Genetic Differences among Established Populations of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) in Japan: Suggestion of Multiple Introductions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EPPO. Aromia bungii. EPPO Bull. 2015, 45, 4–8. [Google Scholar] [CrossRef]
- Huang, B.K.; Zhao, H.Q. Aromia bungii Faldermann. In Forest Insects of China; Xiao, G., Ed.; China Forestry Press: Beijing, China, 1992; pp. 465–466. (In Chinese) [Google Scholar]
- Shoda-Kagaya, E. Invasion of the red-necked longicorn beetle, Aromia bungii: Damages of Rosaceae trees and practical control methods. Tree For. Health 2018, 22, 68–72. (In Japanese) [Google Scholar]
- Hougen, T.; Kitajima, H.; Katsuki, T. Growth of an alien long-horned beetle, Aromia bungii hatchlings inoculated into smart twigs of montane rosaceous tree species. For. Pests 2019, 68, 99–103. (In Japanese) [Google Scholar]
- Special Report on Forecast of Pest Occurrence. No. 2. Available online: https://www.pref.aichi.jp/uploaded/attachment/319462.pdf (accessed on 5 July 2020). (In Japanese).
- Iwata, R. Aromia bungii (Coleoptera: Cerambycidae): Taxonomy, distribution, biology and eradication. For. Pests 2018, 67, 189–216. (In Japanese) [Google Scholar]
- Tamura, S.; Shoda-Kagaya, E. The expansion of Aromia bungii in Japan. Shinrin Kagaku 2020, 89, 21–25. (In Japanese) [Google Scholar] [CrossRef]
- Kano, M.; Nonaka, T.; Kiriyama, S.; Iwata, R. Aromia bungii (Coleoptera: Cerambycidae), an invasive cerambycid, found at Soka, Saitama Pref., Japan, infesting cherry trees, Cerasus × yedoensis “Somei-yoshino”. For. Pests 2014, 63, 101–105. (In Japanese) [Google Scholar]
- Kiriyama, S.; Iwata, R.; Shoda-Kagaya, E. Newly discovered populations of Aromia bungii (Faldermann), an invasive cerambycid infesting cherry and Japanese apricot trees in Tatebayashi, Gunma Pref., and Fussa, Tokyo Pref. Plant Prot. 2015, 69, 807–809. (In Japanese) [Google Scholar]
- The Ecology and Control Methods of Aromia bungii. Available online: http://www.jppn.ne.jp/osaka/color/Aromia_%0Abungii/Aromia_bungii(H3104).pdf (accessed on 5 July 2020). (In Japanese).
- Nakano, A. Damage caused by the red-necked longhorn beetle, Aromia bungii in Tokushima Prefecture and control measure. Jpn. J. Pestic. Sci. 2018, 43, 12–16. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Brockerhoff, E.G.; Liebhold, A.M. Ecology of forest insect invasions. Biol. Invasions 2017, 19, 3141–3159. [Google Scholar] [CrossRef]
- Cognato, A.I.; Hoebeke, E.R.; Kajimura, H.; Smith, S.M. History of the exotic ambrosia beetles Euwallacea interjectus and Euwallacea validus (Coleoptera: Curculionidae: Xyleborini) in the United States. J. Econ. Entomol. 2015, 108, 1129–1135. [Google Scholar] [CrossRef]
- Bittner, T.D.; Hajek, A.E.; Haavik, L.; Allison, J.; Nahrung, H. Multiple introductions of Sirex noctilio (Hymenoptera: Siricidae) in northeastern North America based on microsatellite genotypes, and implications for biological control. Biol. Invasions 2017, 19, 1431–1447. [Google Scholar] [CrossRef]
- Javal, M.; Lombaert, E.; Tsykun, T.; Courtin, C.; Kerdelhué, C.; Prospero, S.; Roques, A.; Roux, G. Deciphering the worldwide invasion of the Asian long-horned beetle: A recurrent invasion process from the native area together with a bridgehead effect. Mol. Ecol. 2019, 28, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Javal, M.; Roques, A.; Haran, J.; Hérard, F.; Keena, M.; Roux, G. Complex invasion history of the Asian long-horned beetle: Fifteen years after first detection in Europe. J. Pest Sci. 2019, 92, 173–187. [Google Scholar] [CrossRef]
- Wu, Y.; Krishnankutty, S.M.; Vieira, K.A.; Wang, B.; Nadel, H.; Myers, S.W.; Ray, A.M. Invasion of Trichoferus campestris (Coleoptera: Cerambycidae) into the United States characterized by high levels of genetic diversity and recurrent introductions. Biol. Invasions 2020, 22, 1309–1323. [Google Scholar] [CrossRef]
- Pest Risk Analysis for Aromia bungii. Available online: http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm (accessed on 5 July 2020).
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Roehrdanz, R.L. An improved primer for PCR amplification of mitochondrial DNA in a variety of insect species. Insect Mol. Biol. 1993, 2, 89–91. [Google Scholar] [CrossRef]
- Kawai, M.; Shoda-Kagaya, E.; Maehara, T.; Zhou, Z.; Lian, C.; Iwata, R.; Yamane, A.; Hogetsu, T. Genetic structure of pine sawyer Monochamus alternatus (Coleoptera: Cerambycidae) populations in Northeast Asia: Consequences of the spread of pine wilt disease. Environ. Entomol. 2006, 35, 569–579. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program fro Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Paradis, E. Pegas: An R package for population genetics with an integrated-modular approach. Bioinformatics 2010, 26, 419–420. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 1 December 2021).
- Dlugosch, K.M.; Parker, I.M. Founding events in species invasions: Genetic variation, adaptive evolution, and the role of multiple introductions. Mol. Ecol. 2008, 17, 431–449. [Google Scholar] [CrossRef]
- Lee, S.; Cha, D.; Nam, Y.; Jung, J. Genetic diversity of a rising invasive pest in the native range: Population genetic structure of aromia bungii (coleoptera: Cerambycidae) in South Korea. Diversity 2021, 13, 582. [Google Scholar] [CrossRef]
- Hu, C.; Ding, Y.; Sun, K. Research advances of Aromia bungii in China. Agric. Technol. 2007, 27, 63–67. (In Chinese) [Google Scholar]
- Urano, T. Reproductive biology of Aromia bungii (Faldermann) (Coleoptera: Cerambycidae) adults emerged from infested trunks of Cerasus yedoensis “Somei-Yoshino”. For. Pests 2018, 67, 230–236. (In Japanese) [Google Scholar]
- Russo, E.; Nugnes, F.; Vicinanza, F.; Garonna, A.P.; Bernardo, U. Biological and molecular characterization of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae), an emerging pest of stone fruits in Europe. Sci. Rep. 2020, 10, 7112. [Google Scholar] [CrossRef] [PubMed]
- Haack, R.A.; Keena, M.A.; Eyre, D. Life history and population dynamics of cerambycids. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 71–103. ISBN 9781315313245. [Google Scholar]
- Fukaya, M.; Kiriyama, S.; Yasui, H. Mate-location flight of the red-necked longicorn beetle, Aromia bungii (Coleoptera: Cerambycidae): An invasive pest lethal to Rosaceae trees. Appl. Entomol. Zool. 2017, 52, 559–565. [Google Scholar] [CrossRef]
- Xu, T.; Yasui, H.; Teale, S.A.; Fujiwara-Tsujii, N.; Wickham, J.D.; Fukaya, M.; Hansen, L.; Kiriyama, S.; Hao, D.; Nakano, A.; et al. Identification of a male-produced sex-aggregation pheromone for a highly invasive cerambycid beetle. Aromia Bungii. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Emiljanowicz, L.M.; Hager, H.A.; Newman, J.A. Traits related to biological invasion: A note on the applicability of risk assessment tools across taxa. NeoBiota 2017, 32, 31–64. [Google Scholar] [CrossRef] [Green Version]
- Primack, R.; Higuchi, H. Climate change and cherry tree blossom festivals in Japan. Arnoldia 2007, 65, 14–22. [Google Scholar]
- Sakurai, R.; Jacobson, S.K.; Kobori, H.; Primack, R.; Oka, K.; Komatsu, N.; Machida, R. Culture and climate change: Japanese cherry blossom festivals and stakeholders’ knowledge and attitudes about global climate change. Biol. Conserv. 2011, 144, 654–658. [Google Scholar] [CrossRef]
- Hörren, T. A further proof of the Asian redneck long-horned beetle Aromia bungii (Faldermann, 1835) in Germany (Coleoptera: Cerambycidae, Cerambycinae). Entomol. Zeitschrift 2016, 126, 205–207. (In Germany) [Google Scholar]
- EFSA; de la Peña, E.; Schrader, G.; Vos, S. Pest survey card on Aromia bungii. EFSA Support. Publ. 2019, 2019, EN-17. [Google Scholar] [CrossRef] [Green Version]
- Liebhold, A.M.; Halverson, J.A.; Elmes, G.A. Gypsy moth invasion in North America: A quantitative analysis. J. Biogeogr. 1992, 19, 513–520. [Google Scholar] [CrossRef]
- Muirhead, J.R.; Leung, B.; Van Overdijk, C.; Kelly, D.W.; Nandakumar, K.; Marchant, K.R.; MacIsaac, H.J. Modelling local and long-distance dispersal of invasive emerald ash borer Agrilus planipennis (Coleoptera) in North America. Divers. Distrib. 2006, 12, 71–79. [Google Scholar] [CrossRef]
- Robinet, C.; Imbert, C.E.; Rousselet, J.; Sauvard, D.; Garcia, J.; Goussard, F.; Roques, A. Human-mediated long-distance jumps of the pine processionary moth in Europe. Biol. Invasions 2012, 14, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Kinuura, H.; Shirotsuka, K.; Yamamoto, Y.; Tokoro, M.; Shoda-Kagaya, E. Invasion of the red-necked longicorn beetle, Aromia bungii in Kansai region of Japan. For. Pests 2018, 67, 221–223. (In Japanese) [Google Scholar]
- MAFF Notification No.1352, for Amendment of Import Plant Quarantine Regulation (MAF Notification No. 206, 1950). Available online: http://www.pps.go.jp/english/woodpack/import/Implementation_of_ISPM_No15_E2.pdf (accessed on 27 July 2020).
- Zahid, M.I.; Grgurinovic, C.A.; Walsh, D.J. Quarantine risks associated with solid wood packaging materials receiving ISPM 15 treatments. Aust. For. 2008, 71, 287–293. [Google Scholar] [CrossRef]
- Haack, R.A.; Britton, K.O.; Brockerhoff, E.G.; Cavey, J.F.; Garrett, L.J.; Kimberley, M.; Lowenstein, F.; Nuding, A.; Olson, L.J.; Turner, J.; et al. Effectiveness of the international phytosanitary standard ISPM No. 15 on reducing wood borer infestation rates in wood packaging material entering the United States. PLoS ONE 2014, 9, e96611. [Google Scholar] [CrossRef]
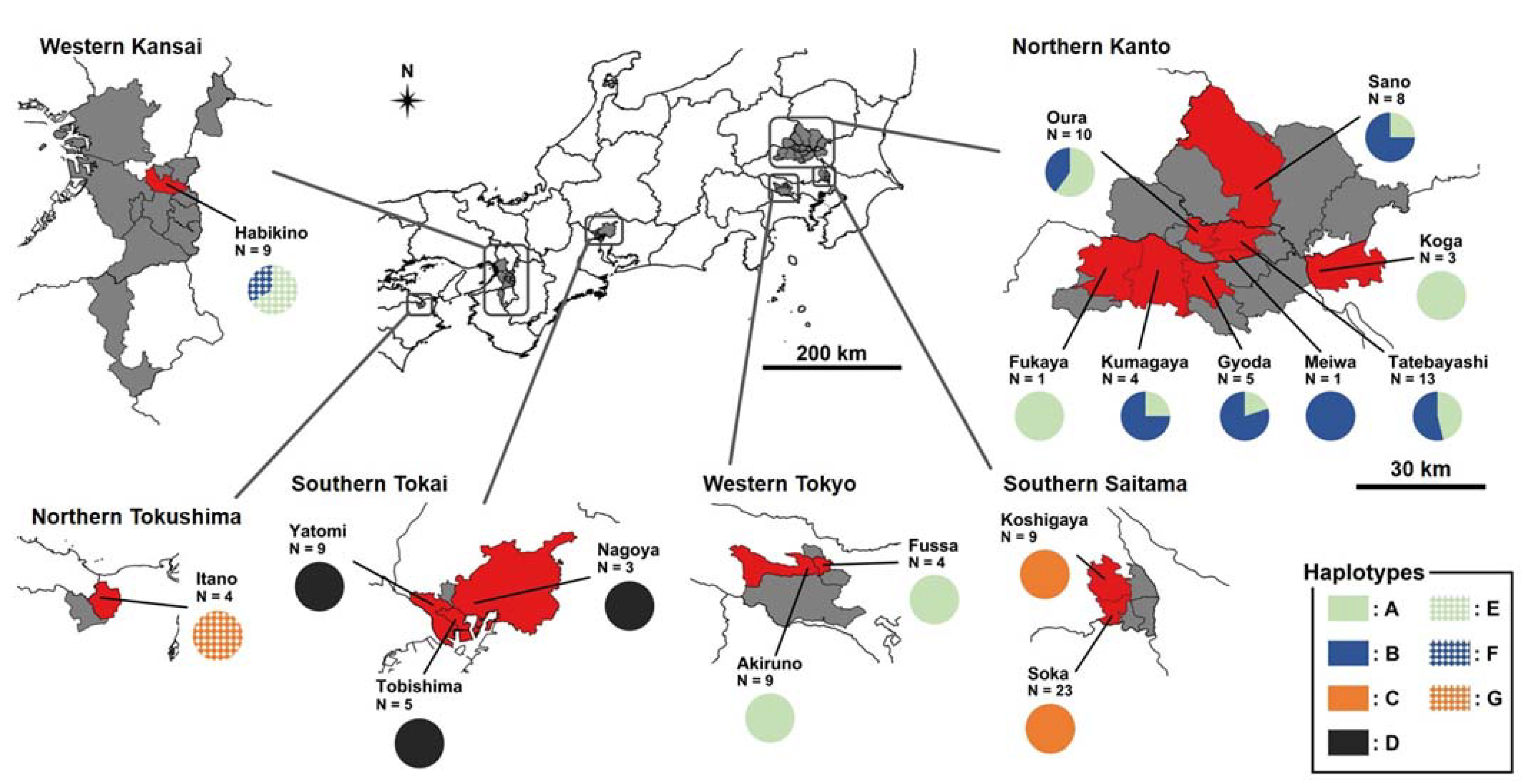



| Introduced Region | Prefecture | Municipality | Site | Longitude | Latitude | Year | Haplotype Composition | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |||||||
| Northern Kanto | Gunma | Tatebayashi | GTa1 | 36°14.6′ N | 139°30.8′ E | 2018 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| GTa2 | 36°14.5′ N | 139°32.8′ E | 2018 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |||
| GTa3 | 36°13.7′ N | 139°32.0′ E | 2018 | 2 | 6 | 0 | 0 | 0 | 0 | 0 | |||
| Oura | GOu1 | 36°14.3′ N | 139°29.5′ E | 2019 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | ||
| GOu2 | 36°15.2′ N | 139°28.3′ E | 2019 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| GOu3 | 36°16.2′ N | 139°26.9′ E | 2019 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | |||
| Meiwa | GMe1 | 36°12.5′ N | 139°31.8′ E | 2018 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| Tochigi | Sano | TSa1 | 36°17.0′ N | 139°32.8′ E | 2018 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 2019 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | ||||||
| Ibaraki | Koga | IKo1 | 36°10.8′ N | 139°42.1′ E | 2019 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Saitama | Fukaya | SFu1 | 36°10.6′ N | 139°13.6′ E | 2019 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Kumagaya | SKu1 | 36°09.7′ N | 139°24.7′ E | 2019 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | ||
| Gyoda | SGy1 | 36°10.9′ N | 139°28.4′ E | 2019 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | ||
| Southern Saitama | Saitama | Soka | SSo1 | 35°50.4′ N | 139°47.7′ E | 2017 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| SSo2 | 35°50.2′ N | 139°49.1′ E | 2018 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | |||
| SSo3 | 35°50.7′ N | 139°49.7′ E | 2018 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | |||
| SSo4 | 35°50.8′ N | 139°49.8′ E | 2018 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |||
| SSo5 | 35°50.8′ N | 139°49.5′ E | 2019 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | |||
| SSo6 | 35°51.6′ N | 139°48.9′ E | 2019 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | |||
| SSo7 | 35°50.3′ N | 139°49.6′ E | 2019 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |||
| SSo8 | 35°50.7′ N | 139°49.5′ E | 2019 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |||
| SSo9 | 35°51.0′ N | 139°49.3′ E | 2019 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | |||
| SSo10 | 35°50.8′ N | 139°49.7′ E | 2019 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | |||
| SSo11 | 35°51.5′ N | 139°50.1′ E | 2019 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |||
| SSo12 | 35°51.6′ N | 139°49.6′ E | 2019 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | |||
| Koshigaya | Sko1 | 35°52.3′ N | 139°48.3′ E | 2019 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | ||
| Sko2 | 35°53.3′ N | 139°48.0′ E | 2019 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |||
| Western Tokyo | Tokyo | Akiruno | TAk1 | 35°43.5′ N | 139°19.5′ E | 2017 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2018 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Fussa | TFu1 | 35°44.1′ N | 139°19.2′ E | 2019 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Southern Tokai | Aichi | Tobishima | ATo1 | 35°04.9′ N | 136°47.2′ E | 2015 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| ATo2 | 35°05.1′ N | 136°46.9′ E | 2017 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |||
| ATo3 | NA | NA | 2019 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | |||
| Yatomi | AYa1 | 35°06.3′ N | 136°44.9′ E | 2019 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | ||
| AYa2 | 35°04.8′ N | 136°45.1′ E | 2019 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | |||
| Nagoya | ANa1 | 35°07.1′ N | 136°52.3′ E | 2019 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | ||
| Western Kansai | Osaka | Habikino | OHa1 | 34°32.1′ N | 135°35.9′ E | 2017 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| 2019 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | ||||||
| OHa2 | 34°32.3′ N | 135°35.5′ E | 2019 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | |||
| Northern Tokushima | Tokushima | Itano | TIt1 | NA | NA | 2017 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Haplotype | Position in Shorter Section | Position in Longer Section | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | 172 | 273 | 345 | 384 | 405 | 450 | 471 | 483 | 513 | 549 | 594 | 606 | 84 | 249 | 306 | 363 | 414 | 534 | 700 | |
| A | A | C | T | A | G | T | C | G | G | T | G | G | A | A | T | A | G | G | A | T |
| B | • | • | • | • | A | C | • | A | • | C | • | • | • | • | • | • | A | • | G | • |
| C | G | • | • | • | A | C | T | • | • | C | • | • | • | G | • | • | A | • | G | • |
| D | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | G | • |
| E | • | T | G | • | • | • | • | • | • | C | • | • | • | • | A | • | A | A | G | C |
| F | • | • | • | • | • | • | • | • | • | C | • | • | • | • | • | G | • | • | G | • |
| G | • | • | • | G | • | • | • | • | A | C | A | A | C | • | • | • | A | • | G | • |
| Source of Variation | df | Sum of Squares | Variance Component | Proportion of Variance | Φ |
|---|---|---|---|---|---|
| Between regions | 1 | 531.9 | 14.0 | 72% | ΦCT = 0.723 (p < 0.001) |
| Among sites within regions | 24 | 151.0 | 0.5 | 3% | ΦSC = 0.092 (p = 0.071) |
| Within sites | 76 | 931.9 | 4.9 | 25% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, S.; Shoda-Kagaya, E. Genetic Differences among Established Populations of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) in Japan: Suggestion of Multiple Introductions. Insects 2022, 13, 217. https://doi.org/10.3390/insects13020217
Tamura S, Shoda-Kagaya E. Genetic Differences among Established Populations of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) in Japan: Suggestion of Multiple Introductions. Insects. 2022; 13(2):217. https://doi.org/10.3390/insects13020217
Chicago/Turabian StyleTamura, Shigeaki, and Etsuko Shoda-Kagaya. 2022. "Genetic Differences among Established Populations of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) in Japan: Suggestion of Multiple Introductions" Insects 13, no. 2: 217. https://doi.org/10.3390/insects13020217
APA StyleTamura, S., & Shoda-Kagaya, E. (2022). Genetic Differences among Established Populations of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae) in Japan: Suggestion of Multiple Introductions. Insects, 13(2), 217. https://doi.org/10.3390/insects13020217






