Chemical Compounds Emitted from Mentha spicata Repel Aromia bungii Females
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
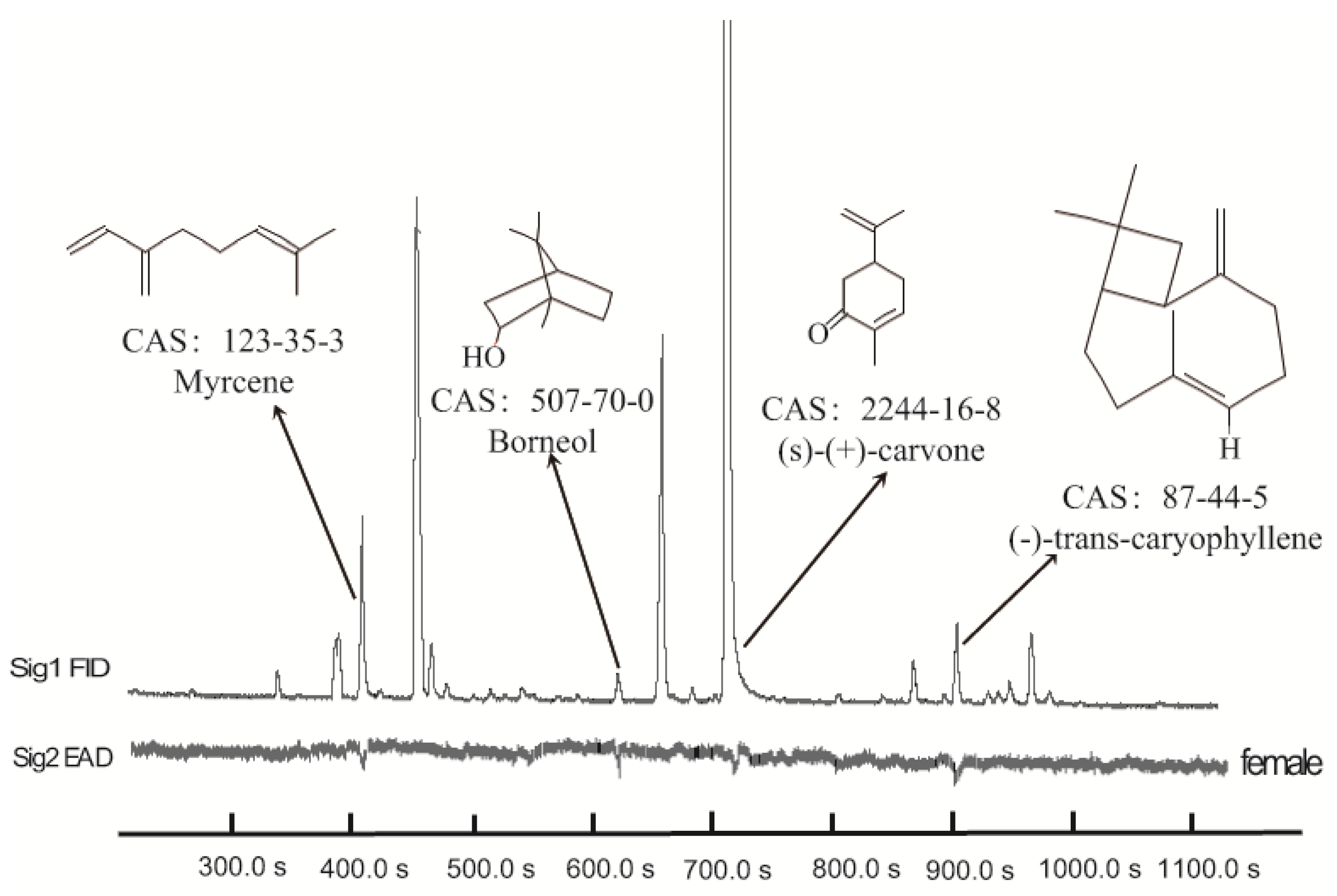
2.2. Coupled Gas Chromatography-Electroantennogram Detection (GC-EAD)
2.2.1. Collection of Volatiles
2.2.2. GC-EAD Analysis of Volatiles
2.3. GC-MS Analysis of Volatiles
2.4. Olfactory Response of A. bungii Females to Four Synthetic Chemicals
2.4.1. Compounds Used
2.4.2. Semi-Field Cage Bioassays of A. bungii Females’ Responses to Synthetic Chemicals
2.5. Statistical Analyses
3. Results
3.1. Identification of Active Chemicals from M. spicata Volatiles
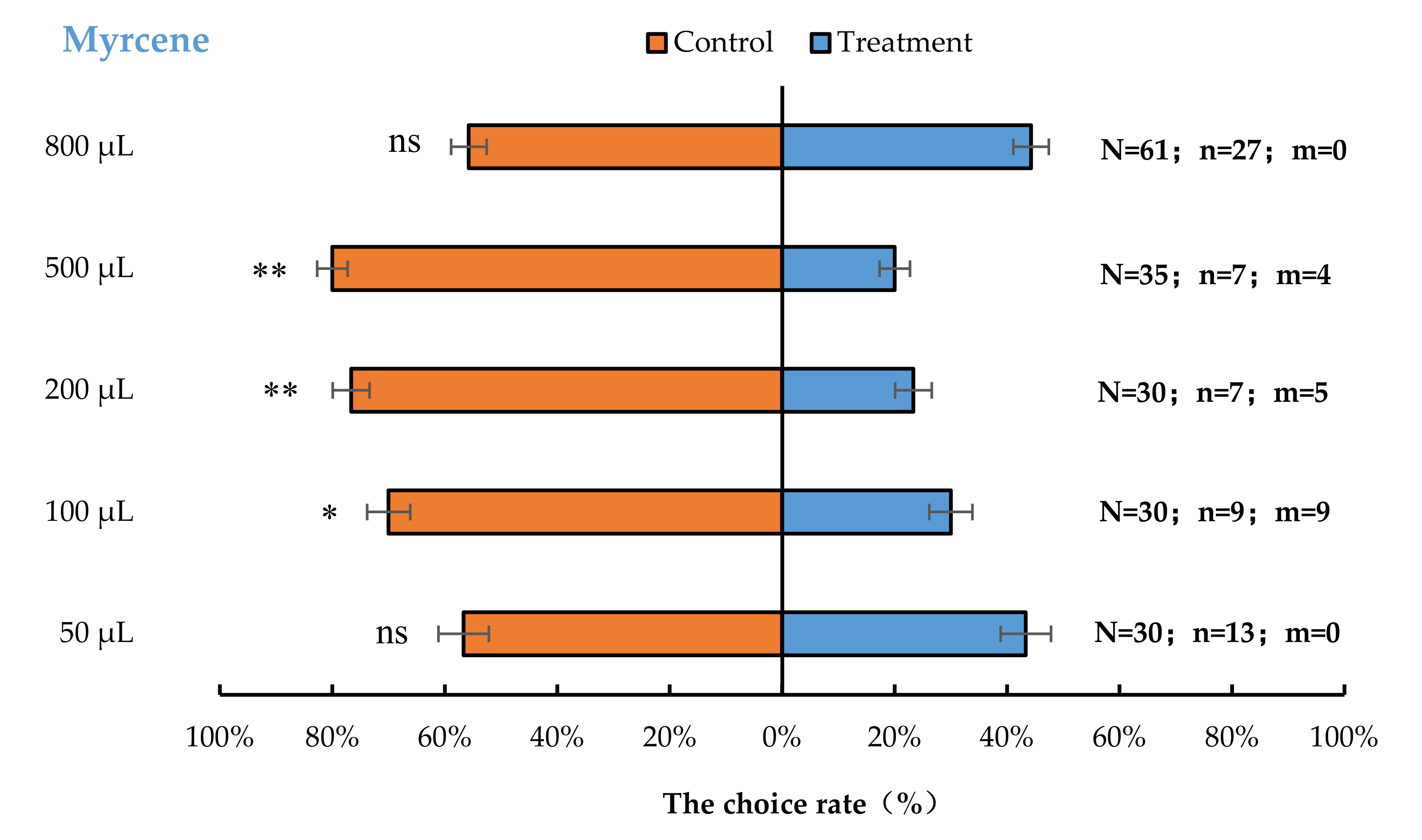
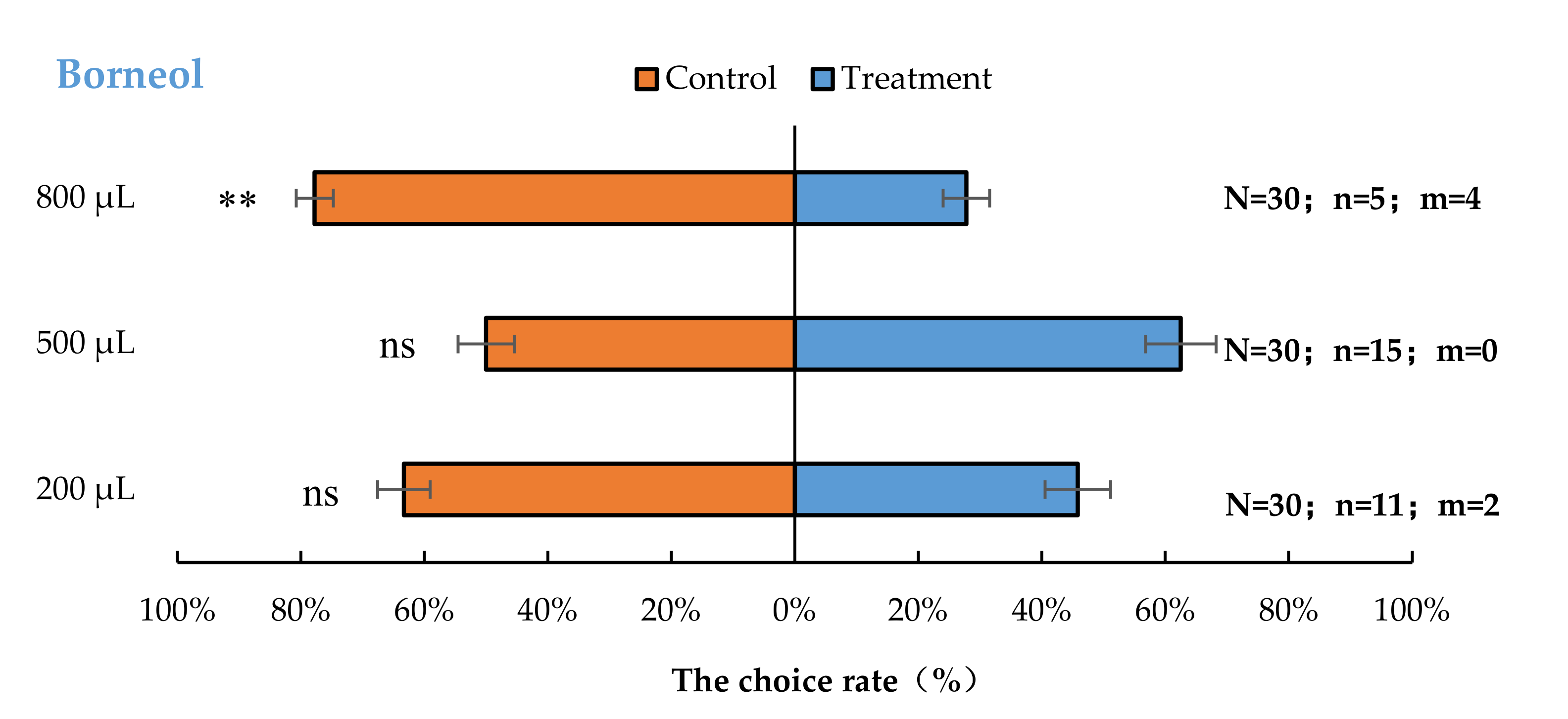
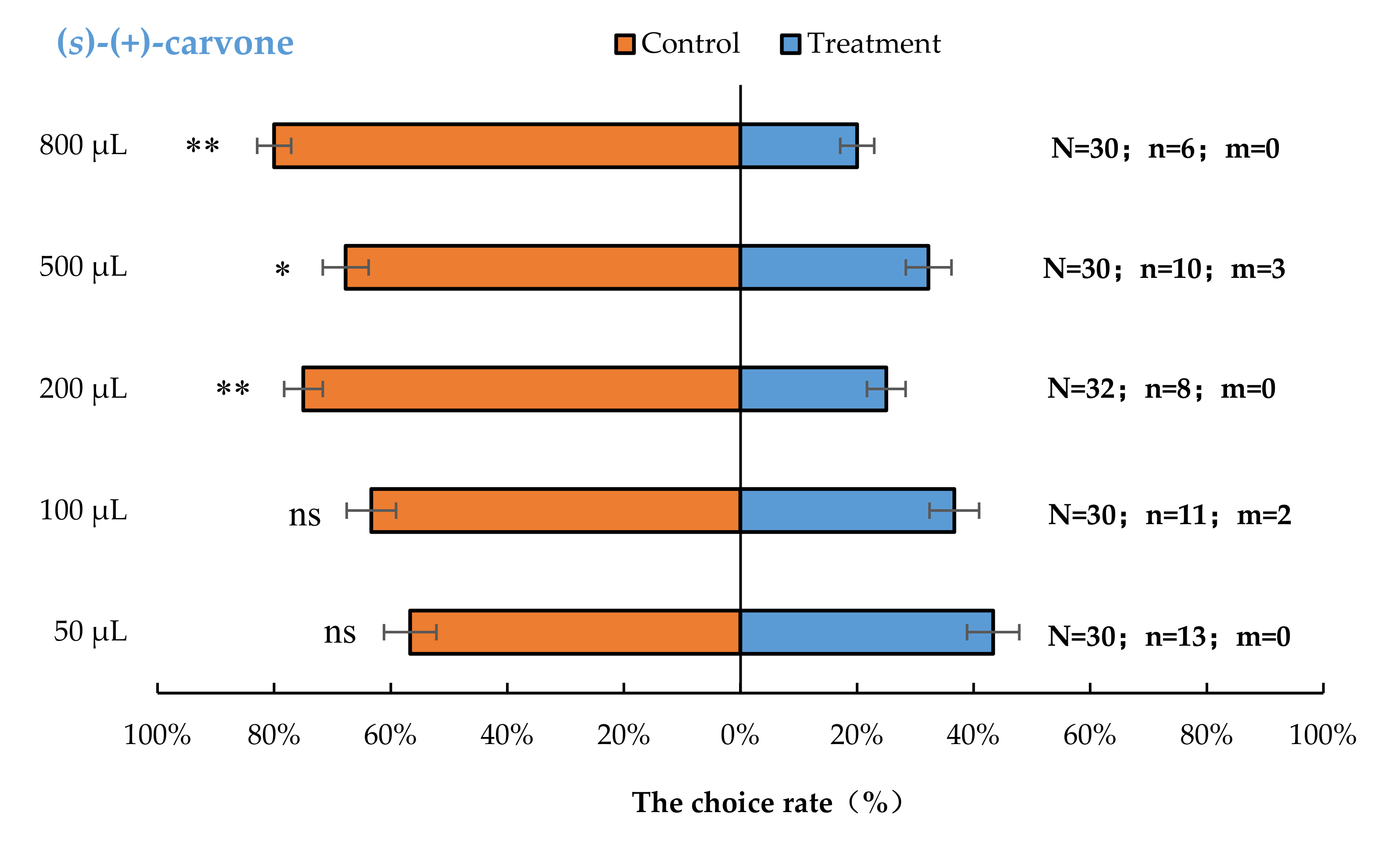
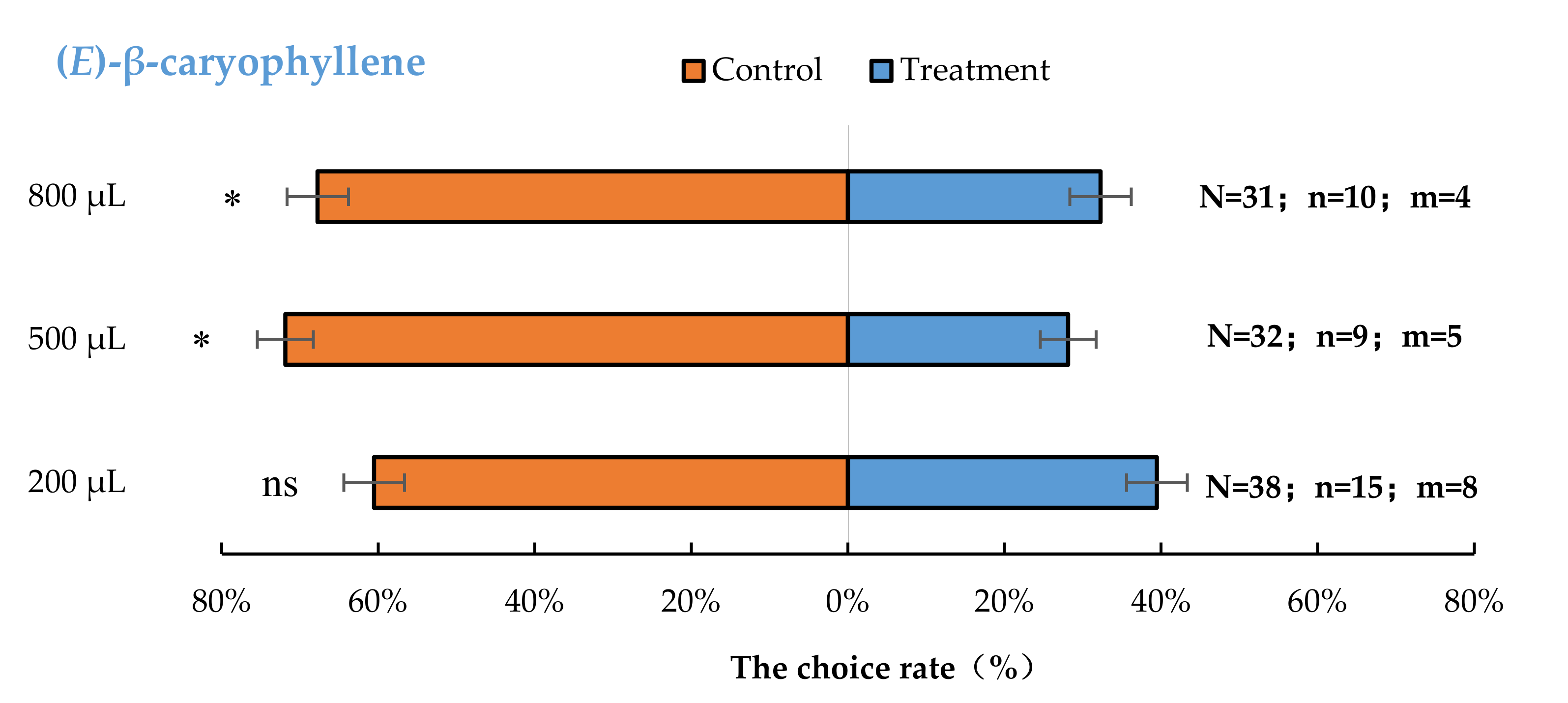
3.2. Semi-Field Cage Bioassay of A. bungii Females to Different Quantities of Four Synthetic Chemicals
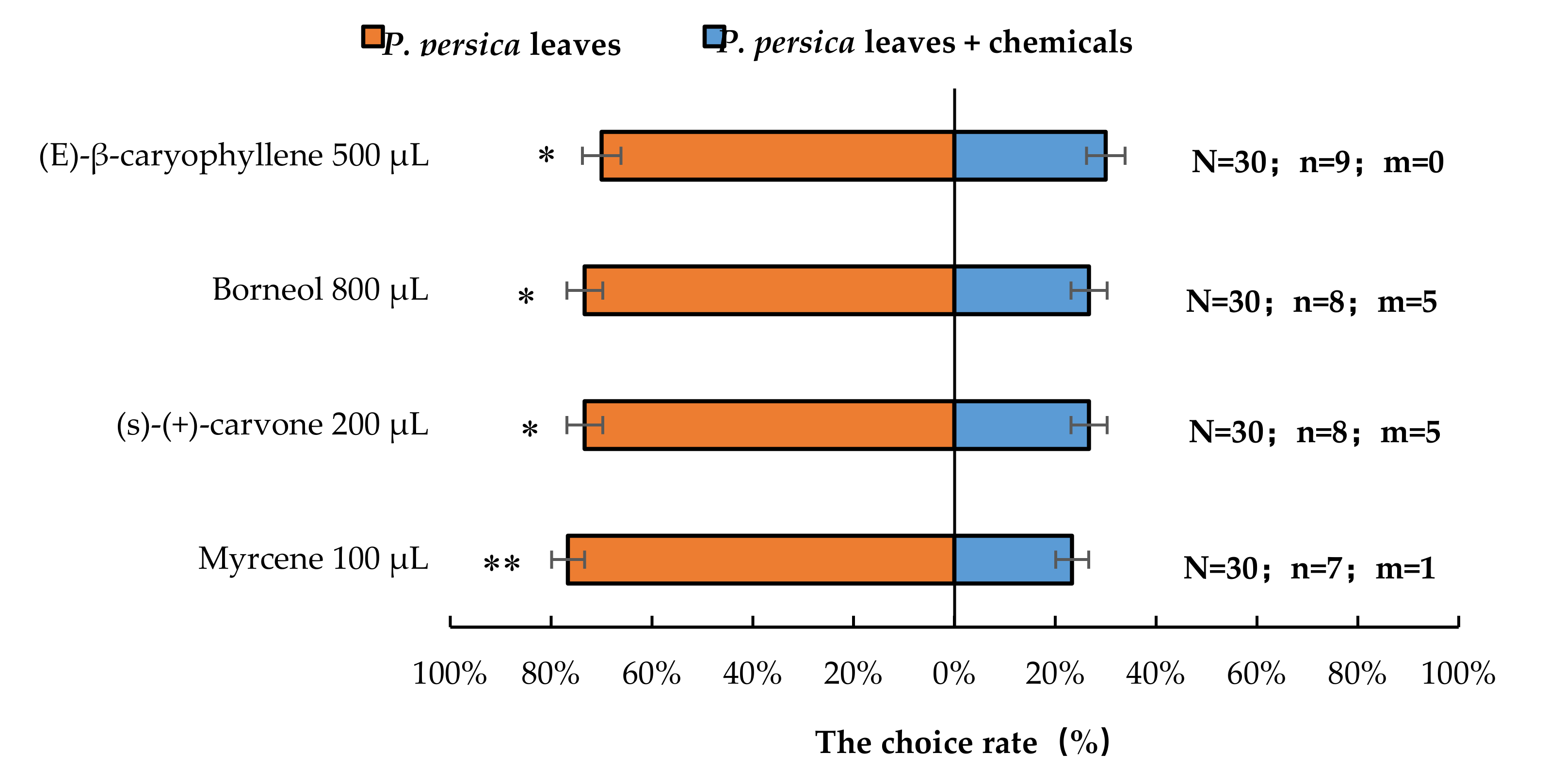
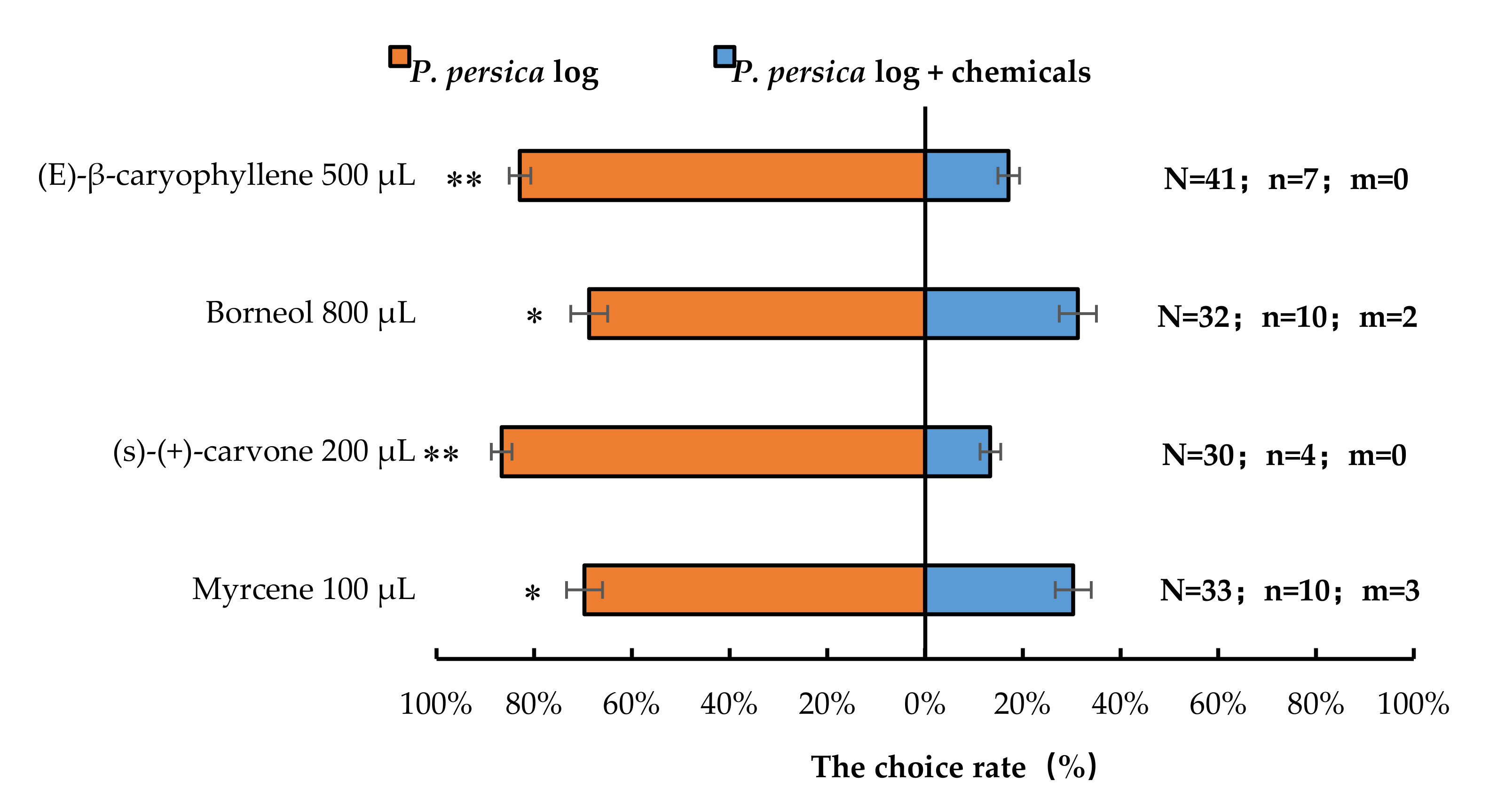
3.3. Semi-Field Cage Bioassay of A. bungii Females’ Responses to Host Plant in the Presence of Synthetic Chemicals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Union. Commission Implementing Decision (EU) 2018/1503 of 8 October 2018 as regards measures to prevent the introduction into and the spread within the Union of Aromia bungii (Faldermann). Off. J. EU 2018, L254, 9–18. [Google Scholar]
- Wang, Q. Cerambycid pests in agricultural and horticultural crops. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 409–562. [Google Scholar]
- Germinara, G.S.; Pistillo, M.; Griffo, R.; Garonna, A.P.; Palma, A.D. Electroantennographic responses of Aromia bungii (Faldermann 1835) (Coleoptera, Cerambycidae) to a range of volatile compounds. Insects 2019, 10, 274. [Google Scholar] [CrossRef] [Green Version]
- Cocquempot, C. Aromia bungii. EPPO datasheet on pests recommended for regulation. EPPO Bull. 2015, 45, 4–8. [Google Scholar]
- EPPO. Pest Risk Analysis for Aromia bungii; EPPO: Paris, France, 2014. [Google Scholar]
- CABI. Aromia bungii. In Invasive Species Compendium; CAB International: Wallingford, UK, 2019. [Google Scholar]
- Peña, E.; Schrader, G.; Vos, S. Pest Survey Card on Aromia bungii; European Food Safety Authority (EFSA): Parma, Italy, 2019; pp. 1–25. [Google Scholar]
- Ma, W.H.; Sun, L.Y.; Yu, L.G.; Wang, J.T.; Chen, J.Y. Study on the occurrence and life history in Aromia bungii (Faldermann). Acta Agric. Boreali Sin. 2007, 22, 247–249, (In Chinese with English Abstract). [Google Scholar]
- Bai, R.X.; Wang, Y.H.; Ma, Z.S.; Jia, Y.Y.; Lv, D.Z. Advance in research of Aromia bungii Faldermann. For. Pest Dis. 2017, 36, 5–9, (In Chinese with English Abstract). [Google Scholar]
- Men, J.; Cao, D.D.; Zhao, B.; Wang, W.C.; Liu, P.C.; Wei, J.R. Behavioral responses of adults of Dastarcus helophoroides (Coleoptera: Bothrideridae) populations originated from different hosts to larval frass of Aromia bungii (Coleoptera: Cerambycidae) and their control effect on A. bungii population. Acta Entomol. Sin. 2017, 60, 229–236, (In Chinese with English Abstract). [Google Scholar]
- Zou, Y.; Hansen, L.; Xu, T.; Teale, S.A.; Hao, D.; Millar, J.G. Optimizing pheromone-based lures for the invasive red-necked longhorn beetle, Aromia bungii. J. Pest Sci. 2019, 92, 1217–1225. [Google Scholar] [CrossRef]
- Men, J.; Zhao, B.; Cao, D.D.; Wang, W.C.; Wei, J.R. Evaluating host location in three native Sclerodermus species and their ability to cause mortality in the wood borer Aromia bungii (Coleoptera: Cerambycidae) in laboratory. Biol. Control 2019, 134, 95–102. [Google Scholar] [CrossRef]
- Wang, J.X.; Yan, X.W.; Cao, D.D.; Yang, B.J.; Zhao, Z.P.; Wei, J.R. Study on biological control of Aromia bungii in the peach orchard by Dastarcus helophoroides. For. Pest Dis. 2021, 40, 16–21, (In Chinese with English Abstract). [Google Scholar]
- Wei, J.R.; Liu, X.B.; Niu, Y.L.; Wang, J.J. Identification of volatiles released from the living adult Aromia bungii Faldermann. For. Pest Dis. 2013, 32, 8–10, (In Chinese with English Abstract). [Google Scholar]
- Xu, T.; Yasui, H.; Teale, S.A.; Fujiwara-Tsujii, N.; Wickham, J.D.; Fukaya, M.; Hansen, L.; Kiriyama, S.; Hao, D.; Nakano, A.; et al. Identification of a male-produced sex-aggregation pheromone for a highly invasive cerambycid beetle, Aromia bungii. Sci. Rep. 2017, 7, 7330. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, M.; Kiriyama, S.; Yasui, H. Mate-location fight of the red-necked longicorn beetle, Aromia bungii (Coleoptera: Cerambycidae): An invasive pest lethal to Rosaceae trees. Appl. Entomol. Zool. 2017, 52, 559–565. [Google Scholar] [CrossRef]
- Mori, K. Pheromone synthesis. Part 263: Synthesis of the racemate and the enantiomers of (E)-cis-6,7-epoxy-2-nonenal, the male-produced pheromone of the red-necked longhorn beetle, Aromia bungii. Tetrahedron 2018, 74, 1444–1448. [Google Scholar] [CrossRef]
- Yasui, H.; Fujiwara-Tsujii, N.; Yasuda, T.; Fukaya, M.; Kiriyama, S.; Nakano, A.; Watanabe, T.; Mori, K. Electroantennographic responses and field attraction of an emerging invader, the red-necked longicorn beetle Aromia bungii (Coleoptera: Cerambycidae), to the chiral and racemic forms of its male-produced aggregation-sex pheromone. Appl. Entomol. Zool. 2018, 54, 109–114. [Google Scholar] [CrossRef]
- Wang, W.C.; Cao, D.D.; Men, J.; Wei, J.R. (R)-(+)-citronellal identified as a female-produced sex pheromone of Aromia bungii Faldermann (Coleoptera: Cerambycidae). Egypt. J. Biol. Pest Control 2018, 28, 77. [Google Scholar] [CrossRef]
- Dardouri, T.; Gomez, L.; Schoeny, A.; Costagliola, G.; Gautier, H. Behavioural response of green peach aphid Myzus persicae (Sulzer) to volatiles from different rosemary (Rosmarinus officinalis L.). Agric. For. Entomol. 2019, 21, 336–345. [Google Scholar] [CrossRef]
- Li, X.H.; Garvey, M.; Kaplan, I.; Li, B.P.; Carrillo, J. Domestication of tomato has reduced the attraction of herbivore natural enemies to pest-damaged plants. Agric. For. Entomol. 2018, 20, 390–401. [Google Scholar] [CrossRef]
- Thacker, J.R.M. An Introduction to Arthropod Pest Control; Cambridge University Press: New York, NY, USA, 2002; pp. 1–360. [Google Scholar]
- Ware, G.W.; Whitacre, D.M. The Pesticide Book, 6th ed.; Meister Media Worldwide: Willoughby, OH, USA, 2004; 496p. [Google Scholar]
- Li, X.W.; Zhang, Z.J.; Hafeez, M.; Huang, J.; Zhang, J.M.; Wang, L.K.; Lu, Y.B. Rosmarinus officinialis L. (Lamiales: Lamiaceae), a promising repellent plant for thrips management. J. Econ. Entomol. 2021, 114, 131–141. [Google Scholar] [CrossRef]
- Smith, W.E.C.; Shivaji, R.; Williams, W.P.; Luthe, D.S.; Sandoya, G.V.; Smith, C.L.; Sparks, D.L.; Brown, A.E. A maize line resistant to herbivory constitutively releases (E)-β-Caryophyllene. J. Econ. Entomol. 2012, 105, 120–128. [Google Scholar] [CrossRef]
- Lawrence, B.M. Mint: The Genus Mentha; CRC Press: Boca Raton, FL, USA, 2006; Volume 44, p. 547. [Google Scholar]
- Wu, L.J.; Li, S.; Xie, J.X.; Sun, X.J.; Yan, X.Z.; Hao, C. Effects of stem and leaf ethanol extracts from Mentha spicata L. on antifeeding and antioviposition of diamondback moth Plutella xylostella. J. Plant Prot. 2016, 43, 1007–1013, (In Chinese with English Summary). [Google Scholar]
- Tripathi, A.K.; Prajapati, V.; Ahmad, A.; Aggarwal, K.K.; Khanuja, S.P.S. Piperitenone oxide as toxic, repellent, and reproduction retardant toward malarial vector Anopheles stephensi (Diptera: Anophelinae). J. Med. Entomol. 2004, 41, 691–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sertkaya, E.; Kaya, K.; Soylu, S. Acaricidal activities of the essential oils from several medicinal plants against the carmine spider mite Tetranychus cinnabarinus Boisd (Acarina: Tetranychidae). Ind. Crops Prod. 2010, 31, 107–112. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal properties of Mentha species: A review. Ind. Crops Prod. 2011, 34, 802–817. [Google Scholar] [CrossRef]
- Stepanycheva, E.A.; Chermenskaya, T.D.; Pavela, R.; Petrova, M.O. Effects of volatiles of essential oils on behavior of the western flower thrips Frankliniella occidentalis Perg (Thysanoptera, Thripidae). Entomol. Rev. 2018, 98, 801–806. [Google Scholar] [CrossRef]
- Bright, L.Z.; Handley, M.; Chien, I.; Curi, S.; Brownworth, L.A.; D’hers, S.; Bernier, U.R.; Gurman, P.; Elman, N.M. Analytical models integrated with satellite images for optimized pest management. Precis. Agric. 2016, 17, 628–636. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, A.K. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front. Plant. Sci. 2018, 9, 1295. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, Y.X.; Zhang, J.H.; Dou, L.D.; Ye, B.H.; Chen, N.Z. Research and application of semiochemicals of Platypus spp. Plant Quar. 2015, 29, 22–25, (In Chinese with English Abstract). [Google Scholar]
- Hao, D.J.; Ma, L.J. Analysis of volatile components of Pinus teada L. by gas chromatography-mass spectrometry with solid-phase microextraction. J. Anal. Sci. 2008, 24, 88–90, (In Chinese with English Abstract). [Google Scholar]
- Schröder, R.; Hilker, M. The relevance of background odor in resource location by insects: A behavioral approach. Bioscience 2008, 58, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Diabate, S.; Martin, T.; Murungi, L.K.; Fiaboe, K.K.M.; Subramanian, S.; Wesonga, J.; Deletre, E. Repellent activity of Cymbopogon citratus and Tagetes minuta and their specific volatiles against Megalurothrips sjostedti. J. Appl. Entomol. 2019, 143, 855–866. [Google Scholar] [CrossRef]
- Buleandra, M.A.; Oprea, E.B.; Popa, D.E.A.; David, I.G.A.; Moldovan, Z.A.; Mihai, I.A.; Badea, I.A.A. Comparative chemical analysis of Mentha piperita and M. spicata and a fast assessment of commercial peppermint teas. Nat. Prod. Commun. 2016, 11, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Yeom, H.J.; Kang, J.S.; Kim, G.H.; Park, I.K. Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica). J. Agric. Food Chem. 2012, 60, 7194–7203. [Google Scholar] [CrossRef]
- Iglesias, J.; Ester, A. Laboratory evaluation of potential molluscicides for the control of eggs of the pest slug Deroceras reticulatum (Pulmonata: Limacidae). Int. J. Pest Manag. 2002, 48, 19–23. [Google Scholar] [CrossRef]
- Saxena, D.B.; Goswami, B.K. Nematicidal activity of some essential oils against Meloidogyne incognita. India Perfum. 1987, 31, 150–154. [Google Scholar]
- Bestmann, C.B. Plant insecticidal VIII Synergistic activity of (−)-carvone and pyrethrine in the essential oil of Chrysanthemum balsamital. J. Appl. Entomol. 1998, 106, 144–149. [Google Scholar] [CrossRef]
- Watanabe, F.; Tadaki, S.; Takaoka, M.; Ishino, M.; Morimoto, I. Killing activities of the volatiles emitted from essential oils for Dermatophagoides pteronyssinus, Dermatophagoides farine and Tyrophagus putrescentiae. Shoyakugaku Zasshi 1989, 43, 163–168. [Google Scholar]
- Frank, T.; Biert, K.; Speiser, B. Feeding deterrent effect of carvone a compound from caraway seeds on the slug Arion lubitanicus. Ann. Appl. Biol. 2002, 14, 93–100. [Google Scholar] [CrossRef]
- Monzote, L.; Stamberg, W.; Staniek, K.; Gille, L. Toxic effects of carvacrol, caryophyllene oxide, and ascaridole from essential oil of Chenopodium ambrosioides on mitochondria. Toxicol. Appl. Pharmacol. 2009, 240, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Luo, L.; Liu, Z.L.; Yuan, Z.L. Biological activity of β-caryophyllene on Reticulitermes flaviceps (Isoptera: Rhinotermitidae). China Plant Prot. 2017, 37, 12–16, (In Chinese with English Abstract). [Google Scholar]
- Liu, Y.Q.; Xue, M.; Zhang, Q.C.; Zhou, F.Y.; Wei, J.Q. Toxicity of β-caryophyllene from Vitex negundo (Lamiales: Verbenaceae) to Aphis gossypii Glover (Homoptera: Aphididae) and its action mechanism. Acta Entomol. Sin. 2010, 53, 396–404. [Google Scholar]
- Silva, R.C.S.; Milet-Pinheiro, P.; Silva, P.C.B.; Silva, A.G.; Silva, M.V.; Navarro, D.M.A.F.; Silva, N.H. (E)-caryophyllene and α-humulene: Aedes aegypti oviposition deterrents elucidated by gas chromatography-electrophysiological assay of Commiphora leptophloeos leaf oil. PLoS ONE 2015, 10, e0144586. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.F. The preliminary study on mechanism of odor perception of Agrilus zanthoxylumi to volatiles from Zanthoxylum bungeanum. Master’s Thesis, Northwest Agriculture & Forest University, Xi’an, China, 2016. [Google Scholar]
- Guo, S.S.; Yuan, G.H.; Chai, X.L.; Guo, X.R.; Teng, X.H.; Li, W.Z. Interactive effect of (E)-β-farnesene and (−)-β-caryophyllene on chemical communication between Myzus persicae and Coccinella septempunctata. J. Henan Agric. Univ. 2017, 51, 42–47, (In Chinese with English Abstract). [Google Scholar]
- Liu, Y.; Xie, D.S.; Hu, C.H.; Dong, W.X.; Zhang, F.; Zhang, J.P.; Xiao, C. Influence of β-caryophyllene on the behaviors of female Drosophila suzukii. Environ. Entomol. 2018, 40, 684–689. [Google Scholar]
- Roland, J.; Denford, K.E.; Jimenez, L. Borneol as an attractant for Cyzenis albicans, a tachinid parasitoid of the winter moth, Operophtera brumata L. (Lepidoptera: Geometridae). Can. Entomol. 1995, 127, 413–421. [Google Scholar] [CrossRef]
- Katz, T.M.; Miller, J.H.; Hebert, A.A. Insect repellents: Historical perspectives and new developments. J. Am. Acad. Dermatol. 2008, 58, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, D.; Liu, J.; Zhao, Z.; Yan, X.; Wang, W.; Wei, J. Chemical Compounds Emitted from Mentha spicata Repel Aromia bungii Females. Insects 2022, 13, 244. https://doi.org/10.3390/insects13030244
Cao D, Liu J, Zhao Z, Yan X, Wang W, Wei J. Chemical Compounds Emitted from Mentha spicata Repel Aromia bungii Females. Insects. 2022; 13(3):244. https://doi.org/10.3390/insects13030244
Chicago/Turabian StyleCao, Dandan, Jianfeng Liu, Zhengping Zhao, Xuewu Yan, Weichao Wang, and Jianrong Wei. 2022. "Chemical Compounds Emitted from Mentha spicata Repel Aromia bungii Females" Insects 13, no. 3: 244. https://doi.org/10.3390/insects13030244
APA StyleCao, D., Liu, J., Zhao, Z., Yan, X., Wang, W., & Wei, J. (2022). Chemical Compounds Emitted from Mentha spicata Repel Aromia bungii Females. Insects, 13(3), 244. https://doi.org/10.3390/insects13030244





