-order predation. Ecol. Monogr. 2001, 71, 93–116. [Google Scholar]
- Greenstone, M.H.; Tillman, P.G.; Hu, J.S. Predation of the newly invasive pest Megacopta cribraria (Hemiptera: Plataspidae) in soybean habitats adjacent to cotton by a complex of predators. J. Econ. Entomol. 2014, 107, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Lahiri, S.; Reisig, D.D. Ecology and management of kudzu bug (Hemiptera: Plataspidae) in Southeastern soybeans. J. Integr. Pest Manag. 2016, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Grant, J.I. Phenology and Cold Tolerance of Megacopta cribraria: An Invasive Soybean Pest at Its Northern Limit. Master’s Thesis, University of Maryland, College Park, MA, USA, 2016. [Google Scholar]
- Morrill, A.W. Plant-bugs injurious to cotton bolls. US Dep. Agric. Bur. Entomol. 1910, 86, 1–110. [Google Scholar]
- Tillman, P.G.; Greenstone, M.H.; Hu, J.S. Predation of stink bugs (Hemiptera: Pentatomidae) by a complex of predators in cotton and adjoining soybean habitats in Georgia, USA. Fla. Entomol. 2015, 98, 1114–1126. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.M. Biology, Ecology, and Economics of Brown Stink Bug, Euschistus servus (Heteroptera: Pentatomidae), in Desert Cotton Agroecosystems. Master’s Thesis, University of Arizona, Tucson, AZ, USA, 2017; p. 75. [Google Scholar]
- Clancy, D.W. Natural enemies of some Arizona cotton insects. J. Econ. Entomol. 1946, 39, 326–328. [Google Scholar] [CrossRef]
- Ehler, L.E. An evaluation of some natural enemies of Nezara viridula in northern California. BioControl 2002, 47, 309–325. [Google Scholar] [CrossRef]
- Knowlton, G.F. Observation on the feeding of some predaceous Hemiptera. Proc. Utah Acad. Sci. 1944, 21, 37–39. [Google Scholar]
- Rosenheim, J.; Wilhoit, L. Why lacewings may fail to suppress aphids… Predators that eat other predators disrupt cotton aphid control. Calif. Agric. 1993, 47, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Ehler, L.E.; Long, R.F.; Kinsey, M.G.; Kelley, S.K. Potential for augmentative biological control of black bean aphid in California sugarbeet. Entomophaga 1997, 42, 241. [Google Scholar] [CrossRef]
- Ambrose, D.P. Assassin bugs (Reduviidae excluding Triatominae). In Heteroptera of Economic Importance; Schaefer, C.W., Panizzi, A.R., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2000; pp. 695–712. [Google Scholar]
- Hagler, J.R.; Blackmer, F.; Spurgeon, D.W. Accuracy of a prey-specific DNA assay and a generic prey-immunomarking assay for detecting predation. Methods Ecol. Evol. 2015, 6, 1426–1434. [Google Scholar] [CrossRef]
- Hagler, J.R.; Blackmer, F. Evidence of intraguild predation on a key member of the cotton predator complex. Food Webs 2015, 4, 8–13. [Google Scholar] [CrossRef]
- Yaherwandi, Y.; Diratika, M. Kelimpahan kepik predator (Hemiptera: Reduviidae) di daerah endemik serangan ulat api pada perkebunan kelapa sawit rakyat. J. Penelit. Pertan. Terap. 2020, 20, 1–10. [Google Scholar]
- Tuttle, D.M.; Wene, G.P.; Sheets, L.W. The cotton leaf perforator and its control in Arizona. J. Econ. Entomol. 1961, 54, 67–70. [Google Scholar] [CrossRef]
- Orphanides, G.M.; Gonzalez, D.; Bartlett, B.R. Identification and evaluation of pink bollworm predators in southern California. J. Econ. Entomol. 1971, 64, 421–424. [Google Scholar] [CrossRef]
- Sarwar, M. Biological parameters of pink bollworm Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae): A looming threat for cotton and its eradication opportunity. Int. J. Res. Agric. Forest. 2017, 4, 25–36. [Google Scholar]
- Atkins, E.L., Jr.; Frost, M.H., Jr.; Anderson, L.D.; Deal, A.S. The “omnivorous leaf roller,” Platynota stultana Wlshm., on cotton in California: Nomenclature, life history, and bionomics (Lepidoptera, Tortricidae). Ann. Entomol. Soc. Am. 1957, 50, 251–259. [Google Scholar] [CrossRef]
- Horton, J.R. A swallowtail butterfly injurious to California orange trees. Mon. Bull. Calif. State Hortic. Comm. 1922, 11, 377–387. [Google Scholar]
- Ewing, K.P.; Ivy, E.E. Some factors influencing bollworm populations and damage. J. Econ. Entomol. 1943, 36, 602–606. [Google Scholar] [CrossRef]
- Lingren, P.D.; Ridgway, R.L.; Jones, S.L. Consumption by several common arthropod predators of eggs and larvae of two Heliothis species that attack cotton. Ann. Entomol. Soc. Am. 1968, 61, 613–618. [Google Scholar] [CrossRef]
- Ali, A.S.A.; Watson, T.F. Effect of temperature on development and survival of Zelus renardii. Environ. Entomol. 1978, 7, 889–890. [Google Scholar] [CrossRef]
- Cohen, A.C. Organization of digestion and preliminary characterization of salivary trypsin-like enzymes in a predaceous heteropteran, Zelus renardii. J. Insect Physiol. 1993, 39, 823–829. [Google Scholar] [CrossRef]
- Shrestha, R.B.; Parajulee, M.N.; Blanco, C. Functional response of selected cotton arthropod predators to bollworm eggs in the laboratory. In Proceeding of Beltwide Cotton Conference, San Antonio, TX, USA, 5–9 January 2004; pp. 5–9. [Google Scholar]
- Wille, J.E. Biological control of certain cotton insects and the application of new organic insecticides in Peru. J. Econ. Entomol. 1951, 44, 13–18. [Google Scholar] [CrossRef]
- Ables, J.R. Feeding behavior of an assassin bug, Zelus renardii. Ann. Entomol. Soc. Am. 1978, 71, 476–478. [Google Scholar] [CrossRef]
- Cohen, A.C.; Tang, R. Relative prey weight influences handling time and biomass extraction in Sinea confusa and Zelus renardii (Heteroptera: Reduviidae). Environ. Entomol. 1997, 26, 559–565. [Google Scholar] [CrossRef]
- Ehler, L.E. An evaluation of some natural enemies of Spodoptera exigua on sugarbeet in northern California. BioControl 2004, 49, 121–135. [Google Scholar] [CrossRef]
- Eisenring, M.; Romeis, J.; Naranjo, S.E.; Meissle, M. Multitrophic Cry-protein flow in a dual-gene Bt-cotton field. Agric. Ecosyst. Environ. 2017, 247, 283–289. [Google Scholar] [CrossRef]
- Bisabri-Ershadi, B.; Ehler, L.E. Natural biological control of western yellow-striped armyworm, Spodoptera praefica (Grote) in hay alfalfa in northern California. Hilgardia 1981, 49, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Su, H.H.; Tian, J.C.; Naranjo, S.E.; Romeis, J.; Hellmich, R.L.; Shelton, A.M. Bacillus thuringiensis plants expressing Cry1Ac, Cry2Ab and Cry1F are not toxic to the assassin bug, Zelus renardii. J. Appl. Entomol. 2015, 139, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Van Zwaluwenburg, R.H. Recent immigrant insects. Hawaii. Plant Rec. 1946, 50, 11–17. [Google Scholar]
- Mbata, K.J.; Hart, E.R.; Lewis, R.K. Reproductive behavior in Zelus renardii Kolenati 1857 (Hemiptera: Reduviidae). Iowa State J. Res. 1987, 62, 261–265. [Google Scholar]
- Severin, H.H.; Severin, H.C.; Hartung, W.J. The ravages, life history, weights of stages, natural enemies and methods of control of the melon fly (Dacus cucurbitae Coq.). Ann. Entomol. Soc. Am. 1914, 7, 177–207. [Google Scholar] [CrossRef]
- Faúndez, E.I. La chinche asesina Zelus renardii (Kolenati, 1856) (Heteroptera: Reduviidae) en Chile: Comentarios después de 15 años de su llegada al país. Bol. Soc. Entomol. Arag. 2015, 57, 421–423. [Google Scholar]
- Hinckley, A.D. The klu beetle, Mimosestes sallaei (Sharp), in Hawaii (Coleoptera: Bruchidae). Proc. Hawaii Entomol. Soc. 1960, 17, 260–269. [Google Scholar]
- Campbell, R.E. The broad-bean weevil. USDA Bull. 1920, 807, 1–23. [Google Scholar]
- Mezani, S. Bioécologie de la Bruche de la Fève Bruchus rufimanus Boh. (Coleoptera: Bruchidae) Dans des Parcelles de Variétés de Fèves Différentes et de Féverole Dans la Région de Tizi-Rached (Tizi-Ouzou). Master’s Thesis, University Mouloud Mammeri de Tizi Ouzou, Tizi Ouzou, Algeria, 2011. [Google Scholar]
- Mezani, S. Suivi des Populations de Bruchus rufimanus (Coleoptera: Chrysomelidae) Dans les Lieux de Diapause et Dans des Parcelles de Variétés de Fève Différentes (Aguadulce, Séville et Féverole) Dans la Région de Tizi-Ouzou. Ph.D. Thesis, University Mouloud Mammeri de Tizi Ouzou, Tizi Ouzou, Algeria, 2016. [Google Scholar]
- Moran, P.J. Lack of establishment of the Mediterranean tamarisk beetle Diorhabda elongata (Coleoptera: Chrysomelidae) on athel (Tamarix aphylla) (Tamaricaceae) in South Texas. Southwest Entomol. 2010, 35, 129–145. [Google Scholar] [CrossRef]
- Hernandez, M.; Masonick, P.; Weirauch, C. Crowdsourced online images provide insights into predator-prey interactions of putative natural enemies. Food Webs 2019, 21, e00126. [Google Scholar] [CrossRef]
- Drees, B.M.; Jackman, J. Field Guide to Texas Insects. Texas A&M Agrilife Extension (Blog). 1999. Available online: https://texasinsects.tamu.edu/ (accessed on 31 August 2021).
- Muir, F.A.G. Introduction. In Handbook of the Insects and Other Invertebrates of Hawaiian Sugarcane Fields; Williams, F.X., Ed.; Experiment Station of the Hawaiian Sugar Planters’ Association: Honolulu, HI, USA, 1931; pp. 11–32. [Google Scholar]
- Curkovik, T.; Araya, J.E.; Baena, M.; Guerrero, M.A. Presencia de Zelus renardii Kolenati (Heteroptera: Reduviidae) en Chile. Bol. Soc. Entomol. Arag. 2004, 34, 163–165. [Google Scholar]
- Heimpel, G.E.; Rosenheim, J.A.; Mangel, M. Predation on adult Aphytis parasitoids in the field. Oecologia 1997, 110, 346–352. [Google Scholar] [CrossRef]
- Cogni, R.; Freitas, A.V.L.; Amaral Filho, B.F. Influence of prey size on predation success by Zelus longipes L. (Het., Reduviidae). J. Appl. Entomol. 2002, 126, 74–78. [Google Scholar] [CrossRef]
- Zhang, G.; Hart, E.R.; Weirauch, C. A taxonomic monograph of the assassin bug genus Zelus Fabricius (Hemiptera: Reduviidae): 71 species based on 10,000 specimens. Biodivers. Data J. 2016, 4, e8150. [Google Scholar] [CrossRef]
- Van der Heyden, T. First records of Zelus renardii (Kolenati, 1856) (Hemiptera: Heteroptera: Reduviidae: Harpactorinae) for Albania. Arq. Entomol. 2017, 18, 49–50. [Google Scholar]
- Sahayaraj, K. Reduviids and Their Merits in Biological Control. In Basic and Applied Aspects of Biopesticides; Sahayaraj, K., Ed.; Springer: New Delhi, India, 2014; pp. 195–214. [Google Scholar]
- Sahayaraj, K.; Balasubramanian, R. Reduviid: An important biological control agent. In Artificial Rearing of Reduviid Predators for Pest Management; Sahayaraj, K., Balasubramanian, R., Eds.; Springer: New Delhi, India, 2016; pp. 1–28. [Google Scholar]
- Weirauch, C.; Alvarez, C.; Zhang, G. Zelus renardii and Z. tetracanthus (Hemiptera: Reduviidae): Biological attributes and the potential for dispersal in two assassin bug species. Fla. Entomol. 2012, 95, 641–649. [Google Scholar] [CrossRef]
- Kolenati, F.A. Meletemata Entomologica Hemipterorum Heteropterorum Caucasi. Harpagocorisiae, Monographice Dispositae. Fasciculum IV. Bull. Soc. Nat. Moscou 1856, 1–502. [Google Scholar]
- Henry, T.J. Biodiversity of Heteroptera. In Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H.S., Eds.; Wiley-Blackwell: Oxford, UK, 2009; pp. 223–254. [Google Scholar]
- Baena, M.; Torres, J.L. Nuevos datos sobre heterópteros exóticos en España y Francia: Tempyra biguttula Stål, 1874, Belonochilus numenius (Say, 1832) y Zelus renardii (Kolenati, 1856) (Heteroptera: Rhyparochromidae, Orsillidae, Reduviidae). Bol. Asoc. Esp. Entomol. 2012, 36, 351–360. [Google Scholar]
- Vivas, L. Primera cita en España de la especie Zelus renardii (Kolenati, 1857) (Heteroptera: Reduviidae) que representa la segunda cita en Europa. BVnPC 2012, 1, 34–40. [Google Scholar]
- Dioli, P. Zelus renardii (Kolenati, 1856) (Insecta Heteroptera Reduviidae). Quad. Studi Nat. Romagna 2013, 38, 232–233. [Google Scholar]
- Putchkov, P.V. Invasive true bugs (Heteroptera) established in Europe. Ukr. Entomol. J. 2013, 2, 11–28. [Google Scholar]
- Pinzari, M.; Cianferoni, F.; Martellos, S.; Dioli, P. Zelus renardii (Kolenati, 1856), a newly established alien species in Italy (Hemiptera: Reduviidae, Harpactorinae). Fragm. Entomol. 2018, 50, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Swezey, O.H. Leaf-hoppers and their natural enemies (Pt. VII. Orthoptera, Coleoptera, Hemiptera). Rept. Expt. Sta. Hawaiian Sugar Planters Assoc. Bull. Div. Entomol. 1905, 7, 209–238. [Google Scholar]
- Kirkaldy, G.W. Biological notes on the Hemiptera of the Hawaiian Isles No. 1. Proc. Hawaii Entomol. Soc. 1907, 1, 135–161. [Google Scholar]
- Kirkaldy, G.W. On some Hawaiian Hemiptera-Heteroptera. Can. Entomol. 1907, 39, 244–248. [Google Scholar] [CrossRef]
- Perkins, R.C.L. Introduction being a review of the land-fauna of Hawaii. In Fauna Hawaiiensis or the Zoology of the Sandwich (Hawaiian) Isles; Sharp, D., Ed.; Cambridge University Press: Cambridge, UK, 1913; pp. 1–16. [Google Scholar]
- Hart, E.R. A Systematic Revision of the Genus Zelus Fabricius (Hemiptera: Reduviidae). Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1972. [Google Scholar]
- Hart, E.R. Genus Zelus Fabricius in the United States, Canada, and northern Mexico (Hemiptera: Reduviidae). Ann. Entomol. Soc. Am. 1986, 79, 535–548. [Google Scholar] [CrossRef]
- Hart, E.R. The genus Zelus Fabricius in the West Indies (Hemiptera: Reduviidae). Ann. Entomol. Soc. Am. 1987, 80, 293–305. [Google Scholar] [CrossRef]
- Werner, F.G.; Butler, J.R. The reduviids and nabids associated with Arizona crops. Univ. Ariz. Agr. Exp. Sta. Tech. Bul. 1957, 133, 1–12. [Google Scholar]
- Çerçi, B.; Koçak, Ö. Contribution to the knowledge of Heteroptera (Hemiptera) fauna of Turkey. J. Insect Biodivers. 2016, 4, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Van der Heyden, T. First record of Zelus renardii Kolenati (Heteroptera: Reduviidae: Harpactorinae) in Israel. Rev. Chil. Entomol. 2018, 44, 463–465. [Google Scholar]
- Kök, Ş.; Tomanović, Ž.; Nedeljković, Z.; Şenal, D.; Kasap, İ. Biodiversity of the natural enemies of aphids (Hemiptera: Aphididae) in Northwest Turkey. Phytoparasitica 2020, 48, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Kıyak, S. The new record invasive alien species (IAS) Zelus renardii (Kolenati, 1856) (Hemiptera: Heteroptera: Reduviidae) in central Anatolia (Turkey). J. Het. Turk. 2020, 2, 47–52. [Google Scholar]
- Perkins, R.C.L. The introduction of beneficial insects into the Hawaiian Islands. Nature 1897, 55, 499–500. [Google Scholar] [CrossRef] [Green Version]
- Petrakis, P.V.; Moulet, P. First record of the nearctic Zelus renardii (Heteroptera, Reduviidae, Harpactocorinae) in Europe. Entomol. Hell. 2011, 20, 75–81. [Google Scholar] [CrossRef]
- Simov, N.; Gradinarov, D.; Davranoglou, L.R. Three new assassin bug records (Hemiptera: Heteroptera: Reduviidae) for the Balkan Peninsula. Ecol. Montenegrina 2017, 13, 25–29. [Google Scholar] [CrossRef]
- Van der Heyden, T. A recent record of Zelus renardii (Kolenati, 1856) on Crete/Greece (Hemiptera: Heteroptera: Reduviidae: Harpactorinae). BVnPC 2015, 4, 55–59. [Google Scholar]
- Battaglia, D.; Mele, G. La cimice assassina, Zelus renardii, in Basilicata. Agrifoglio 2020, 98, 1–5. [Google Scholar]
- Rattu, A.; Dioli, P. Prima segnalazione di Zelus renardii (Kolenati, 1856) in Sardegna (Hemiptera, Reduviidae). Revta. Gad. Entom. 2020, 11, 119–125. [Google Scholar]
- Bella, S. The Nearctic bug Zelus renardii (Kolenati) (Hemiptera Reduviidae) in northern Italy and Sicily. J. Zool. 2020, 103, 87–88. [Google Scholar] [CrossRef]
- Garrouste, R. Zelus renardii (Kolenati, 1856): Une réduve nouvelle pour la France (Hemiptera, Reduviidae, Harpactorinae). Bull. Soc. Entomol. Fr. 2019, 124, 335–336. [Google Scholar] [CrossRef]
- Van der Heyden, T.; Grosso-Silva, J.M. First record of Zelus renardii Kolenati, 1856 in Portugal (Heteroptera: Reduviidae: Harpactorinae). Arq. Entomol. 2020, 22, 347–349. [Google Scholar]
- Van der Heyden, T. Erstfund von Zelus renardii Kolenati, 1856 in Deutschland (Heteroptera: Reduviidae). Heteropteron 2021, 61, 31–32. [Google Scholar]
- Van der Heyden, T. On the recent northern European dispersion of Zelus renardii Kolenati (Hemiptera: Heteroptera: Reduviidae) via human activity. Isr. J. Entomol. 2021, 51, 43–46. [Google Scholar]
- Rodríguez-Lozano, B.; Gómez-de Dios, M.Á. The invasive species Zelus renardii (Kolenati, 1857) (Hemiptera, Reduviidae) in Spain and comments about its global expansion. Trans. Am. Entomol. Soc. 2018, 144, 551–558. [Google Scholar] [CrossRef]
- Goula, M.G.; Lizana, F.; Núñez, A.M. New records of the Nearctic leafhopper assassin bug, Zelus renardii Kolenati, 1857 in the Iberian Peninsula (Hemiptera: Heteroptera: Reduviidae). Bull. Inst. Catalana Hist. Nat. 2019, 83, 219–222. [Google Scholar]
- Pérez-Gómez, Á.; Sánchez, Í.; Baena, M. Nuevos registros de hemípteros (Insecta: Hemiptera) alóctonos en Andalucía (sur de España). Rev. Soc. Gad. Hist. Nat. 2020, 14, 9–19. [Google Scholar]
- Pyšek, P.; Richardson, D.M. Traits associated with invasiveness in alien plants: Where do we stand? In Biological Invasions; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 97–125. [Google Scholar]
- Van Kleunen, M.; Dawson, W.; Schlaepfer, D.; Jeschke, J.M.; Fischer, M. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol. Lett. 2010, 13, 947–958. [Google Scholar] [CrossRef]
- Whitcomb, W.H.; Bell, K.O. Predaceous insects, spiders, and mites of Arkansas cotton fields. Agric. Exp. Sta. Div. Agric. Univ. Ark. 1964, 690, 1–84. [Google Scholar]
- Hagler, J.R.; Casey, M.T.; Machtley, S.A. A procedure for pinpointing cannibalism, intraguild predation, and life stage-specific feeding events. BioControl 2020, 1–8. [Google Scholar] [CrossRef]
- Stoner, A.; Metcalfe, A.M.; Weeks, R.E. Plant feeding by Reduviidae, a predaceous family (Hemiptera). J. Kans. Entomol. Soc. 1975, 48, 185–188. [Google Scholar]
- Van den Bosch, R.; Hagen, K.S. Predaceous and parasitic arthropods in California cotton fields. Calif. Agric. Exp. Sta. Bull. 1966, 820, 1–32. [Google Scholar]
- Law, Y.H.; Sediqi, A. Sticky substance on eggs improves predation success and substrate adhesion in newly hatched Zelus renardii (Hemiptera: Reduviidae) instars. Ann. Entomol. Soc. Am. 2010, 103, 771–774. [Google Scholar] [CrossRef]
- Dejean, A.; Revel, M.; Azémar, F.; Roux, O. Altruism during predation in an assassin bug. Naturwissenschaften 2013, 100, 913–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.L. California Cotton Insects; University of California Experiment Station: Berkeley, CA, USA, 1942. [Google Scholar]
- Ambrose, D.P.; Ambrose, A.D. Predation, copulation, oviposition and functional morphology of tibia, rostrum and eggs as tools in the biosystematics of Reduviidae (Hemiptera). Indian J. Entomol. 2009, 71, 1–17. [Google Scholar]
- Zhang, G.; Weirauch, C. Sticky predators: A comparative study of sticky glands in harpactorine assassin bugs (Insecta: Hemiptera: Reduviidae). Acta Zool. 2013, 94, 1–10. [Google Scholar] [CrossRef]
- Cohen, A.C. Feeding adaptations of some predaceous Hemiptera. Ann. Entomol. Soc. Am. 1990, 83, 1215–1223. [Google Scholar] [CrossRef]
- Greenop, A. Using Species Traits to Understand the Mechanisms Driving Pollination and Pest Control Ecosystem Services. Ph.D. Thesis, Lancaster University, Lancaster, UK, 2020. [Google Scholar]
- Force, D.C. r-and K-strategists in endemic host-parasitoid communities. Bull. Entomol. Soc. Am. 1972, 18, 135–137. [Google Scholar] [CrossRef]
- Greenstone, M.H. Spider feeding behaviour optimises dietary essential amino acid composition. Nature 1979, 282, 501–503. [Google Scholar] [CrossRef]
- Carey, J.R. Insect biodemography. Annu. Rev. Entomol. 2001, 46, 79–110. [Google Scholar] [CrossRef]
- Peterson, R.K.; Davis, R.S.; Higley, L.G.; Fernandes, O.A. Mortality risk in insects. Environ. Entomol. 2009, 38, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Plagens, M.J.; Whitcomb, W.H. Corn residue as an overwintering site for spiders and predaceous insects in Florida. Fla. Entomol. 1986, 69, 665–671. [Google Scholar] [CrossRef]
- Foster, S.P.; Harris, M.O. Behavioral manipulation methods for insect pest-management. Annu. Rev. Entomol. 1997, 42, 123–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mafra-Neto, A.; de Lame, F.M.; Fettig, C.J.; Munson, A.S.; Perring, T.M.; Stelinski, L.L.; Stoltman, L.L.; Mafra, L.E.J.; Borges, R.; Vargas, R.I. Manipulation of insect behavior with specialized pheromone and lure application technology (SPLAT®). In Pest Management with Natural Products; Beck, J.J., Coats, J.R., Duke, S.O., Koivunen, M.S., Eds.; American Chemical Society: Columbus, OH, USA, 2013; pp. 31–58. [Google Scholar]
- Fand, B.B.; Amala, U.; Yadav, D.S.; Rathi, G.; Mhaske, S.H.; Upadhyay, A.; Ahammed Shabeer, T.P.; Kumbhar, D.R. Bacterial volatiles from mealybug honeydew exhibit kairomonal activity toward solitary endoparasitoid Anagyrus dactylopii. J. Pest Sci. 2020, 93, 195–206. [Google Scholar] [CrossRef]
- Lozano-Soria, A.; Picciotti, U.; Lopez-Moya, F.; Lopez-Cepero, J.; Porcelli, F.; Lopez-Llorca, L.V. Volatile organic compounds from entomopathogenic and nematophagous fungi, repel banana black weevil (Cosmopolites sordidus). Insects 2020, 11, 509. [Google Scholar] [CrossRef]
- Orzali, L.; Mosconi, F.; Roversi, P.F.; Bergamaschi, V.; Riccioni, L. First report of the invasive pest Drosophila suzukii (Matsumura, 1931) (Diptera Drosophilidae) on Olea europaea L. in Italy. Redia-G. Di Zool. 2020, 103, 109–112. [Google Scholar] [CrossRef]
- Nugnes, F.; Laudonia, S.; Jesu, G.; Jansen, M.G.M.; Bernardo, U.; Porcelli, F. Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in some European countries: Diffusion, hosts, molecular characterization, and natural enemies. Insects 2020, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Bubici, G.; Prigigallo, M.I.; Garganese, F.; Nugnes, F.; Jansen, M.G.M.; Porcelli, F. First report of Aleurocanthus spiniferus on Ailanthus altissima: Profiling of the insect microbiome and microRNAs. Insects 2020, 11, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, K.W.; Reid, W. Surface morphology of legs in the assassin bug Zelus longipes (Hemiptera: Reduviidae): A scanning electron microscopy study with an emphasis on hairs and pores. Ann. Entomol. Soc. Am. 2001, 94, 457–461. [Google Scholar] [CrossRef]
- Fischer, P.; Grozinger, C.M. Pheromonal regulation of starvation resistance in honey bee workers (Apis mellifera). Naturwissenschaften 2008, 95, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Strangways-Dixon, J. Hormonal control of selective feeding in female Calliphora erythrocephala Meig. Nature 1959, 184, 2040–2041. [Google Scholar] [CrossRef]
- House, H.L. Insect nutrition. Ann. Rev. Biochem. 1962, 31, 653–672. [Google Scholar] [CrossRef]
- Robinson, W.H. Urban insects and arachnids. In A Handbook of Urban Entomology; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Miralles-Núñez, A.; Pradera, C.; Pujol-Fructuoso, J.A. La problemática de las especies exóticas: El caso de las picaduras producidas por Zelus renardii Kolenati, 1857 (Hemiptera: Reduviidae) en España. Arq. Entomol. 2021, 24, 133–138. [Google Scholar]
- Pereira-dos Santos, C.E.; de Souza, J.R.; Zanette, R.A.; da Silva, F.J.; Strussmann, C. Bite caused by the assassin bug Zelus Fabricius, 1803 (Hemiptera; Heteroptera: Reduviidae) in a human. Wilderness Environ. Med. 2019, 30, 63–65. [Google Scholar] [CrossRef] [Green Version]
- Rassi Jr., A.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Jurberg, J.; Galvão, C.; Weirauch, C.; Moreira, F.F.F. Hematophagous bugs (Reduviidae, Triatominae). In True bugs (Heteroptera) of the Neotropics. Entomology in Focus; Panizzi, A., Grazia, J., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 2, pp. 353–393. [Google Scholar]




















| Locations | Date | GPS | Plant Exploitation | Associate Insect |
|---|---|---|---|---|
| “2 Giugno” Public Park, Bari, Italy | 24 September 2014 | 41.10250055° N; 16.8747697° E | Quercus ilex L., 1753 | Nidularia pulvinata Planchon, 1864; Kermes vermilio Planchon, 1864 |
| Campus “E. Quagliariello” University of Bari Aldo Moro, Italy | 26 September 2014 | 41.103763° N; 16.859629° E | Citrus spp. L., 1753; Pyracantha coccinea M. Roem., 1847; Pyrus communis L., 1753; Olea europaea L., 1753; Ficus carica L., 1753 | Aleurocanthus spiniferus (Quaintance, 1903); Aleurothrixus floccosus (Maskell, 1895); Saissetia oleae Olivier, 1791; Homotoma ficus (L., 1758) |
| “A. De Gasperi” Avenue 268, Bari, Italy | 6 August 2015 | 41.104161° N; 16.871717° E | Ailanthus altissima, (Mill) Swingle; Capsicum annuum L., 1753; Aloysia citriodora Palau, 1784 | Aleurocanthus spiniferus (Quaintance, 1903), Aphis gossypii Glover, 1877; Aphis spp. L., 1758 |
| “Papa Pio XII” Avenue 32, Bari, Italy | 21 August 2015 | 41.103763° N; 16.859629° E | Ficus spp. L., 1753 | Macrohomotoma gladiata Kuwayama, 1908 |
| “L. De Laurentis” Avenue, Bari, Italy | 22 September 2015 | 41.093992° N; 16.864317° E | Quercus ilex L., 1753 | Nidularia pulvinata Planchon, 1864; Kermes vermilio Planchon, 1864 |
| S.S. 271, Km 9 Bari-Bitritto, Italy | 15 April 2016 | 41.066386° N; 16.826862° E | Olea europaea L., 1753 | Saissetia oleae Olivier, 1791 |
| “G. Mazzini” Avenue, Valenzano, Italy | 19 April 2016 | 41.04405° N; 16.88717° E | Cupressus spp. L.,1753 | any significant insect presence |
| “Roma” Avenue, Copertino, Lecce, Italy | 18 May 2016 | 40.268763° N; 18.050435° E | Quercus ilex L., 1753 | Nidularia pulvinata Planchon, 1864; Kermes vermilio Planchon, 1864 |
| “G. Toma” street 17, Bari, Italy | 19 September 2017 | 41.108673° N; 16.872666° E | Laurus nobilis L.,1753 | Trioza alacris Flor, 1861 |
| Campus “E. Quagliariello” University of Bari Aldo Moro, Italy | 20 September 2017 | 41.103763° N; 16.859629° E | Laurus nobilis L., 1753 | Trioza alacris Flor, 1861; Protopulvinaria pyriformis (Cockerell, 1894) |
| “Azienda Martucci” farm University of Bari Aldo Moro, Valenzano, Bari, Italy | 22 September 2017 | 41.022620° N; 16.904295° E | Laurus nobilis L., 1753 | Trioza alacris Flor, 1861 |
| “E. Montale” Street, Copertino, Lecce, Italy | 3 March 2018 | 40.263238° N; 18.056953° E | Laurus nobilis L., 1753 | Trioza alacris Flor, 1861 |
| “Piazza dei Martiri” Gioia del Colle, Bari, Italy | 29 September 2018 | 40.800365° N; 16.922870° E | Chamaerops humilis L., 1753 | Ommatissus binotatus Fieber, 1875 |
| “XXV Luglio” Avenue, Lecce, Italy | 2 October 2018 | 40.353825° N; 18.173999° E | Chamaerops humilis L., 1753 | Ommatissus binotatus Fieber, 1875 |
| “Carrer Portugal” Alicante, Spain | 15 September 2018 | 38.337504° N; 0.492043° E | Oleae europaea L., 1753 | Saissetia coffeae (Walker, 1852) |
| “Demokracia” Street, Tirana, Albania | 1 March 2019 | 41.3715552° N; 19.7776646° E | Ocimum basilicum L., 1753 | any significant insect presence |
| “A. Jashari” Street, Laknas, Albania | 4 March 2019 | 41.3687091° N; 19.7426623° E | Oleae europaea L., 1753 | any significant insect presence |
| Prey Species | Prey Taxa | Cage Area | Cage Volume | Prey Density | Notes |
|---|---|---|---|---|---|
| Macrohomotoma gladiata | (Hemiptera: Psyllidae) | 9.18 × 7.12 × 8.9 cm | Approx. 580 cm3 | >0.06 prey/cm3 | Nymphs aggregate on deformed twigs. Adults are vagrant. |
| Planococcus citri | (Hemiptera: Pseudococcidae) | 8.55 cm (∅); 1.42 cm (h) | 81 cm3 | 0.06 prey/cm3 | Zelus only preys on moving individuals |
| Planococcus ficus | (Hemiptera: Pseudococcidae) | 8.55 cm (∅); 1.42 cm (h) | 81 cm3 | 0.06 prey/cm3 | Zelus only preys on moving individuals |
| Aleurothrixus floccosus | (Hemiptera: Aleyrodidae) | 9.18 × 7.12 × 8.9 cm | Approx. 580 cm3 | circa 0.6 prey/cm3 | Approx. 50 individuals per leaf |
| Aleurocanthus spiniferus | (Hemiptera: Aleyrodidae) | 9.18 × 7.12 × 8.9 cm | Approx. 580 cm3 | circa 0.6 prey/cm3 | Approx. 50 individuals per leaf |
| Latilica sp. | (Hemiptera: Issidae) | 8.55 cm (∅); 1.42 cm (h) | 81 cm3 | 0.01 prey/cm3 | In mixed prey ensemble |
| Kermes vermilio | (Hemiptera: Kermesidae) | 9.18 × 7.12 × 8.9 cm | Approx. 580 cm3 | min. 1.2 prey/cm3 | 2–3 adult females and hundreds of crawlers |
| Nidularia pulvinata | (Hemiptera: Kermesidae) | 9.18 × 7.12 × 8.9 cm | Approx. 580 cm3 | min. 1.2 prey/cm3 | 2–3 adult females, hundreds of crawlers |
| Saissetia oleae | (Hemiptera: Coccidae) | 9.18 × 7.12 × 8.9 cm | Approx. 580 cm3 | From 0.02 to 0.03 prey/cm3 | 2–3 nymphs underside each olive leaf |
| Harmonia axyridis | (Coleoptera: Coccinellidae) | 8.55 cm (∅); 1.42 cm (h) | 81 cm3 | 0.01 prey/cm3 | LAB preys the ladybeetle without killing it |
| Apis mellifera | (Hymenoptera: Apidae) | 8.55 cm (∅); 1.42 cm (h) | 81 cm3 | 0.01 prey/cm3 | Workers |
| Philaenus spumarius | (Hemiptera: Aphrophoridae) | 10 × 10 × 5 cm | Approx. 500 cm3 | 0.02 prey/cm3 | As in [11] |
| Neophilaenus campestris | (Hemiptera: Aphrophoridae) | 10 × 10 × 5 cm | approx. 500 cm3 | 0.02 prey/cm3 | As in [11] |
| Philaenus spumarius | (Hemiptera: Aphrophoridae) | Falcon | 60 cm3 | from 0.15 to 0.25 prey/cm3 | In mixed prey ensemble |
| Neophilaenus campestris | (Hemiptera: Aphrophoridae) | Falcon | 60 cm3 | from 0.15 to 0.25 prey/cm3 | In mixed prey ensemble |
| Issidae | (Hemiptera) | Falcon | 60 cm3 | from 0.15 to 0.25 prey/cm3 | In mixed prey ensemble |
| Miridae | (Hemiptera) | Falcon | 60 cm3 | from 0.15 to 0.25 prey/cm3 | In mixed prey ensemble |
| Bactrocera oleae | (Diptera: Tephritidae) | 9.18 × 7.12 × 8.9 cm | Approx. 580 cm3 | 0.03 prey/cm3 | LAB preys the olive fly |
| Host Plant | Associated Hemipteran | LAB Instars | ||||||
|---|---|---|---|---|---|---|---|---|
| Egg | N1 | N2 | N3 | N4 | N5 | Ad | ||
| Quercus ilex | Nidularia pulvinata | 4 | ||||||
| Quercus ilex | Kermes vermilio | 1 | ||||||
| Olea europaea | Saissetia oleae | 1 | ||||||
| Olea europaea | Saissetia coffeae | 2 | ||||||
| Ficus microcarpa | Macrohomotoma gladiata | 1 | 1 | |||||
| Ficus carica | Homotoma ficus | 2 | 1 | 4 | ||||
| Ailanthus altissima | Aleurocanthus spiniferus | 7 | 1 | 2 | 1 | |||
| Pyracantha coccinea | Aleurocanthus spiniferus | 1 | 1 | 2 | 5 | |||
| Pyrus communis | Aleurocanthus spiniferus | 14 | ||||||
| Citrus CV | Aleurocanthus spiniferus | 1 | 2 | 3 | 18 | |||
| Citrus CV | Aleurothrixus floccosus | 12 | 1 | 3 | 4 | |||
| Aloysia citriodora | Aphis spp. | 1 | 1 | |||||
| Capsicum annuum | Aphis spp. | 2 | ||||||
| Chamaerops humilis | Ommatissus binotatus | 6 | 1 | 2 | 5 | |||
| Laurus nobilis | Trioza alacris | 11 | 1 | 1 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahbib, N.; Picciotti, U.; Sefa, V.; Boukhris-Bouhachem, S.; Porcelli, F.; Garganese, F. Zelus renardii Roaming in Southern Italy. Insects 2022, 13, 158. https://doi.org/10.3390/insects13020158
Lahbib N, Picciotti U, Sefa V, Boukhris-Bouhachem S, Porcelli F, Garganese F. Zelus renardii Roaming in Southern Italy. Insects. 2022; 13(2):158. https://doi.org/10.3390/insects13020158
Chicago/Turabian StyleLahbib, Nada, Ugo Picciotti, Valdete Sefa, Sonia Boukhris-Bouhachem, Francesco Porcelli, and Francesca Garganese. 2022. "Zelus renardii Roaming in Southern Italy" Insects 13, no. 2: 158. https://doi.org/10.3390/insects13020158
APA StyleLahbib, N., Picciotti, U., Sefa, V., Boukhris-Bouhachem, S., Porcelli, F., & Garganese, F. (2022). Zelus renardii Roaming in Southern Italy. Insects, 13(2), 158. https://doi.org/10.3390/insects13020158










