Mass Trapping Drosophila suzukii, What Would It Take? A Two-Year Field Study on Trap Interference
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Traps and Attractants
2.2. Trap Interference Trials
2.2.1. Spring 2018―5 m Inter-Trap Spacing
2.2.2. Summer 2018―5 m Inter-Trap Spacing
2.2.3. Summer 2018―10 m Inter-Trap Spacing
2.2.4. Spring 2020―5 m Inter-Trap Spacing
2.2.5. Summer 2020―5 m Inter-Trap Spacing
2.2.6. Summer 2020―10 m Inter-Trap Spacing
2.2.7. Summer 2020―15 m Inter-Trap Spacing
2.3. Assessments
2.4. Data Analysis
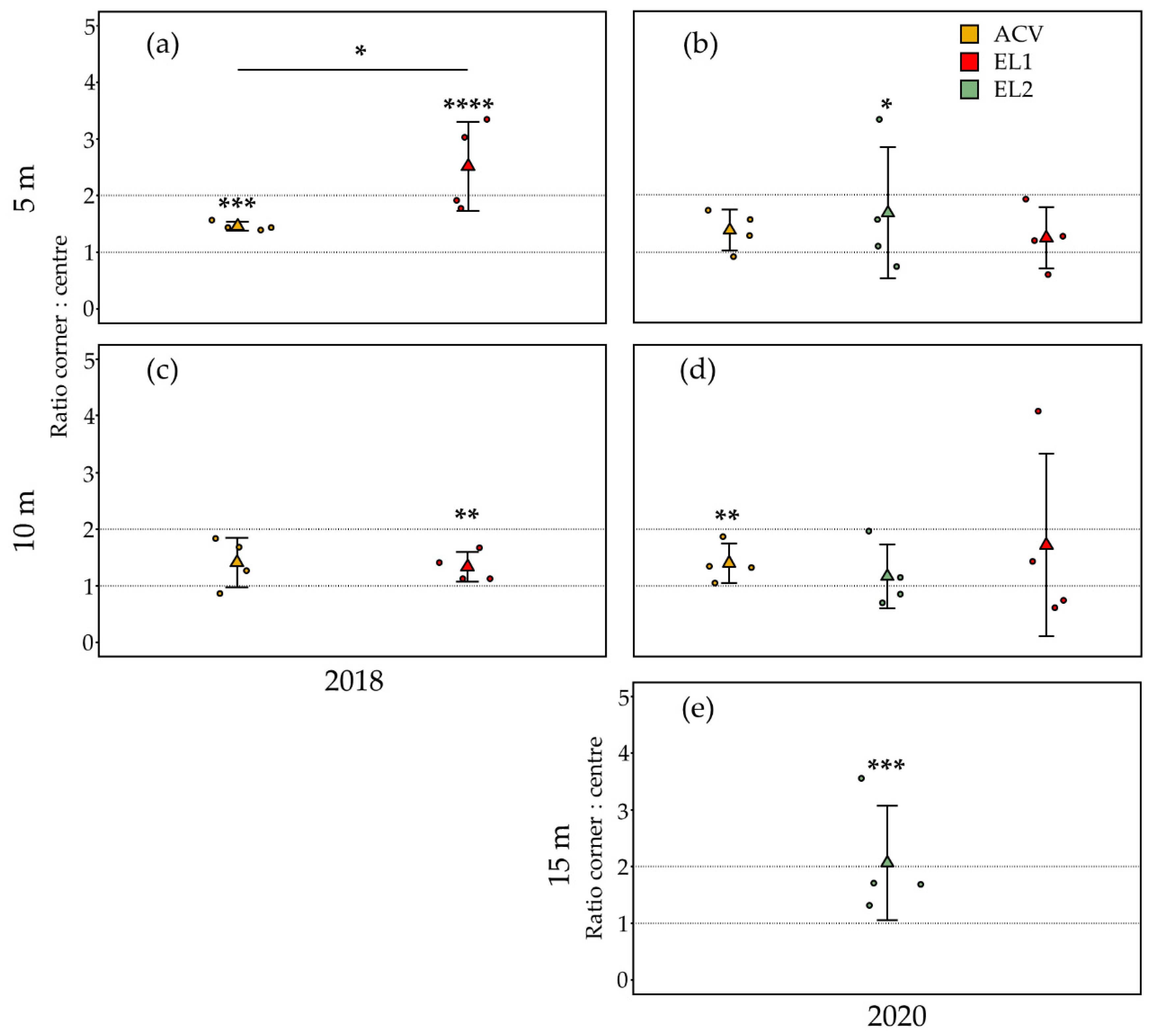
3. Results
3.1. Trap Interference
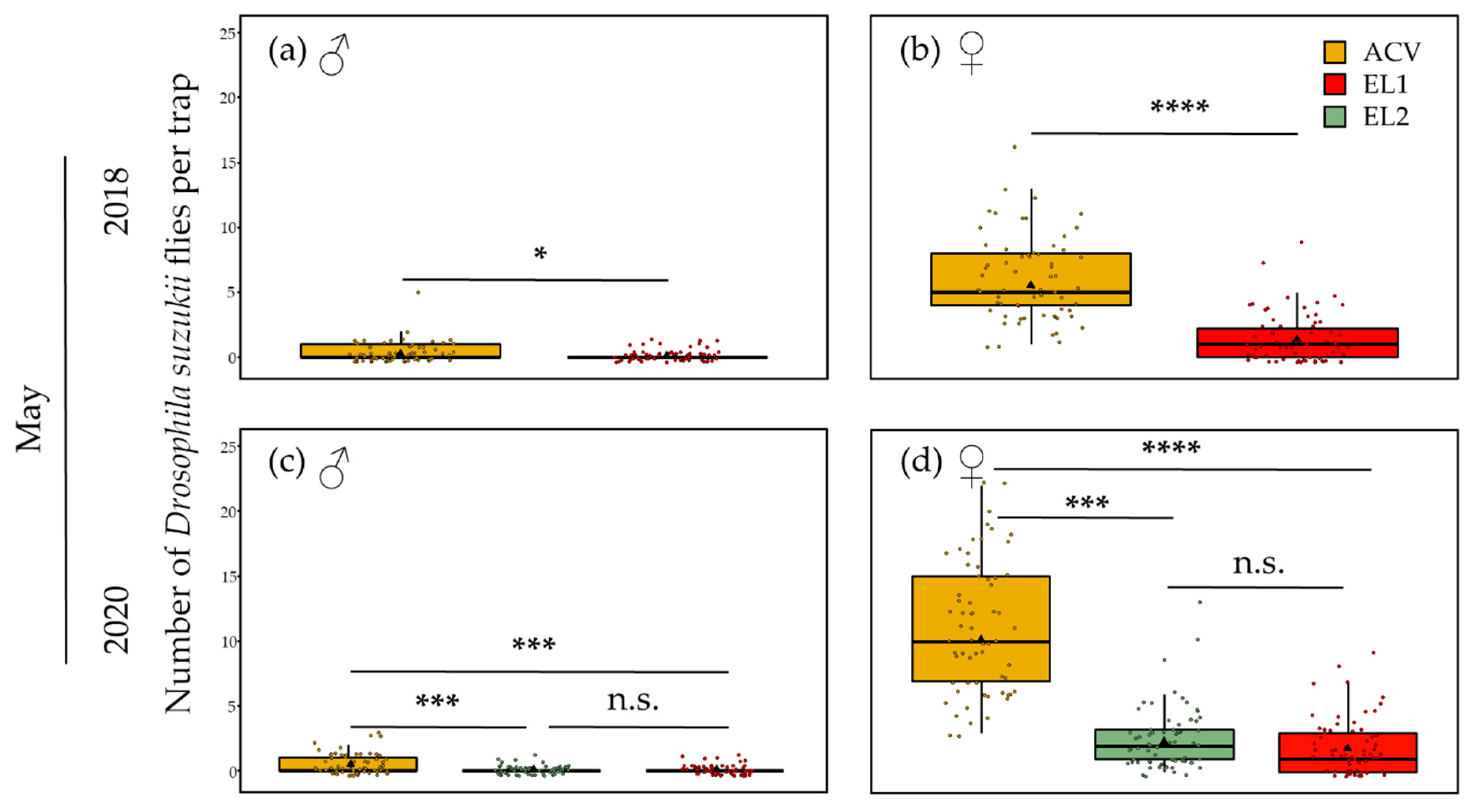
3.1.1. Spring—5 m Inter-Trap Spacing
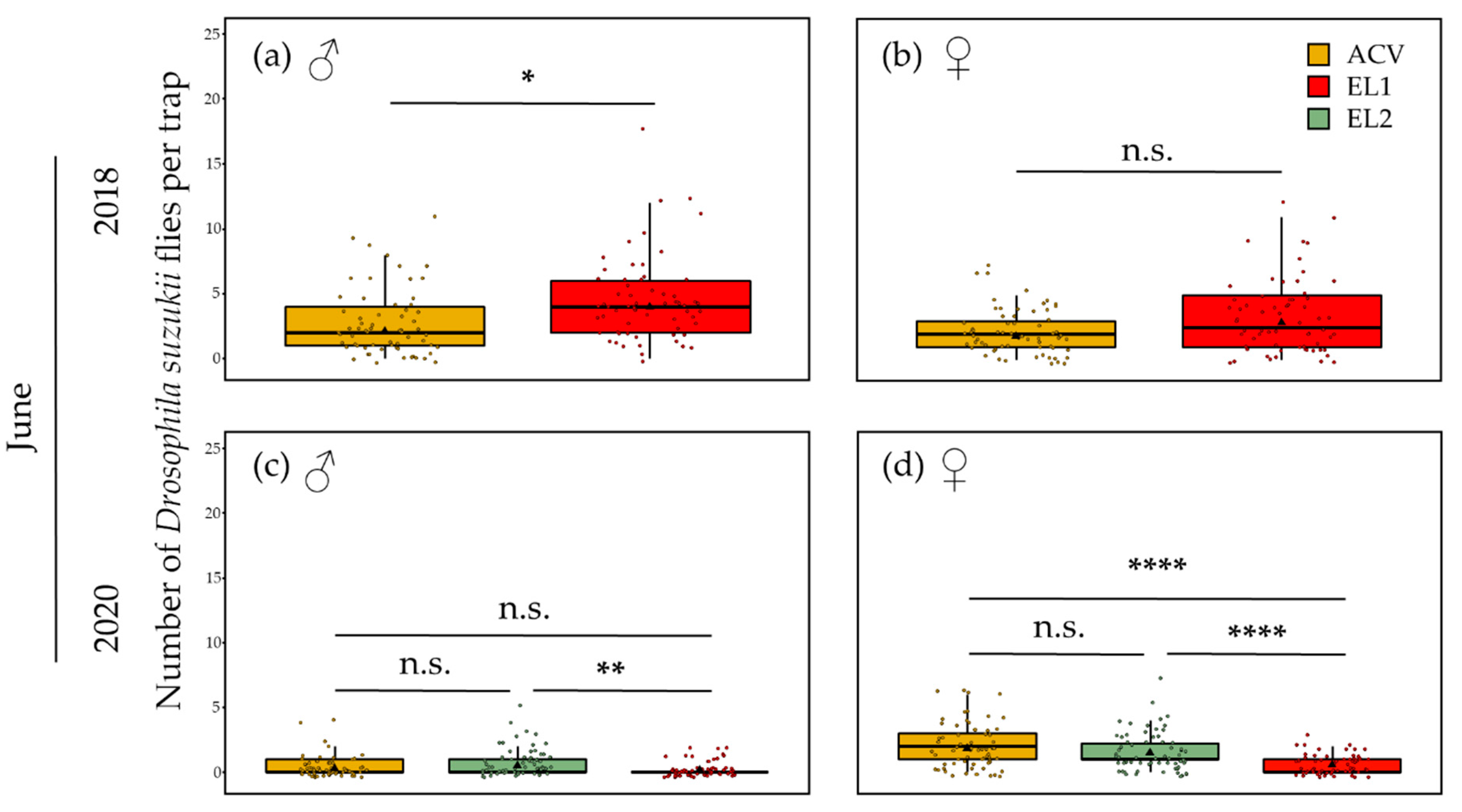
3.1.2. Summer—5 m Inter-Trap Spacing
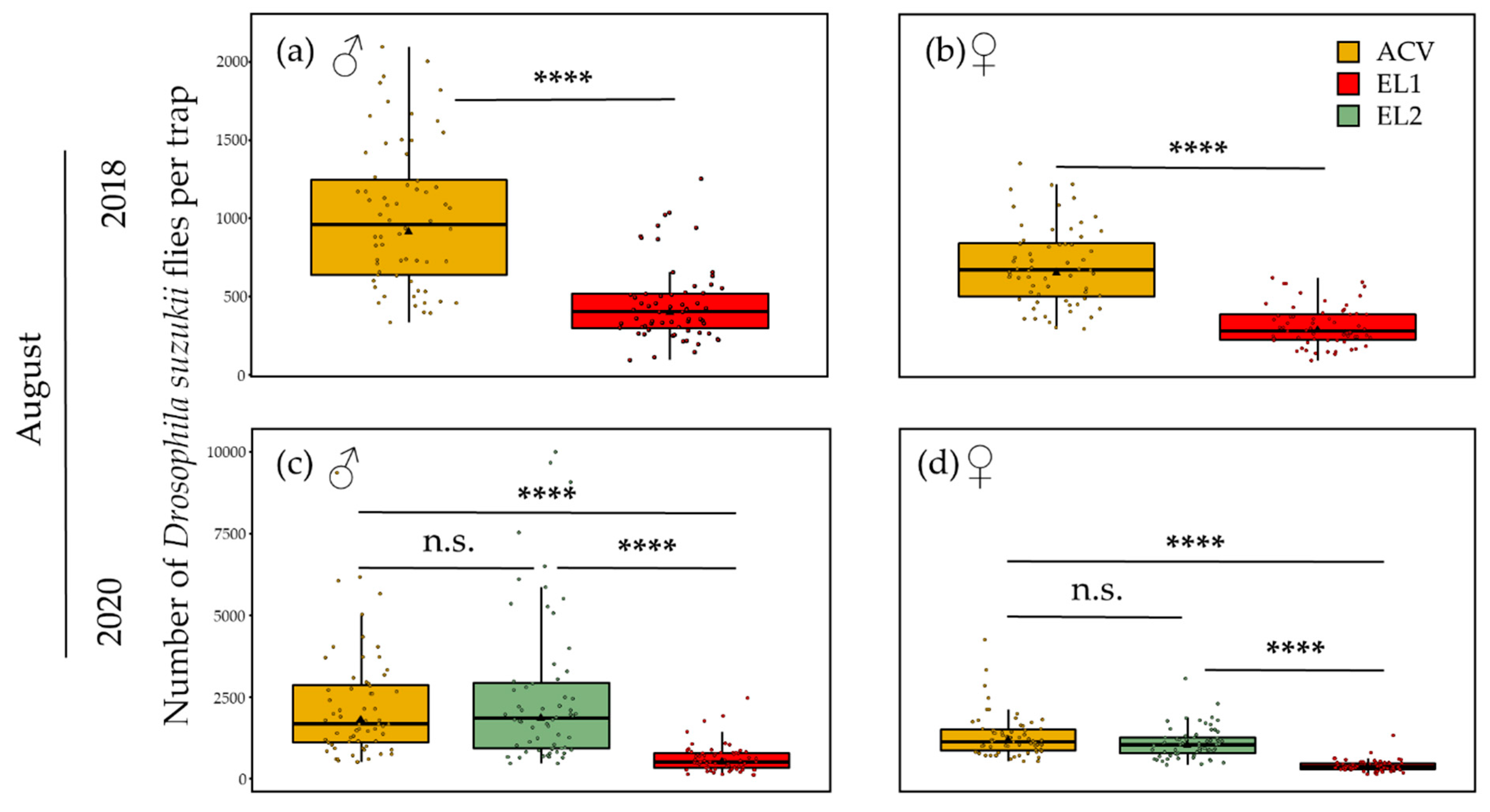
3.1.3. Summer—10 m Inter-Trap Spacing
3.1.4. Summer—15 m Inter-Trap Spacing
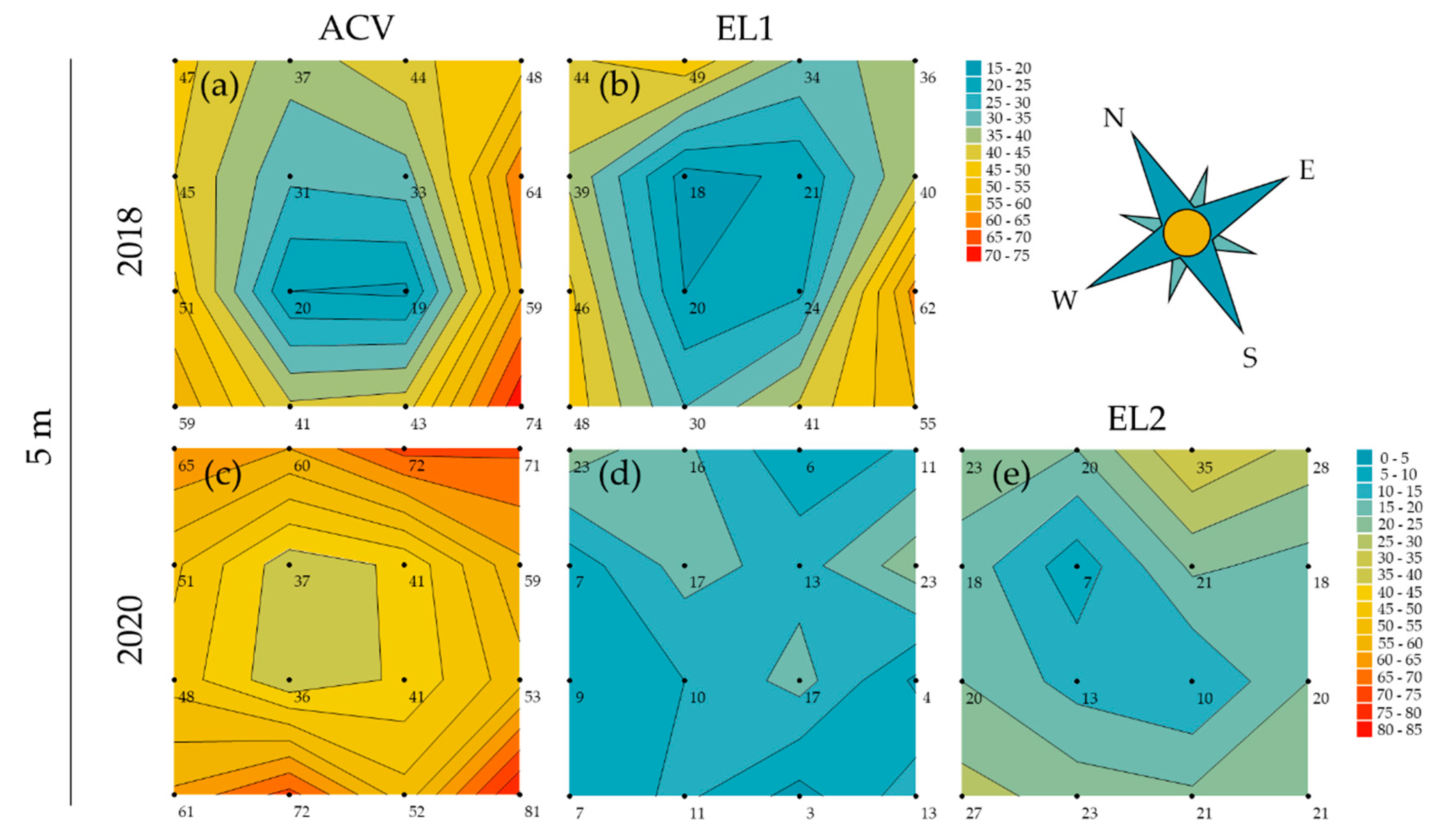
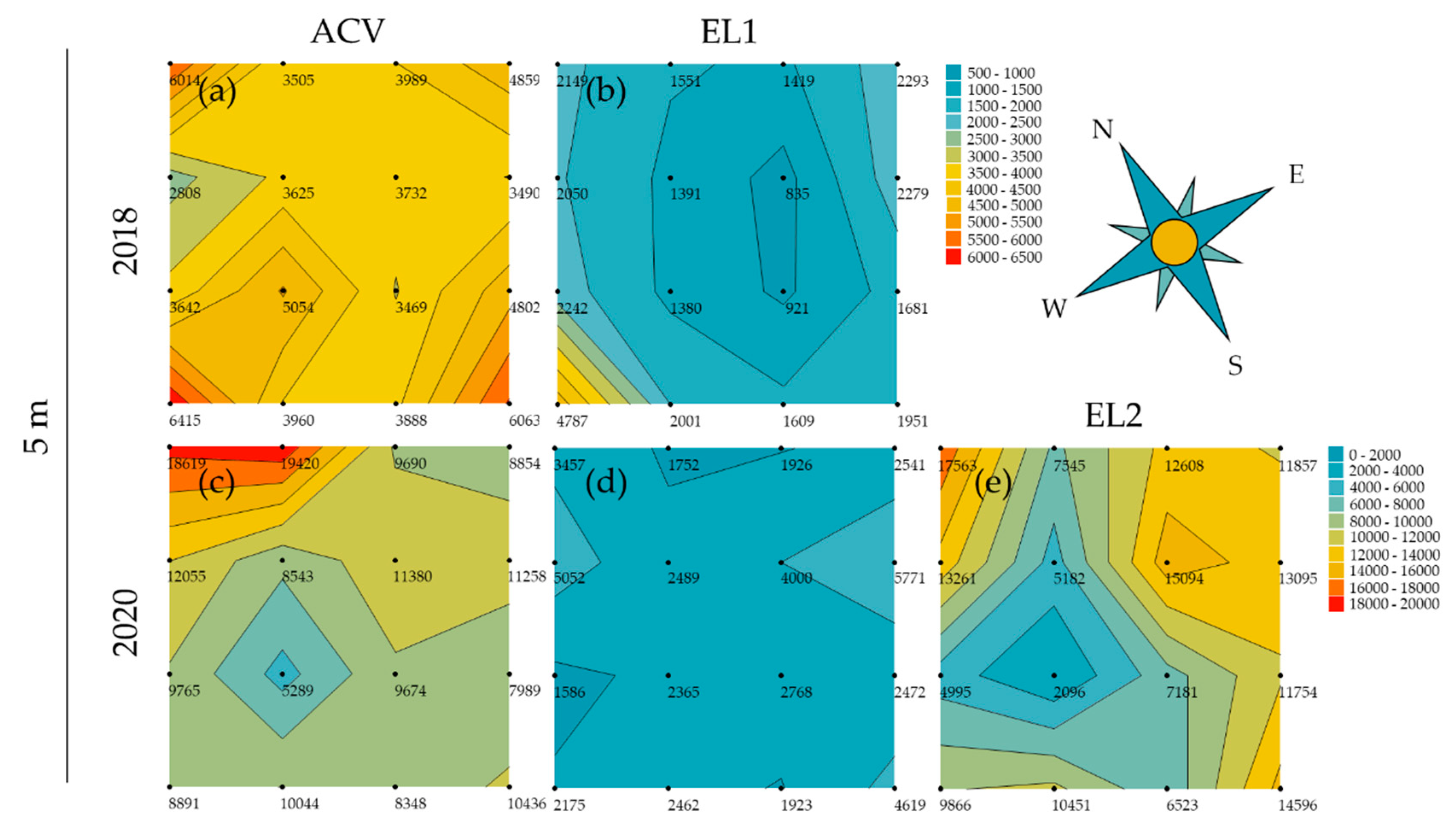
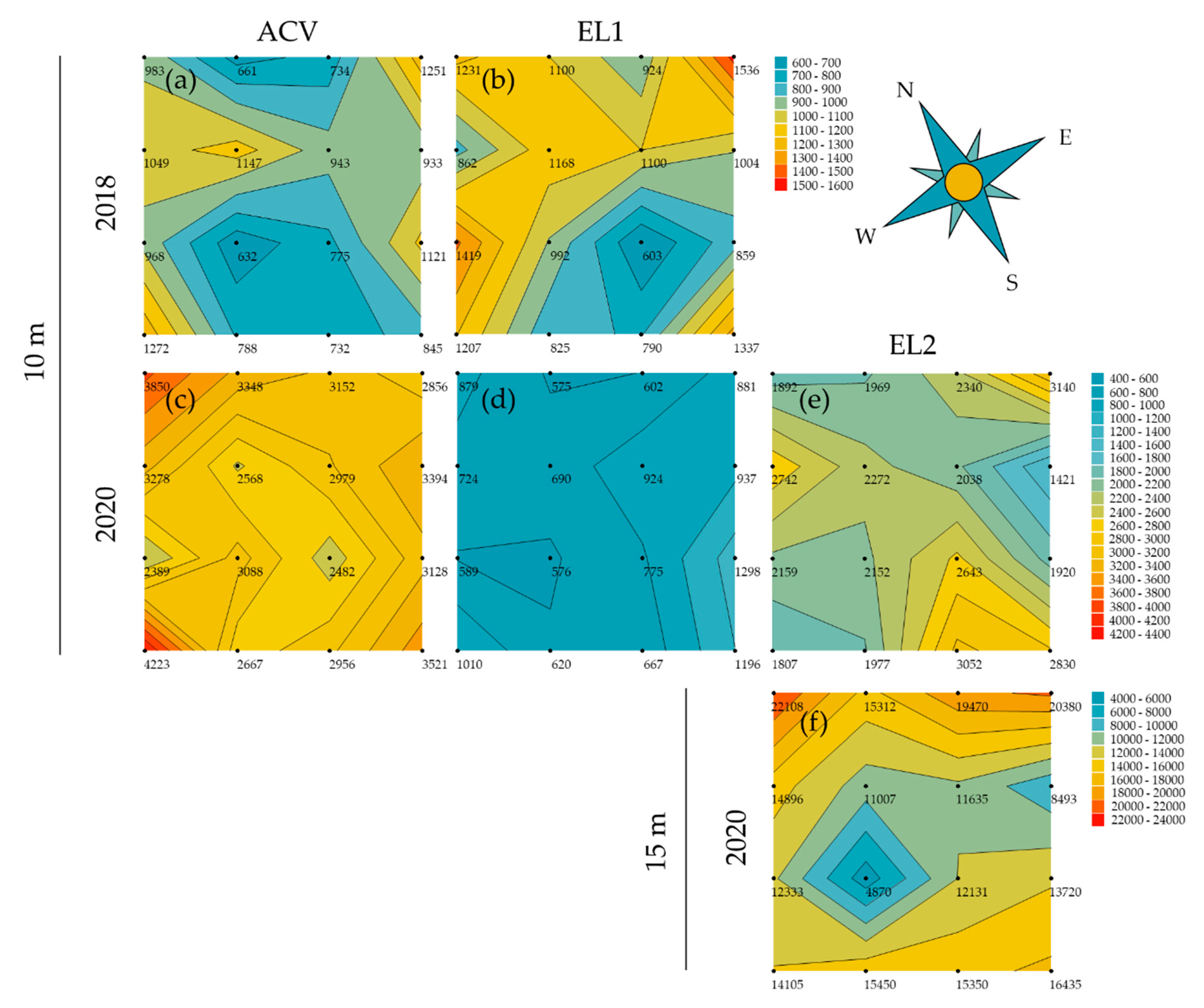
3.2. Spatial Patterns in Trapping Grids
3.3. Attractant Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Ometto, L.; Cestaro, A.; Ramasamy, S.; Grassi, A.; Revadi, S.; Siozios, S.; Moretto, M.; Fontana, P.; Varotto, C.; Pisani, D.; et al. Linking genomics and ecology to investigate the complex evolution of an invasive Drosophila pest. Genome Biol. Evol. 2013, 5, 745–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adrion, J.R.; Kousathanas, A.; Pascual, M.; Burrack, H.J.; Haddad, N.M.; Bergland, A.O.; Machado, H.; Sackton, T.B.; Schlenke, T.A.; Watada, M.; et al. Drosophila suzukii: The genetic footprint of a recent, worldwide invasion. Mol. Biol. Evol. 2014, 31, 3148–3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortelmans, J.; Casteels, H.; Beliën, T. Drosophila suzukii (Diptera: Drosophilidae): A pest species new to Belgium. Belgian J. Zool. 2012, 142, 143–146. [Google Scholar]
- Cini, A.; Ioriatti, C.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectology 2012, 65, 149–160. [Google Scholar]
- Belien, T.; Thys, T.; Fassotte, C.; Walrant, C.; Tomme, M.; Bolen, M.; Bylemans, D. Population dynamics of Drosophila suzukii (Diptera: Drosophilidae) in Belgium in 2013. Commun. Agric. Appl. Biol. Sci. 2014, 79, 169–175. [Google Scholar]
- Andreazza, F.; Bernardi, D.; Baronio, C.A.; Pasinato, J.; Nava, D.E.; Botton, M. Toxicities and effects of insecticidal toxic baits to control Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae). Pest Manag. Sci. 2017, 73, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Dalton, D.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef]
- Digiacomo, G.; Hadrich, J.; Hutchison, W.D.; Peterson, H.; Rogers, M. Economic impact of spotted wing drosophila (Diptera: Drosophilidae) yield loss on Minnesota raspberry farms: A grower survey. J. Integr. Pest Manag. 2019, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Gómez, M.I.; Atallah, S.S.; Conrad, J.M. A bayesian state-space approach for invasive species management: The case of spotted wing drosophila. Am. J. Agric. Econ. 2020, 102, 1227–1244. [Google Scholar] [CrossRef]
- Wollmann, J.; Schlesener, D.C.H.; Ferreira, M.S.; Kruger, A.P.; Bernardi, D.; Garcia, J.A.B.; Nunes, A.M.; Garcia, M.S.; Garcia, F.R.M. Population dynamics of Drosophila suzukii (Diptera: Drosophilidae) in berry crops in southern Brazil. Neotrop. Entomol. 2019, 48, 699–705. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In focus: Spotted wing Drosophila, Drosophila suzukii, across perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef] [PubMed]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. Insects 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolda, M.P.; Goodhue, R.E.; Zalom, F.G. Spotted wing drosophila: Potential economic impact of a newly established pest. Agric. Econ. Res. Upd. 2010, 13, 5–8. [Google Scholar]
- Farnsworth, D.; Hamby, K.; Bolda, M.; Goodhue, R.; Williams, J.; Zalom, F. Economic analysis of revenue losses and control costs associated with the spotted wing drosophila (Drosophila suzukii (Matsumura)) in the California raspberry industry. Pest Manag. Sci. 2017, 73, 1083–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Timmeren, S.; Isaacs, R. Control of spotted wing Drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop Prot. 2013, 54, 126–133. [Google Scholar] [CrossRef]
- Wiman, N.G.; Dalton, D.T.; Anfora, G.; Biondi, A.; Chiu, J.C.; Daane, K.M.; Gerdeman, B.; Gottardello, A.; Hamby, K.A.; Isaacs, R.; et al. Drosophila suzukii population response to environment and management strategies. J. Pest Sci. 2016, 89, 653–665. [Google Scholar] [CrossRef] [Green Version]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A decade of research towards a sustainable integrated pest management program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.R.; Stelinski, L.L. Behavior-modifying strategies in IPM: Theory and practice. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: New York, NY, USA, 2009; pp. 263–315. [Google Scholar]
- Iglesias, L.E.; Nyoike, T.W.; Liburd, O.E. Effect of trap design, bait type, and age on captures of Drosophila suzukii (Diptera: Drosophilidae) in berry crops. J. Econ. Entomol. 2014, 107, 1508–1518. [Google Scholar] [CrossRef] [Green Version]
- Hampton, E.; Koski, C.; Barsoian, O.; Faubert, H.; Cowles, R.S.; Alm, S.R. Use of early ripening cultivars to avoid infestation and mass trapping to manage Drosophila suzukii (Diptera: Drosophilidae) in Vaccinium corymbosum (Ericales: Ericaceae). J. Econ. Entomol. 2014, 107, 1849–1857. [Google Scholar] [CrossRef] [Green Version]
- Lasa, R.; Aguas-Lanzagorta, S.; Williams, T. Agricultural-grade apple cider vinegar is remarkably attractive to Drosophila suzukii (Diptera: Drosophiliadae) in Mexico. Insects 2020, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Hernández, R.A.; Lasa, R.; Montoya, P.; Liedo, P.; Rodríguez, D.; Sánchez, A.; Toledo, J. Efficacy of food-based attractants for monitoring Drosophila suzukii (Diptera: Drosophilidae) in berry crops. Crop Prot. 2021, 150, 105797. [Google Scholar] [CrossRef]
- Landolt, P.J.; Adams, T.; Rogg, H. Trapping spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. J. Appl. Entomol. 2012, 136, 148–154. [Google Scholar] [CrossRef]
- Kanzawa, T. Studies on Drosophila suzukii Mats; Yamanashi Agricultural Experimental Station: Kofu, Janpan, 1939. [Google Scholar]
- Burrack, H.J.; Asplen, M.; Bahder, L.; Collins, J.; Drummond, F.A.; Guédot, C.; Isaacs, R.; Johnson, D.; Blanton, A.; Lee, J.C.; et al. Multistate comparison of attractants for monitoring Drosophila suzukii (Diptera: Drosophilidae) in blueberries and caneberries. Environ. Entomol. 2015, 44, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gut, L.; Grieshop, M. Evaluation of food-based attractants for Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2017, 46, 878–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasa, R.; Tadeo, E.; Toledo-Hérnandez, R.A.; Carmona, L.; Lima, I.; Williams, T. Improved capture of Drosophila suzukii by a trap baited with two attractants in the same device. PLoS ONE 2017, 12, e0188350. [Google Scholar] [CrossRef] [Green Version]
- Tonina, L.; Grassi, A.; Caruso, S.; Mori, N.; Gottardello, A.; Anfora, G.; Giomi, F.; Vaccari, G.; Ioriatti, C. Comparison of attractants for monitoring Drosophila suzukii in sweet cherry orchards in Italy. J. Appl. Entomol. 2018, 142, 18–25. [Google Scholar] [CrossRef]
- Jaffe, B.D.; Avanesyan, A.; Bal, H.K.; Feng, Y.; Grant, J.; Grieshop, M.J.; Lee, J.C.; Liburd, O.E.; Rhodes, E.; Rodriguez-Saona, C.; et al. Multistate comparison of attractants and the impact of fruit development stage on trapping Drosophila suzukii (Diptera: Drosophilidae) in raspberry and blueberry. Environ. Entomol. 2018, 47, 935–945. [Google Scholar] [CrossRef]
- Cai, P.; Yi, C.; Zhang, Q.; Zhang, H.; Lin, J.; Song, X.; Yang, J.; Wang, B.; Ji, Q.; Chen, J. Evaluation of protein bait manufactured from brewery yeast waste for controlling Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2019, 112, 226–235. [Google Scholar] [CrossRef]
- Wollmann, J.; Schlesener, D.C.H.; Vieira, J.G.A.; Bernardi, D.; Garcia, M.S.; Garcia, F.R.M. Evaluation of food baits to capture Drosophila suzukii in the southern of Brazil. An. Acad. Bras. Cienc. 2019, 91, e20180375. [Google Scholar] [CrossRef]
- Scheidler, N.H.; Liu, C.; Hamby, K.A.; Zalom, F.G.; Syed, Z. Volatile codes: Correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci. Rep. 2015, 5, 14059. [Google Scholar] [CrossRef] [Green Version]
- Lasa, R.; Toledo-Hernández, R.A.; Rodríguez, D.; Williams, T. Raspberry as a source for the development of Drosophila suzukii attractants: Laboratory and commercial polytunnel trials. Insects 2019, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzetto, F.; Gonella, E.; Crotti, E.; Vacchini, V.; Syrpas, M.; Pontini, M.; Mangelinckx, S.; Daffonchio, D.; Alma, A. Olfactory attraction of Drosophila suzukii by symbiotic acetic acid bacteria. J. Pest Sci. 2016, 89, 783–792. [Google Scholar] [CrossRef]
- Mori, B.A.; Whitener, A.B.; Leinweber, Y.; Revadi, S.; Beers, E.H.; Witzgall, P.; Becher, P.G. Enhanced yeast feeding following mating facilitates control of the invasive fruit pest Drosophila suzukii. J. Appl. Ecol. 2017, 54, 170–177. [Google Scholar] [CrossRef]
- Lasa, R.; Navarro-De-La-Fuente, L.; Gschaedler-Mathis, A.C.; Kirchmayr, M.R.; Williams, T. Yeast species, strains, and growth media mediate attraction of Drosophila suzukii (Diptera: Drosophilidae). Insects 2019, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Bueno, E.; Martin, K.R.; Raguso, R.A.; Mcmullen, J.G.; Hesler, S.P.; Loeb, G.M.; Douglas, A.E. Response of wild spotted wing drosophila (Drosophila suzukii) to microbial volatiles. J. Chem. Ecol. 2019, 46, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Fountain, M.T.; Günther, C.S.; Eady, P.E.; Goddard, M.R. Separate and combined Hanseniaspora uvarum and Metschnikowia pulcherrima metabolic volatiles are attractive to Drosophila suzukii in the laboratory and field. Sci. Rep. 2021, 11, 1201. [Google Scholar] [CrossRef]
- Alawamleh, A.; Ðurovic, G.; Maddalena, G.; Guzzon, R.; Ganassi, S.; Masqood Hashmi, M.; Wäckers, F.; Anfora, G.; De Cristofaro, A. Selection of lactic acid bacteria species and strains for efficient trapping of Drosophila suzukii. Insects 2021, 12, 153. [Google Scholar] [CrossRef]
- Ðurovic, G.; Alawamleh, A.; Carlin, S.; Maddalena, G.; Guzzon, R.; Mazzoni, V.; Dalton, D.T.; Walton, V.M.; Suckling, D.M.; Butler, R.C.; et al. Liquid baits with Oenococcus oeni increase captures of Drosophila suzukii. Insects 2021, 12, 66. [Google Scholar] [CrossRef]
- Abraham, J.; Zhang, A.; Angeli, S.; Abubeker, S.; Michel, C.; Feng, Y.; Rodriguez-Saona, C. Behavioral and antennal responses of Drosophila suzukii (Diptera: Drosophilidae) to volatiles from fruit extracts. Environ. Entomol. 2015, 44, 356–367. [Google Scholar] [CrossRef] [Green Version]
- Revadi, S.; Vitagliano, S.; Rossi Stacconi, M.V.; Ramasamy, S.; Mansourian, S.; Carlin, S.; Vrhovsek, U.; Becher, P.G.; Mazzoni, V.; Rota-Stabelli, O.; et al. Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol. Entomol. 2015, 40, 54–64. [Google Scholar] [CrossRef]
- Keesey, I.W.; Knaden, M.; Hansson, B.S. Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J. Chem. Ecol. 2015, 41, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Dong, W.; Zhang, F.; Kenis, M.; Griepink, F.; Zhang, J.; Chen, L.; Xiao, C. Identification of active components from volatiles of Chinese bayberry, Myrica rubra attractive to Drosophila suzukii. Arthropod. Plant. Interact. 2018, 12, 435–442. [Google Scholar] [CrossRef]
- Bolton, L.G.; Piñero, J.C.; Barrett, B.A.; Cha, D.H. Electrophysiological and behavioral responses of Drosophila suzukii (Diptera: Drosophilidae) towards the leaf volatile β-cyclocitral and selected fruit-ripening volatiles. Environ. Entomol. 2019, 48, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Urbaneja-Bernat, P.; Cloonan, K.; Zhang, A.; Salazar-Mendoza, P.; Rodriguez-Saona, C. Fruit volatiles mediate differential attraction of Drosophila suzukii to wild and cultivated blueberries. J. Pest Sci. 2021, 94, 1249–1263. [Google Scholar] [CrossRef]
- Dewitte, P.; Van Kerckvoorde, V.; Beliën, T.; Bylemans, D.; Wenseleers, T. Identification of blackberry (Rubus fruticosus) volatiles as Drosophila suzukii attractants. Insects 2021, 12, 417. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Z.; Shi, M.; Kenis, M.; Dong, W.; Zhang, F.; Zhang, J.; Xiao, C.; Chen, L. Antennal and behavioral responses of Drosophila suzukii to volatiles from a non-crop host, Osyris wightiana. Insects 2021, 12, 166. [Google Scholar] [CrossRef]
- Baena, R.; Araujo, E.S.; Souza, J.P.A.; Bischoff, A.M.; Zarbin, P.H.G.; Zawadneak, M.A.C.; Cuquel, F.L. Ripening stages and volatile compounds present in strawberry fruits are involved in the oviposition choice of Drosophila suzukii (Diptera: Drosophilidae). Crop Prot. 2022, 153, 105883. [Google Scholar] [CrossRef]
- Piñero, J.C.; Barrett, B.A.; Bolton, L.G.; Follett, P.A. β-cyclocitral synergizes the response of adult Drosophila suzukii (Diptera: Drosophilidae) to fruit juices and isoamyl acetate in a sex-dependent manner. Sci. Rep. 2019, 9, 10574. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Rogg, H.; Landolt, P.J. Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing drosophila, Drosophila suzukii. J. Chem. Ecol. 2012, 38, 1419–1431. [Google Scholar] [CrossRef]
- Cha, D.H.; Hesler, S.P.; Cowles, R.S.; Vogt, H.; Loeb, G.M.; Landolt, P.J. Comparison of a synthetic chemical lure and standard fermented baits for trapping Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2013, 42, 1052–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, D.H.; Adams, T.; Werle, C.T.; Sampson, B.J.; Adamczyk, J.J.; Rogg, H.; Landolt, P.J. A four-component synthetic attractant for Drosophila suzukii (Diptera: Drosophilidae) isolated from fermented bait headspace. Pest Manag. Sci. 2014, 70, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Bruton, R.; Park, A.; Zhang, A. Identification of attractive blend for spotted wing drosophila, Drosophila suzukii, from apple juice. J. Pest Sci. 2018, 91, 1251–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, N.R.; Strickland, J.; Shields, V.D.C.; Zhang, A. Controlled-release dispenser and dry trap developments for Drosophila suzukii detection. Front. Ecol. Evol. 2020, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Larson, N.R.; Strickland, J.; Shields, V.D.; Rodriguez-Saona, C.; Cloonan, K.; Short, B.D.; Leskey, T.C.; Zhang, A. Field evaluation of different attractants for detecting and monitoring Drosophila suzukii. Front. Ecol. Evol. 2021, 9, 175. [Google Scholar] [CrossRef]
- Larson, N.R.; Strickland, J.; Shields, V.D.; Biondi, A.; Zappalà, L.; Cavallaro, C.; Colazza, S.; Escudero-Colomar, L.A.; Briem, F.; Vogt, H.; et al. Detection and monitoring of Drosophila suzukii in raspberry and cherry orchards with volatile organic compounds in the USA and Europe. Sci. Rep. 2021, 11, 6860. [Google Scholar] [CrossRef]
- Little, C.M.; Dixon, P.L.; Moreau, D.L.; Chapman, T.W.; Hillier, N.K. Assessment of attractant lures and monitoring traps for Drosophila suzukii (Diptera: Drosophidae) using electrophysiology, laboratory choice assays, and field trials. J. Econ. Entomol. 2021, 114, 652–675. [Google Scholar] [CrossRef]
- Lee, J.C.; Burrack, H.J.; Barrantes, L.D.; Beers, E.H.; Dreves, A.J.; Hamby, K.A.; Haviland, D.R.; Isaacs, R.; Richardson, T.A.; Shearer, P.W.; et al. Evaluation of monitoring traps for Drosophila suzukii (Diptera: Drosophilidae) in North America. J. Econ. Entomol. 2012, 105, 1350–1357. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, D.M.; McGhee, P.S.; Hermann, S.L.; Gut, L.J.; Miller, J.R. Alightment of spotted wing drosophila (Diptera: Drosophilidae) on odorless disks varying in color. Environ. Entomol. 2016, 45, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Rice, K.B.; Short, B.D.; Jones, S.K.; Leskey, T.C. Behavioral responses of Drosophila suzukii (Diptera: Drosophilidae) to visual stimuli under laboratory, semifield, and field conditions. Environ. Entomol. 2016, 45, 1480–1488. [Google Scholar] [CrossRef] [Green Version]
- Little, C.M.; Rizzato, A.R.; Charbonneau, L.; Chapman, T.; Hillier, N.R. Color preference of the spotted wing Drosophila, Drosophila suzukii. Sci. Rep. 2019, 9, 16051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renkema, J.M.; Buitenhuis, R.; Hallett, R.H. Optimizing trap design and trapping protocols for Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2014, 107, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Basoalto, E.; Hilton, R.; Knight, A. Factors affecting the efficacy of a vinegar trap for Drosophila suzikii (Diptera: Drosophilidae). J. Appl. Entomol. 2013, 137, 561–570. [Google Scholar] [CrossRef]
- Lee, J.C.; Shearer, P.W.; Barrantes, L.D.; Beers, E.H.; Burrack, H.J.; Dalton, D.T.; Dreves, A.J.; Gut, L.J.; Hamby, K.A.; Haviland, D.R.; et al. Trap designs for monitoring Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2013, 42, 1348–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkpatrick, D.M.; Gut, L.J.; Miller, J.R. Development of a novel dry, sticky trap design incorporating visual cues for Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2018, 111, 1775–1779. [Google Scholar] [CrossRef]
- Van Kerckvoorde, V.; Clymans, R.; Bangels, E.; Alhmedi, A.; De Ketelaere, B.; De Clercq, P.; Bylemans, D.; Belien, T. Tunnel entries and a killing agent uncover the importance of fly retention in Drosophila suzukii traps. Pest Manag. Sci. 2020, 76, 3459–3468. [Google Scholar] [CrossRef]
- Kirkpatrick, D.M.; McGhee, P.S.; Gut, L.J.; Miller, J.R. Improving monitoring tools for spotted wing drosophila, Drosophila suzukii. Entomol. Exp. Appl. 2017, 164, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Clymans, R.; Van Kerckvoorde, V.; Bangels, E.; Akkermans, W.; Alhmedi, A.; De Clercq, P.; Beliën, T.; Bylemans, D. Olfactory preference of Drosophila suzukii shifts between fruit and fermentation cues over the season: Effects of physiological status. Insects 2019, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Del Fava, E.; Ioriatti, C.; Melegaro, A. Cost–benefit analysis of controlling the spotted wing drosophila (Drosophila suzukii (Matsumura)) spread and infestation of soft fruits in Trentino, Northern Italy. Pest Manag. Sci. 2017, 73, 2318–2327. [Google Scholar] [CrossRef]
- Roosjen, P.P.J.; Kellenberger, B.; Kooistra, L.; Green, D.R.; Fahrentrapp, J. Deep learning for automated detection of Drosophila suzukii: Potential for UAV-based monitoring. Pest Manag. Sci. 2020, 76, 2994–3002. [Google Scholar] [CrossRef]
- Navarro-Llopis, V.; Vacas, S. Mass Trapping for Fruit Fly Control. In Trapping and the Detection, Control and Regulation of Tephritid Fruit Flies; Shelly, T.E., Epsky, N., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 513–555. ISBN 9789401791922. [Google Scholar]
- Spies, J.M.; Liburd, O.E. Comparison of attractants, insecticides, and mass trapping for managing Drosophila suzukii (Diptera: Drosophilidae) in blueberries. Florida Entomol. 2019, 102, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Wallingford, A.K.; Cha, D.H.; Loeb, G.M. Evaluating a push-pull strategy for management of Drosophila suzukii Matsumura in red raspberry. Pest Manag. Sci. 2018, 74, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Reinke, M.D.; Miller, J.R.; Gut, L.J. Potential of high-density pheromone-releasing microtraps for control of codling moth Cydia pomonella and obliquebanded leafroller Choristoneura rosaceana. Physiol. Entomol. 2012, 37, 53–59. [Google Scholar] [CrossRef]
- Broumas, T.; Haniotakis, G.; Liaropoulos, C.; Tomazou, T.; Ragoussis, N. The efficacy of an improved form of the mass-trapping method, for the control of the olive fruit fly, Bactrocera oleae (Gmelin) (Dipt., Tephritidae): Pilot-scale feasibility studies. J. Appl. Entomol. 2002, 126, 217–223. [Google Scholar] [CrossRef]
- Navarro-llopis, V.; Primo, J.; Vacas, S. Bait station devices can improve mass trapping performance for the control of the Mediterranean fruit fly. Pest Manag. Sci. 2014, 71, 923–927. [Google Scholar] [CrossRef]
- Navarro-llopis, V.; Primo, J.; Vacas, S. Efficacy of attract-and-kill devices for the control of Ceratitis capitata. Pest Manag. Sci. 2012, 69, 478–482. [Google Scholar] [CrossRef]
- Suckling, D.M.; Walker, J.T.S.; Shaw, P.W.; Manning, L.; Lo, P.; Wallis, R.; Bell, V.; Sandanayaka, W.R.M.; Hall, D.R.; Cross, J.V.; et al. Trapping Dasinuera mali (Diptera:Cecidomyiidae) in apples. J. Econ. Entomol. 2007, 100, 745–751. [Google Scholar] [CrossRef]
- Klick, J.; Lee, J.C.; Hagler, J.R.; Bruck, D.J.; Yang, W.Q. Evaluating Drosophila suzukii immunomarking for mark-capture research. Entomol. Exp. Appl. 2014, 152, 31–41. [Google Scholar] [CrossRef]
- Clymans, R.; Van Kerckvoorde, V.; Beliën, T.; Bylemans, D.; De Clercq, P. Marking Drosophila suzukii (Diptera: Drosophilidae) with fluorescent dusts. Insects 2020, 11, 152. [Google Scholar] [CrossRef] [Green Version]
- Byers, J.A.; Anderbrant, O. Effective attraction radius: A method for comparing species attractants and determining densities of flying insects. J. Chem. Ecol. 1989, 15, 749–765. [Google Scholar] [CrossRef]
- Schlyter, F. Sampling range, attraction range, and effective attraction radius: Estimates of trap efficiency and communication distance in coleopteran pheromone and host attractant systems. J. Appl. Ent. 1992, 114, 439–454. [Google Scholar] [CrossRef]
- Byers, J.A. Modeling distributions of flying insects: Effective attraction radius of pheromone in two and three dimensions. J. Theor. Biol. 2009, 256, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.A. Simulation of mating disruption and mass trapping with competitive attraction and camouflage. Environ. Entomol. 2007, 36, 1328–1338. [Google Scholar] [CrossRef] [Green Version]
- Branco, M.; Jactel, H.; Franco, J.C.; Mendel, Z. Modelling response of insect trap captures to pheromone dose. Ecol. Modell. 2006, 197, 247–257. [Google Scholar] [CrossRef]
- Manoukis, N.C.; Hall, B.; Geib, S.M. A computer model of insect traps in a landscape. Sci. Rep. 2014, 4, 7015. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.G.; Schenker, J.H.; McGhee, P.S.; Gut, L.J.; Brunner, J.F.; Miller, J.R. Maximizing information yield from pheromone-baited monitoring traps: Estimating plume reach, trapping radius, and absolute density of Cydia pomonella (Lepidoptera: Tortricidae) in Michigan apple. J. Econ. Entomol. 2017, 110, 305–318. [Google Scholar] [CrossRef]
- Houseweart, M.W.; Jennings, D.T.; Sanders, C.J. Variables associated with pheromone traps for monitoring spruce budworm populations (Lepidoptera: Tortricidae). Can. Entomol. 1981, 113, 527–537. [Google Scholar] [CrossRef]
- Elkinton, J.S.; Cardé, R.T. Effects of intertrap distance and wind direction on the interaction of gypsy moth (Lepidoptera: Lymantriidae) pheromone-baited traps. Environ. Entomol. 1988, 17, 764–769. [Google Scholar] [CrossRef]
- Wedding, R.; Anderbrant, O.; Jönsson, P. Influence of wind conditions and intertrap spacing on pheromone trap catches of male European pine sawfly, Neodiprion sertifer. Entomol. Exp. Appl. 1995, 77, 223–232. [Google Scholar] [CrossRef]
- Bacca, T.; Lima, E.R.; Picanço, M.C.; Guedes, R.N.C.; Viana, J.H.M. Optimum spacing of pheromone traps for monitoring the coffee leaf miner Leucoptera coffeella. Entomol. Exp. Appl. 2006, 119, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Suckling, D.M.; Stringer, L.D.; Kean, J.M.; Lo, P.L.; Bell, V.; Walker, J.T.S.; Twidle, A.M.; Jiménez-Pérez, A.; El-Sayed, A.M. Spatial analysis of mass trapping: How close is close enough? Pest Manag. Sci. 2015, 71, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.E.S.; Sullivan, N.J.; Brierley, S.; Suckling, D.M.; Sullivan, N.J.; Brierley, S.; Suckling, D.M. Experimental high-density trapping of social wasps: Target kairomones for workers or gynes for drones? N. Z. Entomol. 2020, 43, 65–76. [Google Scholar] [CrossRef]
- Elsensohn, J.E.; Loeb, G.M. Non-crop host sampling yields insights into small-scale population dynamics of of Drosophila suzukii (Matsumura). Insects 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheaterbit. Available online: Weatherbit.io (accessed on 18 January 2022).
- Baker, T.; Carpenter, S.; Gubbins, S.; Newton, R.; Iacono, G.L.; Wood, J.; Harrup, L.E. Can insecticide-treated netting provide protection for equids from Culicoides biting midges in the United Kingdom? Parasit. Vectors 2015, 8, 604. [Google Scholar] [CrossRef] [Green Version]
- R Core Team R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. Vienna, Austria, 2020. Available online: https://www.R-project.org/ (accessed on 18 January 2022).
- Rossi-Stacconi, M.V.; Kaur, R.; Mazzoni, V.; Ometto, L.; Grassi, A.; Gottardello, A.; Rota-Stabelli, O.; Anfora, G. Multiple lines of evidence for reproductive winter diapause in the invasive pest Drosophila suzukii: Useful clues for control strategies. J. Pest Sci. 2016, 89, 689–700. [Google Scholar] [CrossRef]
- Arnó, J.; Solà, M.; Riudavets, J.; Gabarra, R. Population dynamics, non-crop hosts, and fruit susceptibility of Drosophila suzukii in Northeast Spain. J. Pest Sci. 2016, 89, 713–723. [Google Scholar] [CrossRef]
- Briem, F.; Dominic, A.R.; Golla, B.; Hoffmann, C.; Englert, C.; Herz, A.; Vogt, H. Explorative data analysis of Drosophila suzukii trap catches from a seven-year monitoring program in Southwest Germany. Insects 2018, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Zerulla, F.N.; Schmidt, S.; Streitberger, M.; Zebitz, C.P.W.; Zelger, R. On the overwintering ability of Drosophila suzukii in South Tyrol. J. Berry Res. 2015, 5, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Thistlewood, H.M.A.; Gill, P.; Beers, E.H.; Shearer, P.W.; Walsh, D.B.; Rozema, B.M.; Acheampong, S.; Castagnoli, S.; Yee, W.L.; Smytheman, P.; et al. Spatial analysis of seasonal dynamics and overwintering of Drosophila suzukii (Diptera: Drosophilidae) in the Okanagan-Columbia Basin, 2010–2014. Environ. Entomol. 2018, 47, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Haviland, D.R.; Caprile, J.; Rill, S.; Hamby, K.A.; Grant, J.A. Phenology of spotted wing drosophila in the San Joaquin Valley varies by season, crop and nearby vegetation. Calif. Agric. 2016, 70, 24–31. [Google Scholar] [CrossRef]
- Shearer, P.W.; West, J.D.; Walton, V.M.; Brown, P.H.; Svetec, N.; Chiu, J.C. Seasonal cues induce phenotypic plasticity of Drosophila suzukii to enhance winter survival. BMC Ecol. 2016, 16, 11. [Google Scholar] [CrossRef] [Green Version]
- Jakobs, R.; Gariepy, T.D.; Sinclair, B.J. Adult plasticity of cold tolerance in a cool-temperate population of Drosophila suzukii. J. Insect Physiol. 2015, 79, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.T. Cold and heat tolerance of drosophilid flies with reference to their latitudinal distributions. Oecologia 2004, 140, 442–449. [Google Scholar] [CrossRef]
- Swoboda-Bhattarai, K.A.; McPhie, D.R.; Burrack, H.J. Reproductive status of Drosophila suzukii (Diptera: Drosophilidae) females influences attraction to fermentation-based baits and ripe fruits. J. Econ. Entomol. 2017, 110, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.S.; Wallingford, A.K.; Loeb, G.M.; Lee, J.C. Physiological status of Drosophila suzukii (Diptera: Drosophilidae) affects their response to attractive odours. J. Appl. Entomol. 2018, 142, 473–482. [Google Scholar] [CrossRef]
- Wang, X.G.; Stewart, T.J.; Biondi, A.; Chavez, B.A.; Ingels, C.; Caprile, J.; Grant, J.A.; Walton, V.M.; Daane, K.M. Population dynamics and ecology of Drosophila suzukii in Central California. J. Pest Sci. 2016, 89, 701–712. [Google Scholar] [CrossRef]
- Switzer, P.V.; Enstrom, P.C.; Schoenick, C.A. Behavioral explanations underlying the lack of trap effectiveness for small-scale management of Japanese beetles (Coleoptera: Scarabaeidae). J. Econ. Entomol. 2009, 102, 934–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budick, S.A.; Dickinson, M.H. Free-flight responses of Drosophila melanogaster to attractive odors. J. Exp. Biol. 2006, 209, 3001–3017. [Google Scholar] [CrossRef] [Green Version]
- Chow, D.M.; Frye, M.A. Context-dependent olfactory enhancement of optomotor flight control in Drosophila. J. Exp. Biol. 2008, 211, 2478–2485. [Google Scholar] [CrossRef] [Green Version]
- Leitch, K.J.; Ponce, F.V.; Dickson, W.B.; Van Breugel, F.; Dickinson, M.H. The long-distance flight behavior of Drosophila supports an agent-based model for wind-assisted dispersal in insects. Proc. Natl. Acad. Sci. USA 2021, 118, e2013342118. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clymans, R.; Van Kerckvoorde, V.; Thys, T.; De Clercq, P.; Bylemans, D.; Beliën, T. Mass Trapping Drosophila suzukii, What Would It Take? A Two-Year Field Study on Trap Interference. Insects 2022, 13, 240. https://doi.org/10.3390/insects13030240
Clymans R, Van Kerckvoorde V, Thys T, De Clercq P, Bylemans D, Beliën T. Mass Trapping Drosophila suzukii, What Would It Take? A Two-Year Field Study on Trap Interference. Insects. 2022; 13(3):240. https://doi.org/10.3390/insects13030240
Chicago/Turabian StyleClymans, Rik, Vincent Van Kerckvoorde, Tom Thys, Patrick De Clercq, Dany Bylemans, and Tim Beliën. 2022. "Mass Trapping Drosophila suzukii, What Would It Take? A Two-Year Field Study on Trap Interference" Insects 13, no. 3: 240. https://doi.org/10.3390/insects13030240






