Long Non-Coding RNA Bmdsx-AS1 Effects on Male External Genital Development in Silkworm
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Silkworm Breeding and BmE Cell Culture
2.2. Transgenic Vector Construction and Strain Acquisition
2.3. Detection of Genome Extraction and Insertion Sites
2.4. Total RNA Extraction and Quantitative Real-Time RT-PCR (qRT-PCR)
2.5. RNA Interference (RNAi)
2.6. Bioinformatics Analyses of Gene Promoters and Prediction of Transcription Factors
2.7. Construction of a Dual Luciferase Vector
2.8. Luciferase Reporter Assay
2.9. Electrophoretic Mobility Shift Assay (EMSA)
3. Results and Discussion
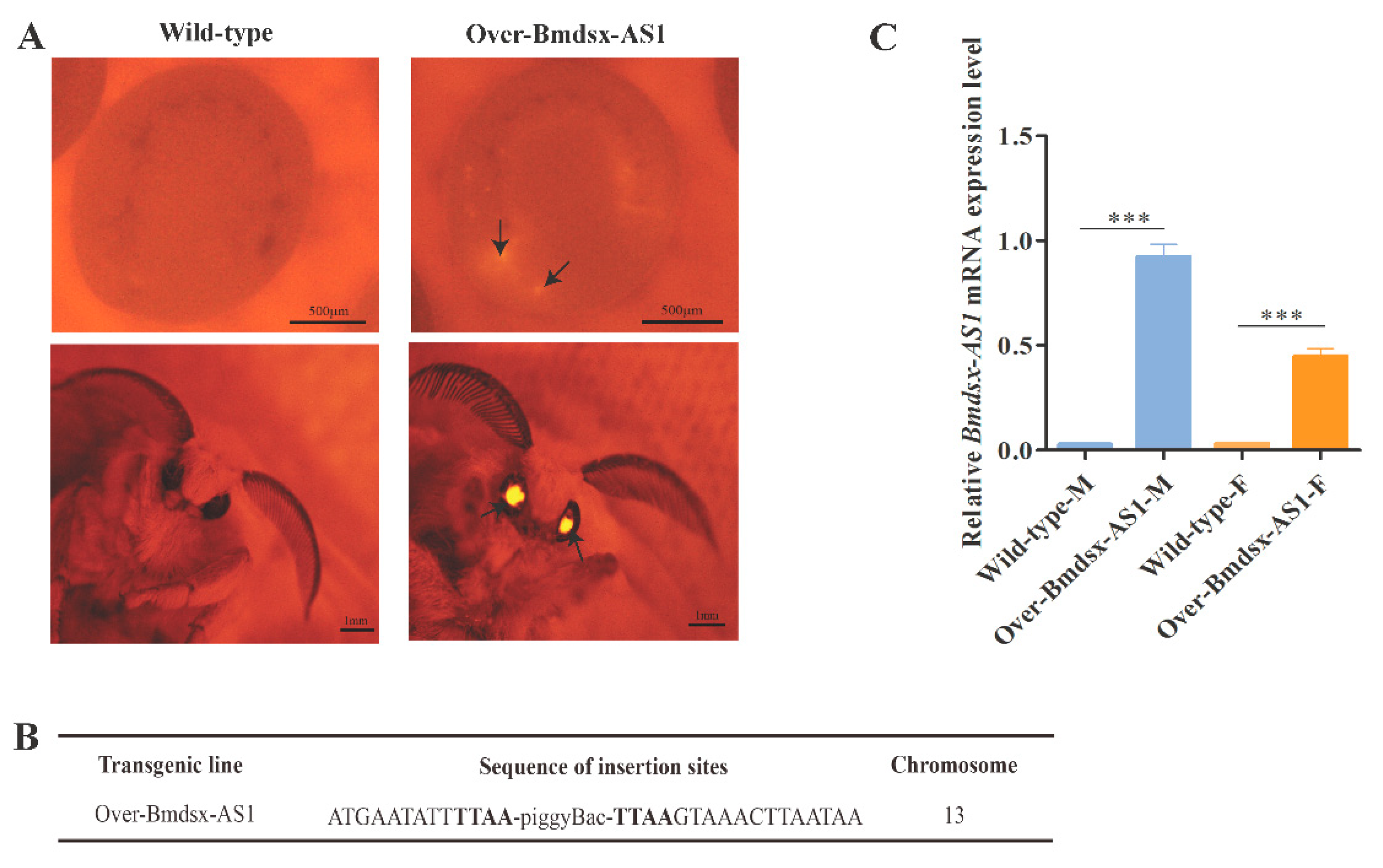
3.1. Construction of the Bmdsx-AS1 Overexpression Transgenic Line
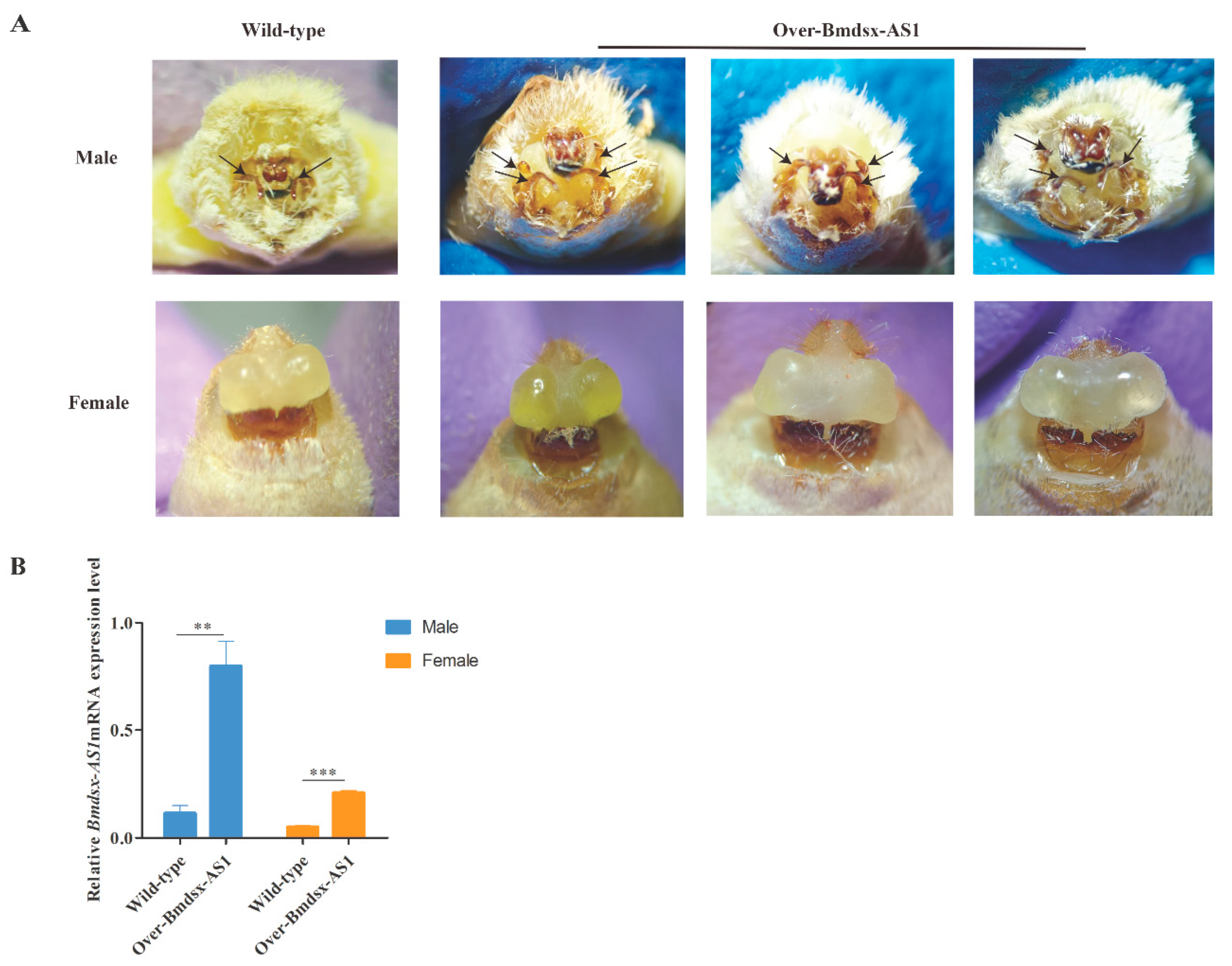
3.2. Phenotype of Over-Bmdsx-AS1 in Genitals
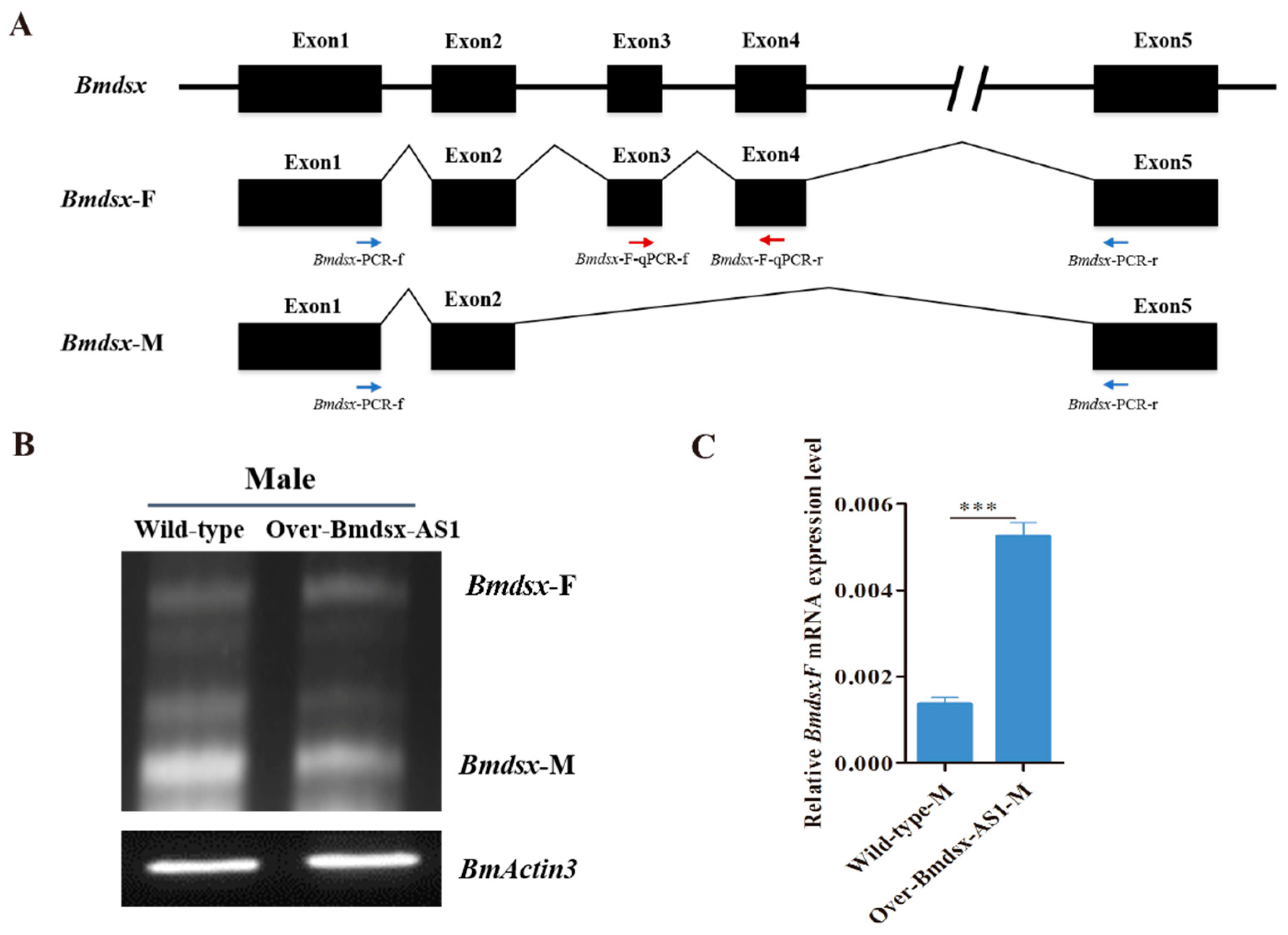
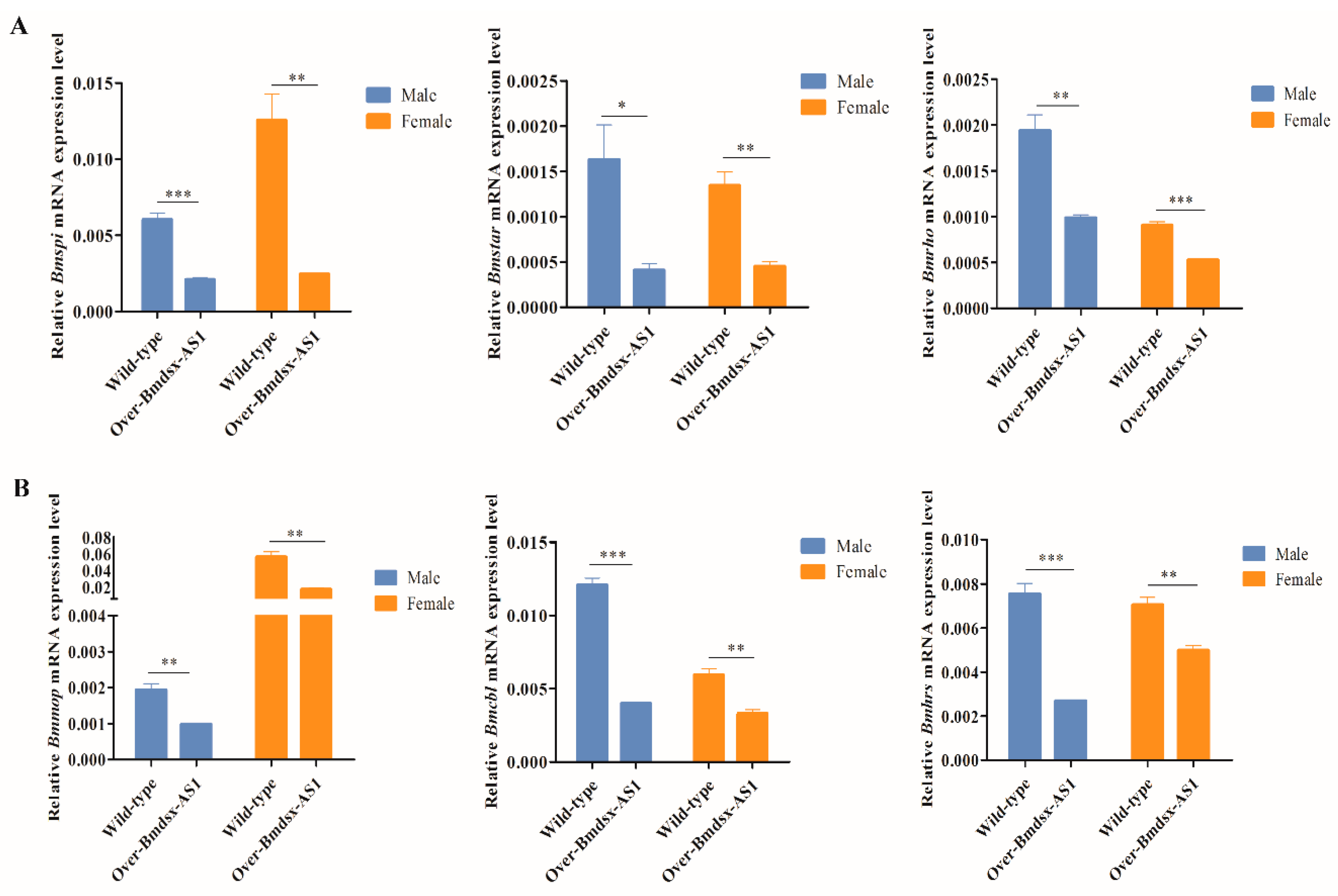
3.3. Overexpression of Bmdsx-AS1 Decreases EGFR Signaling Activity
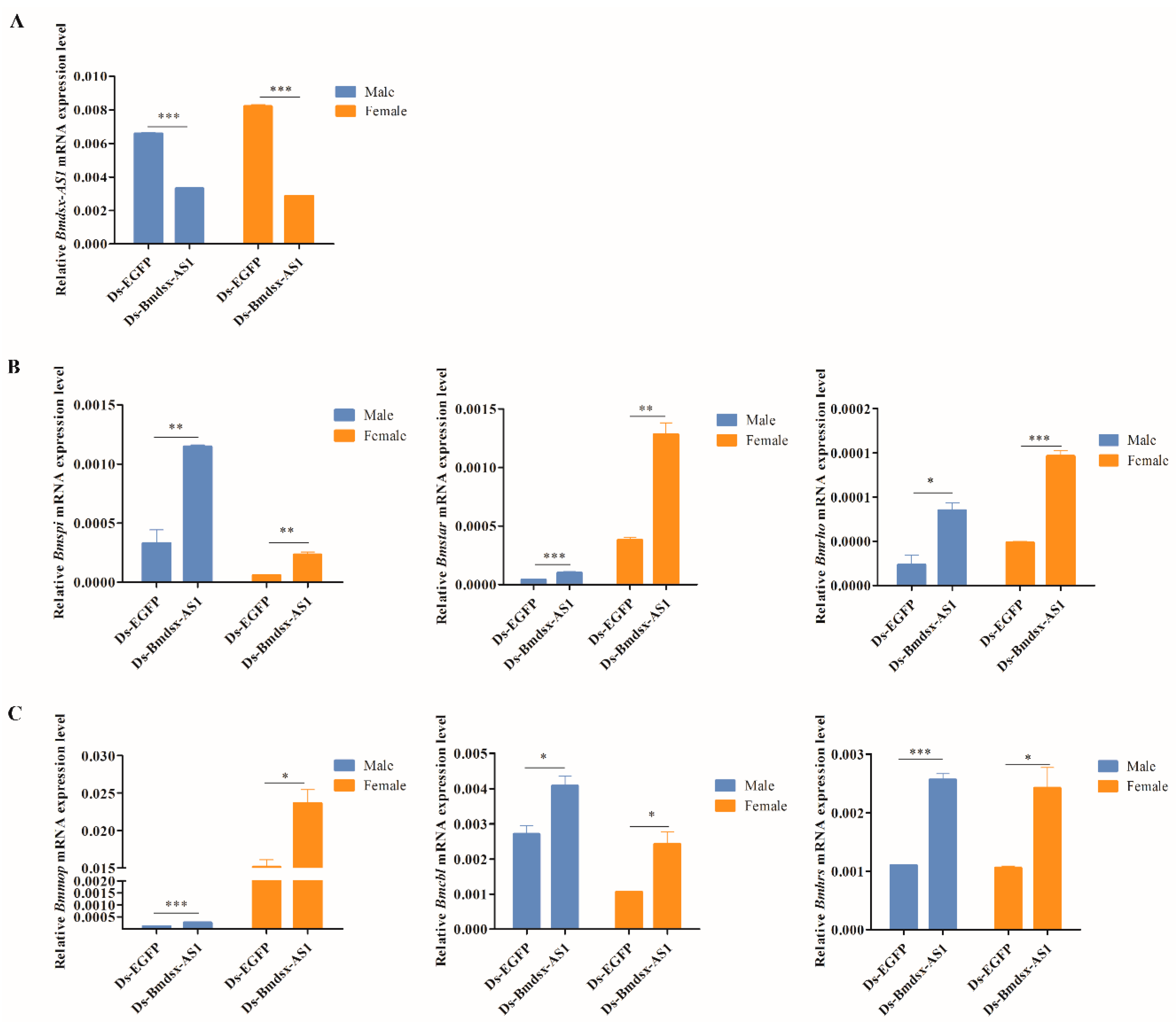
3.4. Knockdown of Bmdsx-AS1 Increases EGFR Signaling Activity
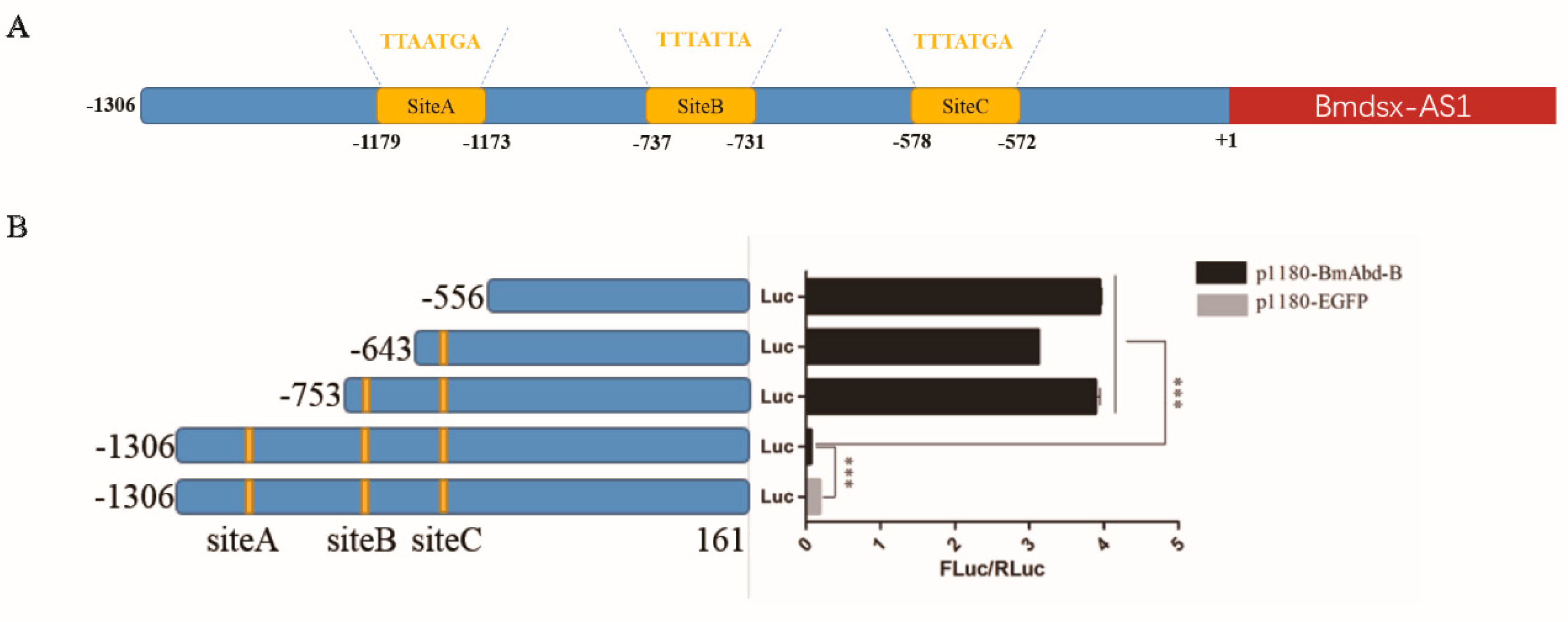
3.5. Promoter of Bmdsx-AS1 Contains BmAbd-B Cis-Element
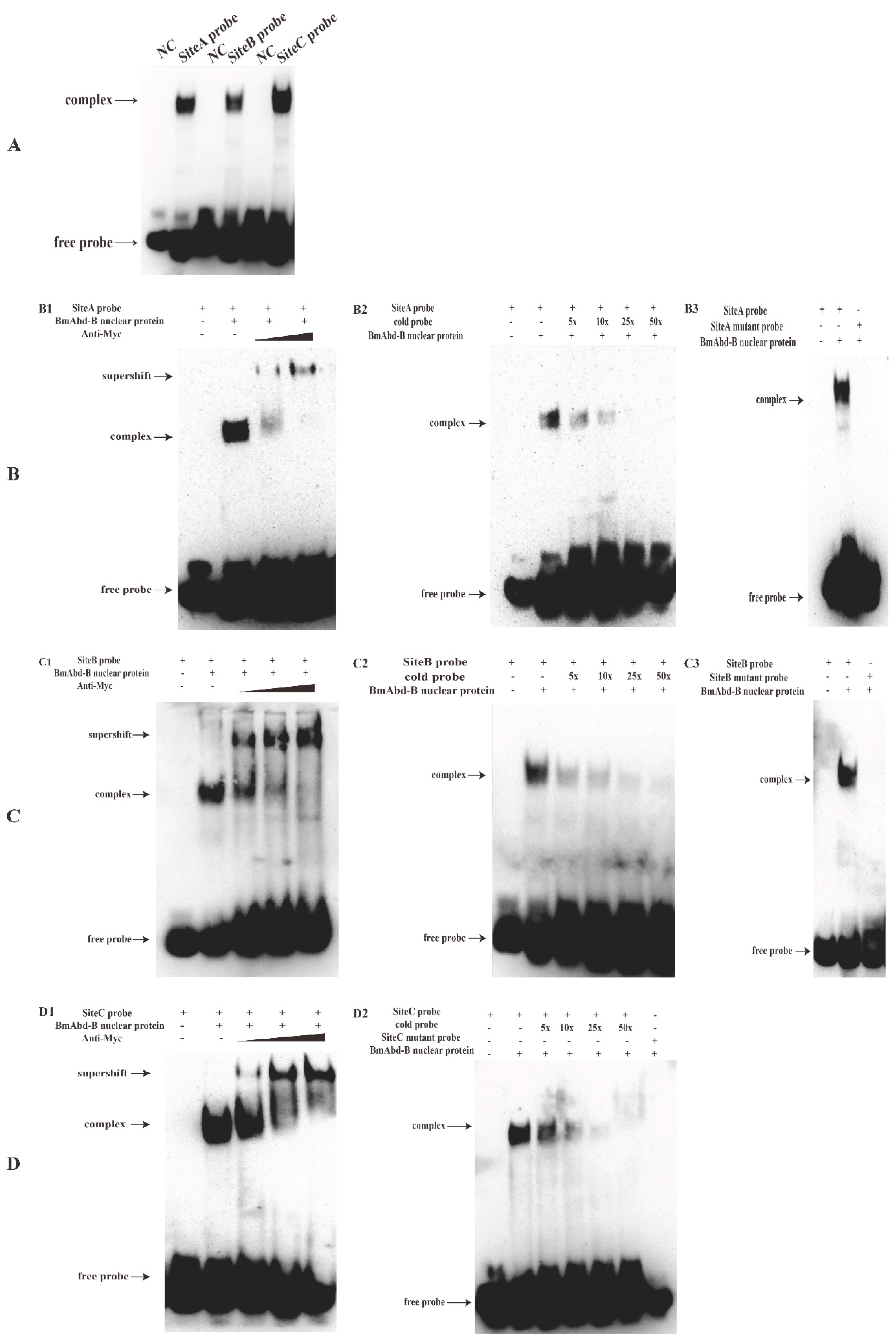
3.6. BmAbd-B Protein Specifically Binds to Promoter of Bmdsx-AS1
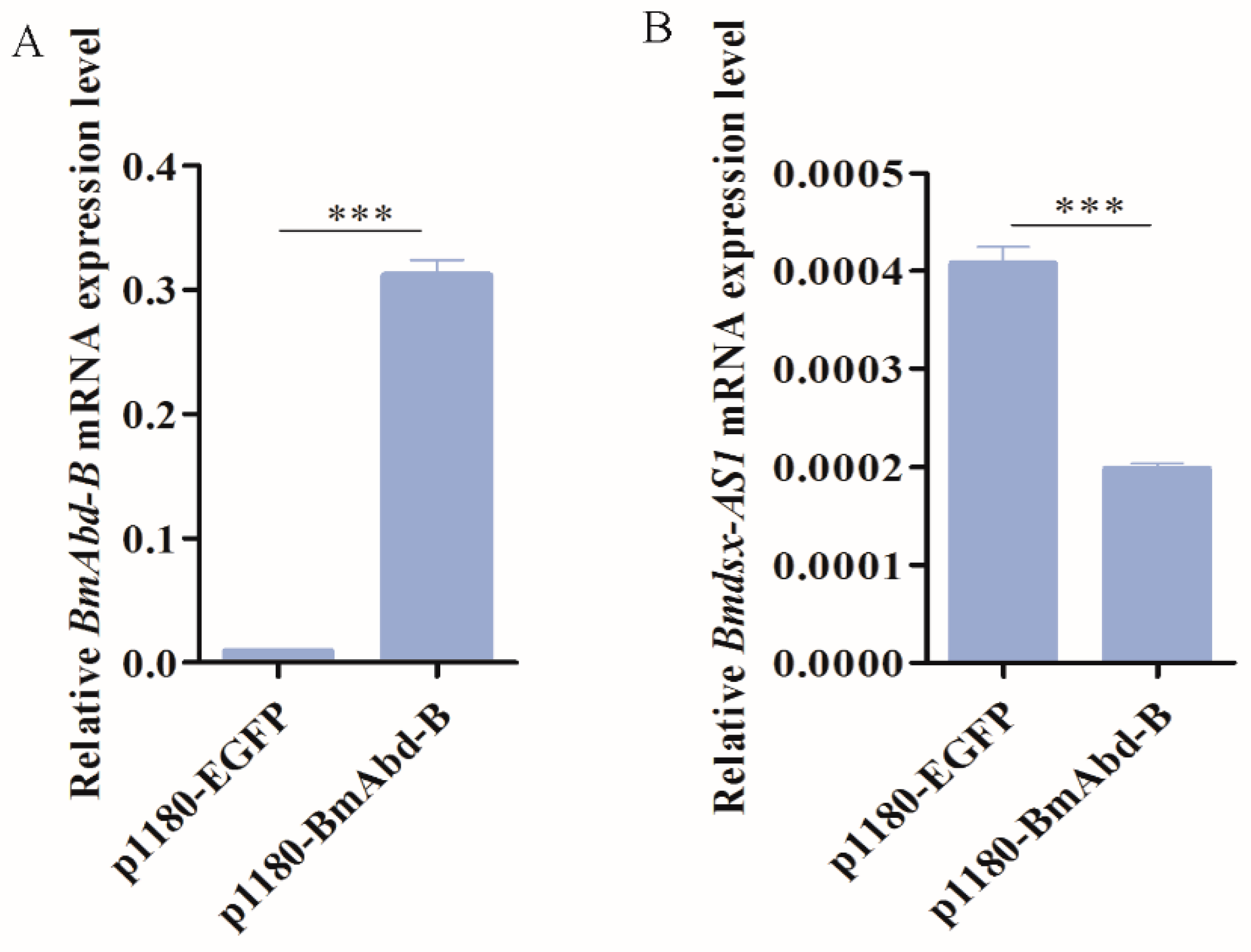
3.7. BmAbd-B Regulates Expression of Bmdsx-AS1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| dsx | Doublesex |
| Abd-B | Abdominal-B |
| SV40 | Terminator of Simian virus 40 |
| M-MLV | Moloney Murine Leukemia Virus |
| PCR | Polymerase Chain Reaction |
| MBP | Maltose binding protein |
| DsRed | Discosoma sp. Red Fluorescent Protein |
| BmE | Embryonic cells of silkworm |
| EGFP | Enhanced green fluorescent protein |
| hrs | Hepatocyte growth factor-regulated tyrosine kinase substrate |
| rho | Rhodopsin |
| spi | spitz |
| cbl | Cbl proto-oncogene |
| EGFR | Epidermal Growth Factor Receptor |
| RTK | Receptor Tyrosine Kinase |
| qPCR | Quantitative Real-Time PCR |
| EMSA | Electrophoretic mobility shift assay |
References
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef]
- Satyavathi, V.; Ghosh, R.; Subramanian, S. Long Non-Coding RNAs Regulating Immunity in Insects. Noncoding RNA 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and Functions of Long Noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef]
- Xia, Q.Y.; Zhou, Z.Y.; Lu, C.; Cheng, D.J.; Dai, F.Y.; Li, B.; Zhao, P.; Zha, X.F.; Cheng, T.C.; Chai, C.L.; et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar]
- Xia, Q.; Guo, Y.; Zhang, Z.; Li, D.; Xuan, Z.; Li, Z.; Dai, F.; Li, Y.; Cheng, D.; Li, R.; et al. Complete resequencing of 40 genomes reveals domestication events and genes in silkworm (Bombyx). Science 2009, 326, 433–436. [Google Scholar] [CrossRef]
- Kawamoto, M.; Jouraku, A.; Toyoda, A.; Yokoi, K.; Minakuchi, Y.; Katsuma, S.; Fujiyama, A.; Kiuchi, T.; Yamamoto, K.; Shimada, T. High-quality genome assembly of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2019, 107, 53–62. [Google Scholar] [CrossRef]
- Tamura, T.; Thibert, C.; Royer, C.; Kanda, T.; Abraham, E.; Kamba, M.; Komoto, N.; Thomas, J.L.; Mauchamp, B.; Chavancy, G.; et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector (vol. 18, pg. 81, 2000). Nat. Biotechnol. 2000, 18, 559. [Google Scholar] [CrossRef]
- Quan, G.X.; Kanda, T.; Tamura, T. Induction of the white egg 3 mutant phenotype by injection of the double-stranded RNA of the silkworm white gene. Insect Mol. Biol. 2002, 11, 217–222. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, Z.Q.; Xu, J.; Zeng, B.S.; Ling, L.; You, L.; Chen, Y.Z.; Huang, Y.P.; Tan, A.J. The CRISPR/Cas System mediates efficient genome engineering in Bombyx mori. Cell Res. 2013, 23, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Y.; Smagghe, G.; Xia, Q.Y. Genome editing in Bombyx mori: New opportunities for silkworm functional genomics and the sericulture industry. Insect Sci. 2019, 26, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, H.; Xiang, Z.; Lu, C.; Dai, F.; Tong, X. Identification and characterization of a new long noncoding RNA iab-1 in the Hox cluster of silkworm, Bombyx mori identification of iab-1. J. Cell Biochem. 2019, 120, 17283–17292. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Iwami, M.; Kiya, T. Identification and characterization of a novel nuclear noncoding RNA, Fben-1, which is prefer entially expressed in the higher brain center of the female silkworm moth, Bombyx mori. Neurosci. Lett. 2011, 496, 176–180. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, T.; Liu, C.; Liu, D.; Zhang, Q.; Long, R.; Zhao, P.; Xia, Q. Systematic Identification and Characterization of Long Non-Coding RNAs in the Silkworm, Bombyx mori. PLoS ONE 2016, 11, e0147147. [Google Scholar] [CrossRef]
- Zhou, Q.Z.; Fang, S.M.; Zhang, Q.; Yu, Q.Y.; Zhang, Z. Identification and comparison of long non-coding RNAs in the silk gland between domestic and wild silkworms. Insect Sci. 2018, 25, 604–616. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, H.; Shen, M.; Huang, H.; Hou, Q.; Zhang, Z.; Zhao, W.; Guo, X.; Wu, P. Analysis of lncRNA-mediated gene regulatory network of Bombyx mori in response to BmNPV infection. J. Invertebr. Pathol. 2020, 170, 107323. [Google Scholar] [CrossRef]
- Zhou, Q.Z.; Zhang, B.; Yu, Q.Y.; Zhang, Z. BmncRNAdb: A comprehensive database of non-coding RNAs in the silkworm, Bombyx mori. BMC Bioinform. 2016, 17, 370. [Google Scholar] [CrossRef]
- Xu, X.; Wang, K.; Zha, X. An antisense lncRNA functions in alternative splicing of Bmdsx in the silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 2019, 516, 639–644. [Google Scholar] [CrossRef]
- Horn, C.; Wimmer, E.A. A versatile vector set for animal transgenesis. Dev. Genes Evol. 2000, 210, 630–637. [Google Scholar] [CrossRef]
- Wang, G.H.; Xia, Q.Y.; Cheng, D.J.; Duan, J.; Zhao, P.; Chen, J.; Zhu, L. Reference genes identified in the silkworm Bombyx mori during metamorphism based on oligonucleotide microarray and confirmed by qRT-PCR. Insect Sci. 2008, 15, 405–413. [Google Scholar] [CrossRef]
- Liu, H.L.; Lin, Y.; Shen, G.W.; Gu, J.J.; Ruan, Y.; Wu, J.X.; Zhang, Y.J.; Li, K.R.; Long, W.; Jia, L.B.; et al. A novel GATA transcrip tion factor GATA beta 4 promotes vitellogenin transcription and egg formation in the silkworm Bombyx mori. Insect Biochem. Mol. 2019, 107, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.D.; Hu, Q.H.; Tong, C.M.; Yang, H.G.; Zheng, S.C.; Feng, Q.L.; Deng, H.M. Transcriptomic analysis reveals the regulation network of BmKruppel homolog 1 in the oocyte development of Bombyx mori. Insect Sci. 2020, 28, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, F.; Suzuki, M.G.; Mita, K.; Okano, K.; Shimada, T. A homologue of the Drosophila doublesex gene is transcribedinto sex-specific mRNA isoforms in the silkworm, Bombyx mori. Comp. Biochem. Phys. B 2001, 128, 145–158. [Google Scholar] [CrossRef]
- Suzuki, M.G.; Ohbayashi, F.; Mita, K.; Shimada, T. The mechanism of sex-specific splicing at the doublesex gene is different between Drosophila melanogaster and Bombyx mori. Insect Biochem. Mol. 2001, 31, 1201–1211. [Google Scholar] [CrossRef]
- Suzuki, M.G.; Funaguma, S.; Kanda, T.; Tamura, T.; Shimada, T. Analysis of the biological functions of a doublesex homologue in Bombyx mori. Dev. Genes Evol. 2003, 213, 345–354. [Google Scholar] [CrossRef]
- Duan, J.; Xu, H.; Ma, S.; Guo, H.; Wang, F.; Zhang, L.; Zha, X.; Zhao, P.; Xia, Q. Ectopic expression of the male BmDSX affects formation of the chitin plate in female Bombyx mori. Mol. Reprod. Dev. 2014, 81, 240–247. [Google Scholar] [CrossRef]
- Schweitzer, R.; Shaharabany, M.; Seger, R.; Shilo, B.-Z. Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes Dev. 1995, 9, 1518–1529. [Google Scholar] [CrossRef]
- Mayer, U.; Nüsslein-Volhard, C. A group of genes required for pattern formation in the ventral ectoderm of the Drosophila embryo. Genes Dev. 1988, 2, 1496–1511. [Google Scholar] [CrossRef]
- Lee, J.R.; Urban, S.; Garvey, C.F.; Freeman, M. Regulated intracellular ligand transport and proteolysis control EGF signal acti vation in Drosophila. Cell 2001, 107, 161–171. [Google Scholar] [CrossRef]
- Urban, S.; Lee, J.R.; Freeman, M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 2001, 107, 173–182. [Google Scholar] [CrossRef]
- Seto, E.S.; Bellen, H.J.; Lloyd, T.E. When cell biology meets development: Endocytic regulation of signaling pathways. Genes Dev. 2002, 16, 1314–1336. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I. Mechanisms Controlling EGF Receptor Endocytosis and Degradation. Biochem. Soc. Trans. 2003, 31, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Stern, K.A.; Smit, G.D.V.; Place, T.L.; Winistorfer, S.; Piper, R.C.; Lill, N.L. Epidermal growth factor receptor fate is controlled by Hrs tyrosine phosphorylation sites that regulate Hrs degradation. Mol. Cell. Biol. 2007, 27, 888–898. [Google Scholar] [CrossRef]
- Miura, G.I.; Roignant, J.-Y.; Wassef, M.; Treisman, J.E. Myopic acts in the endocytic pathway to enhance signaling by the Dro sophila EGF receptor. Development 2008, 135, 1913–1922. [Google Scholar] [CrossRef]
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.L.; Barthel, A.; et al. RNA interference in Lepidoptera: An overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, B.; Ling, L.; Xu, J.; You, L.; Aslam, A.F.; Tan, A.; Huang, Y. Enhancement of larval RNAi efficiency by over-express ing Argonaute2 in Bombyx mori. Int. J. Biol. Sci. 2015, 11, 176–185. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, J.; Wen, M.Y.; Wang, H.; Wang, Y.; Wang, K.X.; Wan, Q.X.; Zha, X.F. A Novel Splice Variant of the Masculinizing Gene Masc with piRNA-Cleavage-Site Defect Functions in Female External Genital Development in the Silkworm, Bombyx mori. Biomolecules 2019, 9, 318. [Google Scholar] [CrossRef]
- Wasserman, J.D.; Freeman, M. An autoregulatory cascade of EGF receptor signaling patterns the Drosophila egg. Cell 1998, 95, 355–364. [Google Scholar] [CrossRef]
- Tsruya, R.; Schlesinger, A.; Reich, A.; Gabay, L.; Sapir, A.; Shilo, B.-Z. Intracellular trafficking by Star regulates cleavage of the Drosophila EGF receptor ligand Spitz. Genes Dev. 2002, 16, 222–234. [Google Scholar] [CrossRef]
- Kiuchi, T.; Koga, H.; Kawamoto, M.; Shoji, K.; Sakai, H.; Arai, Y.; Ishihara, G.; Kawaoka, S.; Sugano, S.; Shimada, T.; et al. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 2014, 509, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Kalis, A.K.; Murphy, M.W.; Zarkower, D. EGL-5/ABD-B plays an instructive role in male cell fate determination in the C. elegans somatic gonad. Dev. Biol. 2010, 344, 827–835. [Google Scholar] [CrossRef]
- Dolle, P.; Izpisua-Belmonte, J.; Brown, J.; Tickle, C.; Duboule, D. HOX-4 genes and the morphogenesis of mammalian genitalia. Genes Dev. 1991, 5, 1767–1776. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foronda, D.; Martin, P.; Sanchez-Herrero, E. Drosophila Hox and sex-determination genes control segment elimination through EGFR and extramacrochetae activity. PLoS Genet. 2012, 8, e1002874. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yoder, J.H. Hox-mediated regulation of doublesex sculpts sex-specific abdomen morphology in Drosophila. Dev. Dyn. 2012, 241, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Herrero, E.; Vernós, I.; Marco, R.; Morata, G. Genetic organization of Drosophila bithorax complex. Nature 1985, 313, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Tiong, S.; Bone, L.M.; Whittle, J.R.S. Recessive lethal mutations within the bithorax-complex in Drosophila. Mol. Gen. Genet. MGG 1985, 200, 335–342. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.-X.; Chen, C.-B.; Wan, Q.-X.; Zha, X.-F. Long Non-Coding RNA Bmdsx-AS1 Effects on Male External Genital Development in Silkworm. Insects 2022, 13, 188. https://doi.org/10.3390/insects13020188
Wang K-X, Chen C-B, Wan Q-X, Zha X-F. Long Non-Coding RNA Bmdsx-AS1 Effects on Male External Genital Development in Silkworm. Insects. 2022; 13(2):188. https://doi.org/10.3390/insects13020188
Chicago/Turabian StyleWang, Kai-Xuan, Chun-Bing Chen, Qiu-Xing Wan, and Xing-Fu Zha. 2022. "Long Non-Coding RNA Bmdsx-AS1 Effects on Male External Genital Development in Silkworm" Insects 13, no. 2: 188. https://doi.org/10.3390/insects13020188
APA StyleWang, K.-X., Chen, C.-B., Wan, Q.-X., & Zha, X.-F. (2022). Long Non-Coding RNA Bmdsx-AS1 Effects on Male External Genital Development in Silkworm. Insects, 13(2), 188. https://doi.org/10.3390/insects13020188





