A Review of Entomopathogenic Nematodes as a Biological Control Agent for Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Biology and Distribution of R. ferrugineus
3. Red Palm Weevil Infestation
4. Control Management of the Red Palm Weevil
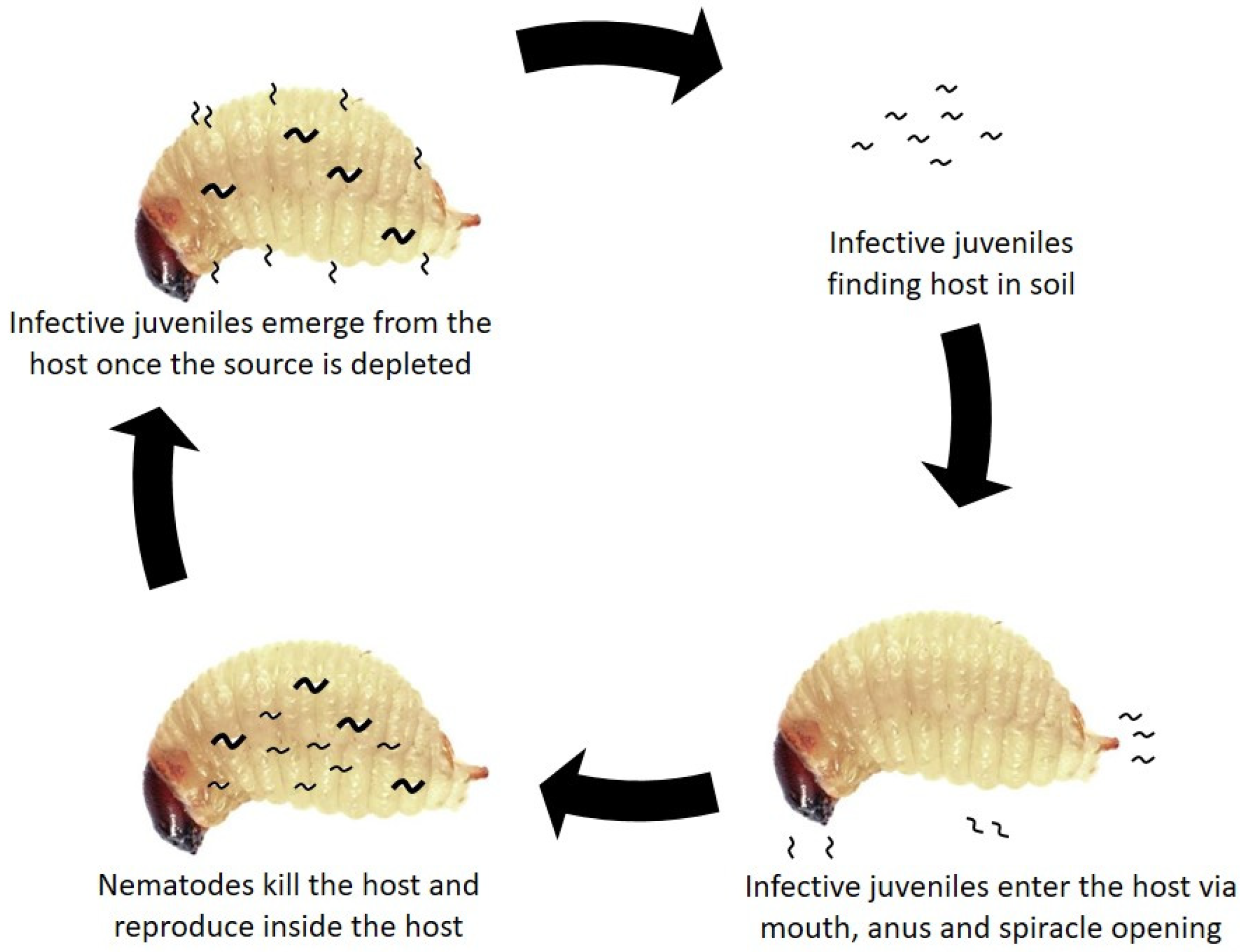
5. Biology of Entomopathogenic Nematodes
6. Mutualistic Symbiotic Bacteria
7. Application of Entomopathogenic Nematodes as Biological Control Agent
8. Biological Assay on Pathogenicity of Entomopathogenic Nematodes against R. ferrugineus
9. Formulation
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- EPPO. Rhynchophorus ferrugineus. 2021. Available online: https://gd.eppo.int (accessed on 2 February 2021).
- Murphy, S.T.; Briscoe, B.R. The red palm weevil as an alien invasive: Biology and the prospects for biological control as a component of IPM. Biocontrol News Inf. 1999, 20, 35–46. [Google Scholar]
- Faleiro, J.R. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci. 2006, 26, 135–154. [Google Scholar] [CrossRef]
- Wattanapongsiri, A. A revision of the genera Rhynchophorus and Dynamis (Coleoptera: Curculionidae); Oregon State University: Corvallis, OR, USA, 1966. [Google Scholar]
- FAO. Red Palm Weevil: Guidelines on Management Practices; FAO: Rome, Italiy, 2020; ISBN 9789251321898. [Google Scholar]
- Wahizatul, A.A.; Chong, J.L.; Hazlina, A.Z.; Norhayati, Y.; Wan Bayani, W.O.; Yong, K.W.; Ainatun, N.Z.; Mohd, H.H. The red palm weevil, Rhynchophorus ferrugineus: Current issues and challenges in Malaysia. Oil Palm Bull. 2017, 74, 17–24. [Google Scholar]
- Milosavljević, I.; El-Shafie, H.A.F.; Faleiro, J.R.; Hoddle, C.D.; Lewis, M.; Hoddle, M.S. Palmageddon: The wasting of ornamental palms by invasive palm weevils, Rhynchophorus spp. J. Pest Sci. 2019, 92, 143–156. [Google Scholar] [CrossRef]
- Faleiro, J.R.; Abdallah, A.B.; El-Bellaj, M.; Al-Ajlan, A.M.; Oihabi, A. Threat of the red palm weevil, Rhynchophorus ferrugineus (Olivier) to date palm plantations in North Africa. Arab J. Plant Prot. 2012, 30, 274–280. [Google Scholar]
- Dembilio, O.; Jacas, J.A. Bio-ecology and integrated management of the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae), in the region of Valencia (Spain). Hell. Plant Prot. J. 2012, 5, 1–12. [Google Scholar]
- Cuthbertson, A.G.S.; Audsley, N. Further screening of entomopathogenic fungi and nematodes as control agents for Drosophila Suzukii. Insects 2016, 7, 24. [Google Scholar] [CrossRef]
- Ruiu, L. Insect pathogenic bacteria in integrated pest management. Insects 2015, 6, 352–367. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.C. Giant Palm Weevils of the Genus Rhynchophorus (Coleoptera: Curculionidae) and Their Threat to Florida Palms; Pest Alert DACS-P-01682; Florida Department of Agriculture and Consumer Services: Tallahassee, FL, USA, 2010; pp. 1–2.
- Giblin-Davis, R.M.; Howard, F.W. Vulnerability of stressed palms to attack by Rhynchophorus cruentatus (Coleoptera: Curculionidae) and insecticidal control of the pest. J. Econ. Entomol. 1989, 82, 1185–1190. [Google Scholar] [CrossRef]
- EPPO. Rhynchophorus palmarum. 2021. Available online: https://gd.eppo.int/ (accessed on 20 February 2021).
- Hoddle, M.S.; Hoddle, C.D. Palmageddon: The invasion of California by the South American palm weevil is underway. CAPCA ADVISER 2017, 20, 40–44. [Google Scholar]
- Giblin-Davis, R.M.; Faleiro, J.R.; Jacas, J.A.; Peña, J.E.; Vidyasagar, P.S.P.V. Biology and management of the red palm weevil, Rhynchophorus ferrugineus. In Potential Invasive Pests of Agricultural Crops; Peña, J.E., Ed.; CAB International: Oxfordshire, UK, 2013; pp. 1–34. ISBN 9781845938291. [Google Scholar]
- Tanyi Tambe, J.; Riolo, P.; Okolle, J.N.; Isidoro, N.; Fanciulli, P.P.; Dallai, R. Sexual size differences and colour polymorphism of Rhynchophorus phoenicis in the southwest region of Cameroon. Bull. Insectol. 2013, 66, 153–159. [Google Scholar]
- Egonyu, J.P.; Gitonga, K.J.; Khamis, F.M.; Copeland, R.S.; Finyange, P.; Odhiambo, R.; Ddamulira, G.; Tanga, C.M.; Subramanian, S. Trapping, identification and rearing of edible palm weevils in Kenya and Uganda. J. Insects Food Feed 2021, 7, 1243–1253. [Google Scholar] [CrossRef]
- Hoddle, M.S.; Hoddle, C.D. How far can the palm weevil, Rhynchophorus vulneratus (Coleoptera: Curculionidae), fly? J. Econ. Entomol. 2016, 109, 629–636. [Google Scholar] [CrossRef]
- Hoddle, M.S.; Hoddle, C.D.; Alzubaidy, M.; Kabashima, J.; Nisson, J.N.; Millar, J.; Dimson, M. The palm weevil Rhynchophorus vulneratus is eradicated from Laguna Beach. Calif. Agric. 2016, 71, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Hallett, R.H.; Crespi, B.J.; Borden, J.H. Synonymy of Rhynchophorus ferrugineus (Olivier), 1790 and R. vulneratus (Panzer), 1798 (Coleoptera, Curculionidae, Rhynchophorinae). J. Nat. Hist. 2004, 38, 2863–2882. [Google Scholar] [CrossRef]
- Rugman-Jones, P.F.; Hoddle, C.D.; Hoddle, M.S.; Stouthamer, R. The lesser of two weevils: Molecular-genetics of pest palm weevil populations confirm Rhynchophorus vulneratus (Panzer 1798) as a valid species distinct from R. ferrugineus (Olivier 1790), and reveal the global extent of both. PLoS ONE 2013, 8, e78379. [Google Scholar] [CrossRef]
- Aman-Zuki, A.; Ghazali, S.Z.; Badrulisham, A.S.; Hazmi, I.R.; Nurul Wahida, O.; Yaakop, S. Proof on the divergence times of two sympatric species, Rhynchophorus ferrugineus and R. vulneratus (Coleoptera: Curculionidae) by molecular clock analysis. J. Entomol. Res. Soc. 2021, 23, 11–26. [Google Scholar] [CrossRef]
- Buxton, P.A. Insect pests of dates and the date palm in Mesopotamia and elsewhere. Bull. Entomol. Res. 1920, 11, 287–303. [Google Scholar] [CrossRef]
- Cox, M.L. Red Palm Weevil, Rhynchophorus ferrugineus in Egypt. FAO Plant Prot. Bull. 1993, 41, 30–31. [Google Scholar]
- Barranco, P.; de la Pena, J.; Cabello, T. Un nuevo curculio’nido tropical para la fauna europa, Rhynchophorus ferrugineus (Olivier 1790), (Curculionidae: Coleoptera). Bol. De La Asoc. Esp. De Entomol. 1995, 20, 257–258. [Google Scholar]
- Jalinas, J.; Güerri-agulló, B.; Dosunmu, O.G.; Llorca, L.V.L.; Mankin, R.W.; Red, T.; Weevil, P.; Olivier, R. Acoustic activity cycles of Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) early instars after Beauveria bassiana (Hypocreales: Clavicipitaceae) treatments. Ann. Entomol. Soc. Am. 2017, 110, 551–557. [Google Scholar] [CrossRef]
- Fiaboe, K.K.M.; Peterson, A.T.; Kairo, M.T.K.; Roda, A.L. Predicting the potential worldwide distribution of the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) using ecological niche modelling. Fla. Entomol. 2012, 95, 659–673. [Google Scholar] [CrossRef]
- Ge, X.; He, S.; Wang, T.; Yan, W.; Zong, S. Potential distribution predicted for Rhynchophorus ferrugineus in China under different climate warming scenarios. PLoS ONE 2015, 10, e0141111. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.V.; al Shuaibi, M.A.; Faleiro, J.R.; Abozuhairah, R.A.; Vidyasagar, P.S.P.V. An integrated management approach for red palm weevil Rhynchophorus ferrugineus Oliv. a key pest of date palm in the Middle East. J. Agric. Mar. Sci. 1998, 3, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.; Juliet, V. A novel approach to identify red palm weevil on palms. Adv. Mater. Res. 2013, 634–638, 3853–3857. [Google Scholar] [CrossRef]
- El-sabea, A.M.R.; Faleiro, J.R.; Abo-el-saad, M.M. The threat of red palm weevil Rhynchophorus ferrugineus to date plantations of the Gulf region in the Middle-East: An economic perspective. Outlooks Pest Manag. 2009, 20, 131–134. [Google Scholar] [CrossRef]
- Al-Nujiban, A.A.; Aldosari, S.A.; Al-Suhaibani, A.M.; Abdel-Azim, M.M.; Mostafa Ibrahim, S.M.; Shukla, P. Effect of date palm cultivar on fecundity and development of Rhynchophorus ferrugineus. Bull. Insectol. 2015, 68, 199–206. [Google Scholar]
- Gozel, U.; Gozel, C.; Yurt, C.; Inci, D. Efficacy of entomopathogenic nematodes on the red palm weevil Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Curculionidae) larvae. Int. J. Bioassay 2015, 4, 4436–4439. [Google Scholar] [CrossRef]
- Yong, W.K.; Aisyah, A.B.; Wahizatul, A.A. Fecundity, fertility and survival of red palm weevil (Rhynchophorus ferrugineus) larvae reared on sago palm. Sains Malays. 2015, 44, 1371–1375. [Google Scholar] [CrossRef]
- Wahizatul, A.A.; Zazali, C.; Abdul Rahman, A.R.; Nurul Izzah, A.G. A new invasive coconut pest in Malaysia: The red palm weevil (Curculionidae: Rhynchophorus ferrugineus). Plant 2013, 89, 97–110. [Google Scholar]
- Witt, A.; Hula, V.; Suleiman, A.S.; van Damme, K. First record of the red palm weevil Rhynchophorus ferrugineus (Olivier) on Socotra Island (Yemen), an exotic pest with high potential for adverse economic impacts. Rend. Lincei. Sci. Fis. E Nat. Vol. 2020, 31, 645–654. [Google Scholar] [CrossRef]
- Dembilio, O.; Jacques, J.A. Biology and management of red palm weevil. In Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges; Wakil, W., Faleiro, J.R., Miller, T.A., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 13–35. ISBN 9783319243979. [Google Scholar]
- Ju, R.-T.; Wang, F.; Wan, F.-H.; Li, B. Effect of host plants on development and reproduction of Rhynchophorus ferrugineus (Olivier) (Coleopteraa: Curculionidae). J. Pest Sci. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Norzainih, J.J.; Harris, M.N.; Nurul Wahida, O.; Salmah, Y.; Norefrina Shafinaz, M.N. Continuous rearing of the red palm weevils, Rhynchophorus ferrugineus (Olivier), 1970 on sugarcane in laboratory for biological studies (Coleoptera: Dryophthoridae). In Proceedings of the 3rd International Conference on Chemical, Agricultural and Medical Sciences, 10–11 December 2015; pp. 38–40. [Google Scholar]
- El-Zoghby, I.R.M.; Abdel-Hameid, N.F. Rearing of the red palm weevil, Rhynchophorus ferrugineus (Olivier) on Different Natural Diets. In Proceedings of the 4th International Conference on Biotechnology Applications in Agriculture (ICBAA), Benha University, Moshtohor and Hurghada, Egypt, 4–7 April 2018; pp. 509–518. [Google Scholar]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Jabr, A.M.; Al-Ayied, H.Y. Managing invasive populations of red palm weevil: A worldwide perspective. J. Food Agric. Environ. 2013, 11, 456–463. [Google Scholar]
- Salama, H.S.; Abdel-Razek, A.S. Development of the red palm weevil, Rhynchophorus ferrugineus (Olivier), (Coleoptera, Curculionidae) on natural and synthetic diets. J. Pest Sci. 2002, 75, 137–139. [Google Scholar] [CrossRef]
- Güerri-Agulló, B.; Gómez-Vidal, S.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: A SEM study. Microsc. Res. Tech. 2010, 73, 714–725. [Google Scholar] [CrossRef]
- Harith-Fadzilah, N.; Harris-Hussain, M.; Idris, A.G.; Azlina, Z.; Samsudin, A.; Zamri, Z.; Wahizatul, A.A.; Jalinas, J.; Maizom, H. Physical and physiological monitoring on red palm weevil—Infested oil palms. Insects 2020, 11, 407. [Google Scholar] [CrossRef]
- Chihaoui-Meridja, S.; Harbi, A.; Abbes, K.; Chaabane, H.; la Pergola, A.; Chermiti, B.; Suma, P. Systematicity, persistence and efficacy of selected insecticides used in endotherapy to control the red palm weevil Rhynchophorus ferrugineus (Olivier, 1790) on Phoenix canariensis. Phytoparasitica 2020, 48, 75–85. [Google Scholar] [CrossRef]
- Al-Dosary, N.M.N.; Al-Dobai, S.; Faleiro, J.R. Review on the management of red palm weevil Rhynchophorus ferrugineus Olivier in date palm Phoenix dactylifera L. Emir. J. Food Agric. 2016, 28, 34–44. [Google Scholar] [CrossRef]
- Haris, M.H.; Nang, M.L.S.; Chuah, T.S.; Wahizatul, A.A. The efficacy of synthethic food baits in capturing red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in campus area of Universiti Malaysia Terengganu. Serangga 2014, 19, 19–35. [Google Scholar]
- Faleiro, J.R.; Al-Shawaf, A.M.; Al-Dandan, A.M.; Al-Odhayb, A.; Al-Rudayni, A.; Abdallah, A.B.; Peixoto, M.P.; Vargas, R.; Bottom, M.; Chidi, S.; et al. Controlled release products for managing insect pests. Outlooks Pest Manag. 2016, 27, 175–180. [Google Scholar] [CrossRef]
- Jalinas, J.; Guerri-Agullo, B.; Dosunmu, O.G.; Haseeb, M.; Lopez-Llorca, L.V.; Mankin, R.W. Acoustic signal applications in detection and management of Rhynchophorus spp. in fruit-crops and ornamental palms. Fla. Entomol. 2019, 102, 475–479. [Google Scholar] [CrossRef]
- Jalinas, J.; Guerri-Agullo, B.; Mankin, R.W.; Lopez-Follana, R.; Lopezz_Llorca, L.V. Acoustic assessment of Beauveria bassiana (Hypocrealess: Clavicipitaceae) effects on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) larval activity and mortality. J. Econ. Entomol. 2015, 108, 444–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankin, R.W. Applications of acoustics in insect pest management. CAB Rev. 2012, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- DOA. Report on Current Status of Attack of the Red Palm Weevil, Rhynchophorus ferrugineus in Terengganu; Department of Agriculture Malaysia: Kuching, Malaysia, 2019. [Google Scholar]
- Santhi, S.V.; Salame, L.; Nakache, Y.; Koltai, H.; Soroker, V.; Glazer, I. Attraction of entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora to the red palm weevil (Rhynchophorus ferrugineus). Biol. Control 2015, 83, 75–81. [Google Scholar] [CrossRef]
- Manzoor, M.; Ahmad, J.; Sharif, Z.M.; Majeed, D.; Kiran, H.; Ali, M.J. Comparative effectiveness of entomopathogenic nematodes against red palm weevil (Rhynchophorus ferrugineus) in Pakistan. J. Entomol. Zool. Stud. 2017, 5, 756–760. [Google Scholar]
- Dembilio, Ó.; Quesada-moraga, E.; Santiago-álvarez, C.; Jacas, J.A. Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus. J. Invertebr. Pathol. 2010, 104, 214–221. [Google Scholar] [CrossRef]
- Yasin, M.; Wakil, W.; Ghazanfar, M.U.; Qayyum, M.A.; Tahir, M.; Bedford, G.O. Virulence of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against red palm weevil, Rhynchophorus ferrugineus (Olivier). Entomol. Res. 2017, 49, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Ishak, I.; Ng, L.C.; Harris-Hussain, M.; Jalinas, J.; Idris, A.B.; Azlina, Z.; Samsudin, A.; Wahizatul, A.A. Pathogenicity of an indigenous strain of the entomopathogenic fungus Metarhizium anisopliae (Hypocrealess: Clavicipitaceae) (MET-GRA4 Strain) as a potential biological control agent against the red palm weevil (Coleoptera: Dryophthoridae). J. Econ. Entomol. 2020, 113, 43–49. [Google Scholar] [CrossRef]
- Gopinadhan, P.B.; Mohandas, N.; Vasudevan Nair, K.P. Cytoplasmic polyhedrosis virus infecting redpalm weevil of coconut. Curr. Sci. 1990, 59, 577–580. [Google Scholar]
- Mazza, G.; Francardi, V.; Simoni, S.; Benvenuti, C.; Cervo, R.; Faleiro, J.R.; Llacer, E.; Longo, S.; Nannelli, R.; Tarasco, E.; et al. An overview on the natural enemies of Rhynchophorus palm weevils, with focus on R. ferrugineus. Biol. Control 2014, 77, 83–92. [Google Scholar] [CrossRef]
- Alfazariy, A.A. Notes on the survival capacity of two naturally occurring entomopathogens on the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Egypt. J. Biol. Pest Control 2004, 14, 423. [Google Scholar]
- Martinez de Altube, M.; Peña, A.M. Use of entomopathogenic nematodes and chitosan against Rhynchophorus ferrugineus and Paysandisia archon in Phoenix canariensis, Pheonix dactilifera and Chamaerops humilis in Spain. Insect Pathog. Insect Parasit. Nematodes IOBC/Wprs Bull. 2009, 45, 369–370. [Google Scholar]
- Yasin, M.; Wakil, W.; Qayyum, M.A.; Ali, S.; Sajjad, A.; Aqueel, M.A.; Shakeel, M. Biocontrol potential of entomopathogenic fungi, nematodes and bacteria against Rhynchophorus ferrugineus (Olivier). Egypt. J. Biol. Pest Control. 2021, 31, 138–149. [Google Scholar] [CrossRef]
- Ali, I.A.; Al-Jabr, A.; Memari, A.R. FDTD Simulation and experimental investigation of controlled microwave irradiation of red palm weevils. In Proceedings of the IEEE Middle East Conference on Antennas and Propagation (MECAP 2010), Cairo, Egypt, 20–22 October 2010; pp. 1–8. [Google Scholar]
- Massa, R.; Panariello, G.; Migliore, M.D.; Pinchera, D.; Schettino, F.; Griffo, R.; Martano, M.; Power, K.; Maiolino, P.; Caprio, E. Microwave heating: A promising and eco-compatible solution to fight the spread of red palm weevil. Arab J. Plant Prot. 2019, 37, 143–148. [Google Scholar] [CrossRef]
- Massa, R.; Panariello, G.; Pinchera, D.; Schettino, F.; Caprio, E.; Griffo, R.; Migliore, M.D. Experimental and numerical evaluations on palm microwave heating for red palm weevil pest control. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Massa, R.; Caprio, E.; de Santis, M.; Griffo, R.; Migliore, M.D.; Panariello, G.; Pinchera, D.; Spigno, P. Microwave treatment for pest control: The case of Rhynchophorus ferrugineus in Phoenix Canar. EPPO Bull. 2011, 41, 128–135. [Google Scholar] [CrossRef]
- Fabrick, J.A.; Yool, A.J.; Spurgeon, D.W. Insecticidal activity of marigold Tagetes patula plants and foliar extracts against the Hemipteran pests, Lygus hesperus and Bemisia tabaci. PLoS ONE 2020, 15, e0233511. [Google Scholar] [CrossRef]
- Samarasekera, R.; Kalhari, K.S. Insecticidal activity of essential oils of Ceylon Cinnamomum and Cymbopogon Species against Musca domestica. J. Essent. Oil Res. 2006, 18, 352–354. [Google Scholar] [CrossRef]
- Doumbia, M.; Yoboue, K.; Kouame, L.K.; Kanko, C.; Kra, D.K.; Kwadjo, K.E.; Douan, B.G.; Dagnogo, M. Toxicity of Cymbopogon nardus (Glumaless: Poacea) against four stored food products insect pests. Int. J. Farming Alllied Sci. 2014, 3, 903–909. [Google Scholar]
- Hernandez-Lambraño, R.; Pajaro-Castro, N.; Caballero-Gallardo, K.; Stashenko, E.; Olivero-Verbel, J. Essential oils from plants of the genus Cymbopogon as natural insecticides to control stored product pests. J. Stored Prod. Res. 2015, 62, 81–83. [Google Scholar] [CrossRef]
- Mona, M.A.D. Insecticidal potential of cardamom and clove extracts on adult red palm weevil Rhynchophorus ferrugineus. Saudi J. Biol. Sci. 2020, 27, 195–201. [Google Scholar] [CrossRef]
- Kanzaki, N.; Kiontke, K.; Tanaka, R.; Hirooka, Y.; Schwarz, A.; Müller-Reichert, T.; Chaudhuri, J.; Pires-Dasilva, A. description of two three-gendered nematode species in the new genus Auanema (Rhabditina) that are models for reproductive mode evolution. Sci. Rep. 2017, 7, 11135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, W.; Robbins, R.T.; Kirkpatrick, T. Molecular characterization of root-knot nematodes (Meloidogyne spp.) from Arkansas, USA. Sci. Rep. 2019, 9, 15680. [Google Scholar] [CrossRef] [Green Version]
- Elawad, S.A.; Gowen, S.R.; Hague, N.G.M. The life cycle of Steinernema abbasi and S. riobrave in Galleria mellonella. Nematology 1999, 1, 762–764. [Google Scholar] [CrossRef]
- Nguyen, K.B.; Smart, G.C. Life cycle of Steinernema scapterisci Nguyen & Smart 1990. J. Entomol. 1992, 24, 160–169. [Google Scholar]
- Tarasco, E.; Clausi, M.; Rappazzo, G.; Panzavolta, T.; Curto, G.; Sorino, R.; Oreste, M.; Longo, A.; Leone, D.; Tiberi, R.; et al. Biodiversity of entomopathogenic nematodes in Italy. J. Helminthol. 2015, 89, 359–366. [Google Scholar] [CrossRef]
- Stuart, R.J.; Barbercheck, M.E.; Grewal, P.S. Entomopathogenic nematodes in the soil environment: Distributions, interactions and the influence of biotic and abiotic factors. In Nematode Pathogenesis of Insects and Other Pests; Campos-Herrera, R., Ed.; Springer International Publishing AG: Cham, Switzerland, 2015; pp. 97–137. ISBN 9783319182667. [Google Scholar]
- Kaya, H.K.; Stock, S.P. Techniques in insect nematology. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: London, UK, 1997; pp. 281–324. [Google Scholar]
- Gozel, U.; Gozel, C. Entomopathogenic nematodes in pest management. In Integrated Pest Management (IPM): Environmentally Sound Pest Management; Gill, H., Ed.; IntechOpen: London, UK, 2016; pp. 55–69. [Google Scholar]
- Stock, S.P.; Hunt, D.J. Morphology and systematics of nematodes used in biocontrol. In Nematodes as Biocontrol Agents; Grewal, P.S., Ehlers, R.U., Shapiro-Ilan, D.I., Eds.; CAB International: Oxfordshire, UK, 2005; pp. 3–45. [Google Scholar]
- Hunt, D.J.; Nguyen, K.B. Advances in Entomopathogenic Nematodes Taxonomy and Phylogeny. In Nematology Monographs and Perspectives; Brill: Leiden, The Netherlands, 2016; p. 2. ISBN 9789004285347. [Google Scholar]
- Bird, A.F.; Bird, J. The Structure of Nematodes; Academic Press: San Diego, CA, USA, 1991; ISBN 0120996510. [Google Scholar]
- Akhurst, R.J. A Xenorhabdus sp. (Eubacteriales: Enterobacteriaceae) symbiotically associated with Steinernema kraussei (Nematoda: Steinernernatidae). Rev. De Nematol. 1982, 5, 277–280. [Google Scholar]
- Kaya, H.K.; Gaugler, R. Nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Lee, D.L. The Biology of Nematodes; Taylor & Francis: New York, NY, USA, 2002; ISBN 0-203-26116-X. [Google Scholar]
- Park, Y.; Kim, Y. Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus nematophilus, the symbiotic bacteria to the entomopathogenic nematode Steinernema carpocapsae. J. Insect Physiol. 2000, 46, 1469–1476. [Google Scholar] [CrossRef]
- Thanwisai, A.; Tandhavanant, S.; Saiprom, N.; Waterfield, N.R.; Ke, P.; Bode, H.B.; Peacock, S.J.; Chantratita, N. Diversity of Xenorhabdus and Photorhabdus spp. and their symbiotic entomopathogenic nematodes from Thailand. PLoS ONE 2012, 7, e43835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yooyangket, T.; Muangpat, P.; Polseela, R.; Tandhavanant, S.; Thanwisai, A.; Vitta, A. Identification of entomopathogenic nematodes and symbiotic bacteria from Nam Nao National Park in Thailand and larvicidal activity of symbiotic bacteria against Aedes aegypti and Aedes albopictus. PLoS ONE 2018, 13, e0195681. [Google Scholar] [CrossRef]
- Griffin, C.T.; Boemare, N.E.; Lewis, E.E. Biology and behaviour. In Nematodes as Biocontrol Agents; Grewal, P.S., Ehlers, R.U., Shapiro-Ilan, D.I., Eds.; CAB International: Oxfordshire, UK, 2005; pp. 47–64. [Google Scholar]
- Tailliez, P.; Laroui, C.; Ginibre, N.; Paule, A.; Pages, S.; Boemarare, N. Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa: X.vietnamensis sp. nov., P. luminescens subsp. caribbeanensis subsp. nov., P. luminescens subsp. hainanensis subsp. nov., P. temperata subsp. khanii subsp. nov., P. temperata subsp. tasmaniensis subsp. nov., and the reclassification of P. luminescens subsp. thracensis as P. temperata subsp. thracensis comb. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 1921–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissfeld, A.S.; Halliday, R.J.; Simmons, D.E.; Trevino, E.A.; Vance, P.H.; OHara, C.M.O.; Sowers, E.G.; Kern, R.; Koy, R.D.; Hodde, K.; et al. Photorhabdus asymbiotica, a pathogen emerging on two continents that proves that there is no substitute for a well-trained clinical microbiologist. J. Clin. Microbiol. 2005, 43, 4152–4155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dix, I.; Burnell, A.M.; Griffin, C.T.; Joyce, S.A.; Nugent, M.J.; Downes, M.J. The identification of biological species in the genus Heterorhabditis (Nematoda: Heterorhabditidae) by cross-breeding second generation amphimictic adults. Parasitology 1992, 104, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Kerry, B.R.; Hominick, W.M. Biological control. In The Biology of Nematodes; Lee, D.L., Ed.; Taylor & Francis: London, UK, 2002; pp. 932–984. [Google Scholar]
- Poinar, G.O. Taxonomy and biology of Steinernematidae and Heterorhabditidae. In Entomopathogenie Nematodes in Biological Control; Gaugler, R., Kaya, H.K., Eds.; Taylor & Francis: Boca Raton, FL, USA, 1990; pp. 23–59. [Google Scholar]
- Forst, S.; Nealson, K. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol. Rev. 1996, 60, 21–43. [Google Scholar] [CrossRef]
- Vashisth, S.; Chandel, Y.S.; Sharma, K. Entomopathogenic nematodes—A review. Agric. Rev. 2013, 34, 163–175. [Google Scholar] [CrossRef]
- Binda-Rossetti, S.; Mastore, M.; Protasoni, M.; Brivio, M.F. Effects of an entomopathogen nematode on the immune response of the insect pest red palm weevil: Focus on the host antimicrobial response. J. Invertebr. Pathol. 2016, 133, 110–119. [Google Scholar] [CrossRef]
- Mastore, M.; Arizza, V.; Manachini, B.; Brivio, M.F. Modulation of immune responses of Rhynchophorus ferrugineus (Insecta: Coleoptera) induced by the entomopathogenic nematode Steinernema carpocapsae (Nematoda: Rhabditida). Insect Sci. 2014, 22, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Gaugler, R. Steinernema glaseri surface coat protein suppresses the immune response of Popillia japonica (Coleoptera: Scarabaeidae) larvae. Biol. Control 1999, 14, 45–50. [Google Scholar] [CrossRef]
- Kucharska, K.; Kucharski, D.; Zajdel, B. Bacteria Xenorhabdus and Photorhabdus, entomopathogenic nematodes and insects—their role in the complex symbiont-parasite-host relationship. Postepy Mikrobiol. 2015, 54, 154–164. [Google Scholar]
- Owuama, C. Entomopathogenic symbiotic bacteria, Xenorhabdus and Photorhabdus of nematodes. World J. Microbiol. Biotechnol. 2001, 17, 505–515. [Google Scholar] [CrossRef]
- Lacey, L.A.; Shapiro-Ilan, D.I. Microbial control of insect pests in temperate orchard systems: Potential for incorporation into IPM. Annu. Rev. Entomol. 2008, 53, 121–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahina, F.; Gulsher, M.; Javed, S.; Khanum, T.A.; Bhatti, M.I. Susceptibility of different life stages of red palm weevil, Rhynchophorus ferrugineus, to entomopathogenic nematodes. Int. J. Nematol. 2009, 19, 232–239. [Google Scholar]
- El Sadawy, H.A.; El Namaky, A.H.; Al Omari, F.; Bahareth, O.M. Susceptibility of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) to entomopathogenic nematodes with regard to its immune response. Biol. Control 2020, 148, 104308. [Google Scholar] [CrossRef]
- Elawad, S.A.; Mousa, S.A.; Shahdad, A.S.; Alawaash, S.A.; Alamiri, A.M.A. Efficacy of entomopathogenic nematodes against red palm weevil in UAE. Acta Hortic. 2007, 736, 415–420. [Google Scholar] [CrossRef]
- Griffin, C.T. Perspectives on the behavior of entomopathogenic nematodes from dispersal to reproduction: Traits contributing to nematode fitness and biocontrol efficacy. J. Entomol. 2012, 44, 177–184. [Google Scholar]
- Atwa, A.A.; Hegazi, E.M. Comparative susceptibilities of different life stages of the red palm weevil (Coleoptera: Curculionidae) treated by entomopathogenic nematodes. J. Econ. Entomol. 2014, 107, 1339–1347. [Google Scholar] [CrossRef]
- Saleh, M.M.E.; Alheji, M. Biological control of red palm weevil with entomopathogenic nematodes in the eastern province of Saudi Arabia. Egypt. J. Biol. Pest Control 2003, 13, 55–59. [Google Scholar]
- Abbas, M.S.T.; Hanonik, S.P. Pathogenicity of entomopathogenic nematodes to red palm weevil, Rhynchophorus ferrugineus. Int. J. Nematol. 1999, 9, 84–86. [Google Scholar]
- Abbas, M.S.T.; Saleh, M.M.E.; Akil, A.M. Laboratory and field evaluation of the pathogenicity of entomopathogenic nematodes to the red palm weevil, Rhynchophorus ferrugineus (Oliv.) (Col.: Curculionidae). J. Pest Sci. 2001, 74, 167–168. [Google Scholar] [CrossRef]
- Wakil, W.; Yasin, M.; Shapiro-ilan, D. Effects of single and combined applications of entomopathogenic fungi and nematodes against Rhynchophorus ferrugineus (Olivier). Sci. Rep. 2017, 7, 5971. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, O.; Tarasco, E. Evaluation of the effects of autochthonous and commercial isolates of Steinernematidae and Heterorhabditidae on Rhynchophorus ferrugineus. Bull. Insectol. 2011, 64, 175–180. [Google Scholar]
- Tapia, G.; Ruiz, M.A.; Tellez, M.M. Recommendations for a preventive strategy to control red palm weevil (Rhynchophorus ferrugineus, Olivier) based on the use of insecticides and entomopathogenic nematodes. EPPO Bull. 2011, 41, 136–141. [Google Scholar] [CrossRef]
- Llácer, E.; de Altube, M.M.; Jacas, J.A. Evaluation of the efficacy of Steinernema carpocapsae in a chitosan formulation against the red palm weevil, Rhynchophorus ferrugineus, in Phoenix canariensis. BioControl 2009, 54, 559–565. [Google Scholar] [CrossRef]
- Ali, M.A.; Bekhiet, H.K.; Ragheb, D.A.; El-Feshaway, A.A. Evaluation of some entomopathogens on the red palm weevil, Rhynchophorus ferrugineus under laboratory and field conditions. Egypt. J. Agric. Res. 2018, 96, 415–430. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Han, R.; Dolinksi, C. Entomopathogenic nematode production and application technology. J. Nematol. 2012, 44, 206–217. [Google Scholar]
- Shapiro-Ilan, D.I.; Morales-Ramos, J.A.; Rojas, M.G. In vivo production of entomopathogenic nematodes. In Microbial-Based Biopesticides: Methods in Molecular Biology; Glare, T.R., Moran-Diez, M.E., Eds.; Springer Science + Business Media: New York, NY, USA, 2016; Volume 1477, pp. 137–158. ISBN 9781493963676. [Google Scholar]
- McMullen John, G.; Stock, S.P. In vivo and in vitro rearing of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae). J. Vis. Exp. 2014, 91, e52096. [Google Scholar] [CrossRef] [Green Version]
- Strauch, O.; Ehlers, R.U. Influence of the aeration rate on the yields of the biocontrol nematode Heterorhabditis megidis in monoxenic liquid cultures. Appl. Microbiol. Biotechnol. 2000, 54, 9–13. [Google Scholar] [CrossRef]
- Grewal, P.S. Formulation and application technology. In Entomopathogenic Nematology; Gaugler, R., Ed.; CAB International: Oxfordshire, UK, 2002; pp. 265–287. [Google Scholar]
- Cruz-Martínez, H.; Ruiz-Vega, J.; Matadamas-Ortíz, P.T.; Cortés-Martínez, C.I.; Rosas-Diaz, J. Formulation of entomopathogenic nematodes for crop pest control—A review. Plant Prot. Sci. 2017, 53, 15–24. [Google Scholar] [CrossRef] [Green Version]
| Author | Species | Bioassay | Result | Symbiotic Bacteria | Origin/ Country |
|---|---|---|---|---|---|
| [55] | S. corpocapsae S. feltiae H. bacteriophora | Concentrations: 100 IJs each larva and adult RPW: 3rd, 6th, 10th larvae, adult Duration: 12 h duration up to 8 days | Mortality: S. corpocapsae 3rd: 96.5%, 6th: 94.7%, 10th: 88.17%, Adult: 3.07% S. feltiae 3rd: 38.68%, 6th: 36.35%, 10th: 35.35%, Adult: 0% H. bacteriophora 3rd: 85.75%, 6th: 78.15%, 10th: 74.4%, Adult: 0.66% | N/A | Pakistan |
| [34] | S. affine S. carpocapsae S. feltiae H. bacteriophora | RPW: Last instar larvae Concentrations: 500 IJs/ larva Duration: mortality recorded after 7th day | Greatest mortality in H. bacteriophora and least in S. affine | N/A | Turkey |
| [108] | H. bacteriophora S. abbasi S. anomali S. carpocapsae S. feltiae S. glaseri S. riobravae Steinernema sp. S. ritterai (EGBS) S. egyptens S. kushidai Heterorhabditis sp. | Concentration: 2000 IJs/mL RPW: 5 weevils in a box (young, medium, full-grown larvae, pupa with cocoon, and adult) Duration: mortality recorded every 2 days for 10 days | Some EPNs showed a preference for certain life stages of weevils. Steinernema sp. showed the highest mortality, and S. feltiae was the least virulent species | N/A | Egypt |
| [98] | S. carpocapsae | The antimicrobial response of RPW larvae on S. carpocapsae and X. nematophila | Living EPNs and symbionts can suppress the antimicrobial response of the RPW | X. nematophila | Netherlands |
| [105] | S.scapterisci Steinernema sp. S. abbasi S. glaseri H. bacteriophora | RPW: Five late instar larvae and an adult. Concentration: (156–2000 IJs/mL) of EPN injected into hemocoel. Duration: 10–13 days | Adults are more resistant than the larva stage. S.glaseri and H. bacteriophora exhibited high virulence toward the RPW larvae | N/A | Egypt |
| [106] | H. indicus S. riobrave S. abbasi | Concentration: (50, 100, 200, 400, and 800 IJs) Duration: 60 h and 6 days | The local isolate of H. indicus is highly pathogenic towards adult RPWs | N/A | UAE |
| [99] | S. carpocapsae | Immune response of the RPW after infection and post-infection of EPN | The EPN can short-term regulate the phenoloxidase activity for its continuity | N/A | Netherlands |
| [54] | S. carpocapsae H. bacteriophora | RPW: Various stages of the RPW (small, medium and large larvae, pupae and adults) Concentration: 50–6000 IJs/0.4 mL water Duration: Mortality recorded after 72 h | Increase size of the host reduces its susceptibility Small larvae—500 IJs Medium larvae—2000/6000 IJs Large larvae—6000 IJs Pupae/adults—2000 IJs | N/A | Germany |
| [104] | S. pakistanense S. asiaticum S. abbasi S. siamkayai S. feltiae H. indica H. bacteriophora | RPW: Eggs, first, third, sixth, final stages larvae, adult Concentration: 50–1500 IJs/mL Period: Mortality was recorded between 24 to 168 h | H. bacteriophora and S. siamkayai showed the highest mortality of larvae while all EPNs showed similar results in adult RPWs | N/A | Pakistan |
| [109] | H. indica S. carpocapsae | Young and grown larvae, adult RPW were infected with EPNs in the laboratory and date palm field | In the lab, the mortality RPWs is from 70% to 100%. In the field, the mortality of adults and larvae is 46% and 60% | N/A | UAE |
| [110] | S. riobravae S. carpocapsae Heterorhabditi sp. | N/A | All species are virulent to larvae and adult RPWs. LC50 of S. riobravae S. carpocapsae Heterorhabditis sp. were 900, 1100, and 1416 IJs/weevil. | N/A | Egypt |
| [111] | S. abbasi S. carpocapsae All S. carpocapsae S2 S. riobravae S. feltiae S. glaseri S. anomali Heterorhabditis sp. IS12 Heterorhabditis sp. S1 H. bacteriophora | RPW: Larvae, pupae, and adults (lab) 2000 IJs/mL Duration: Mortality was calculated after 7 weeks Field trial: 3000 IJs/mL with 300 mL injected into the infected tree Duration: Mortality was calculated after two weeks of treatment | In the lab, all EPNs were virulent to any RPW stages In the field, 66.67% mortality of larvae was caused by H. bacteriophora | N/A | Egypt |
| [112] | H. bacteriophora | RPW: 2nd, 4th, and 6th instar larval of RPW. Method: Beauveria bassiana and Metarhizium anisopliae combined treatment Larval development was recorded. Duration: Mortality of the larvae were recorded weekly after application | Association of H. bacteriophora and B. bassiana produced better results, especially in early larvae and decelerated larval development | N/A | Pakistan |
| [113] | H. bacteriophora H. megidids H. carpocapsae S. feltiae S. glaseri S. affine S. longicaudum S. apuliae S. kraussel | Concentration: 300 IJs in 0.5 mL water RPW: Late instars and adult RPW Duration: Mortality was recorded every 2 days in 10 days | H. bacteriophora, S. longicaudum, and S. carpocapsae were highly virulent towards larvae and adult RPWs. S. glaseri was only highly virulent towards RPW larvae only. | P. luminescens subsp. laumondii P. luminescens X. nematophila X. bovieni X. ehlersii X. kozodoii | New Zealand Italy USA Germany |
| [114] | S.carpocapsae | An alternate application of EPNs and Imidacloprid on the canary palm as a preventive treatment | Combination applied treatments were able to reduce the population RPWs | N/A | Spain |
| [115] | Steinernema carpocapsae | Product Biorend® was sprayed onto the canary palm. Nine larvae each palm. Period: Inspection after 14 and 28 days post-infection | Restorative and inhibitory of EPNs were at 80% and 98%, respectively | X. nematophila | Spain |
| [68] | Heterorhabditis bacteriophora | Concentration: 300 IJs in 1 mL water RPW: 6th instar larvae and adult Duration: Mortality was recorded until 21 days of exposure in laboratory conditions. Treatment combination: H. bacteriophora with Bacillus thuringiesis Kurstaki (70 µg g−1) and H. bacteriophora with Beauveria bassiana (1 × 107 conidia mL−1) | Mortality percentage of RPW larvae and adults was 92.40% and 81.29%, respectively Mortality percentage of RPW larvae: 93.35–100% (EPN + Bt-k) and 100% (EPN + B. bassiana) Mortality percentage of RPW adult: 81.27–94.24% (EPN + Bt-k) and 100% (EPN + B. bassiana) | N/A | Pakistan |
| [116] | Heterorhabditis bacteriophora-HP-88 | Laboratory condition: Concentration: 250, 500, 1000, 1500, and 2000 IJs/mL RPW: 4th, 8th, 11th instars larvae and adults Duration: Mortality was recorded 24 h till 9 days post-treatment Field condition: Concentration: 2000 IJs/mL Infested tree: Five infested date palm, Phoenix dactylifera injected with IJs. Each tree received approximately 2 L of EPN solution. Duration: Infestation was monitored every week until recovery | Mortality percentage of 4th instar larvae was 100% for all concentrations., while LC50 for 8th, 11th, and adults was 435.16 IJs/mL, 1045.34IJs/mL, and 167.90 IJs/mL, respectively No external sign of recovery for three weeks of observations | N/A | Egypt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurashikin-Khairuddin, W.; Abdul-Hamid, S.N.A.; Mansor, M.S.; Bharudin, I.; Othman, Z.; Jalinas, J. A Review of Entomopathogenic Nematodes as a Biological Control Agent for Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insects 2022, 13, 245. https://doi.org/10.3390/insects13030245
Nurashikin-Khairuddin W, Abdul-Hamid SNA, Mansor MS, Bharudin I, Othman Z, Jalinas J. A Review of Entomopathogenic Nematodes as a Biological Control Agent for Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insects. 2022; 13(3):245. https://doi.org/10.3390/insects13030245
Chicago/Turabian StyleNurashikin-Khairuddin, Wan, Siti Noor Aishikin Abdul-Hamid, Mohammad Saiful Mansor, Izwan Bharudin, Zulkefley Othman, and Johari Jalinas. 2022. "A Review of Entomopathogenic Nematodes as a Biological Control Agent for Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae)" Insects 13, no. 3: 245. https://doi.org/10.3390/insects13030245
APA StyleNurashikin-Khairuddin, W., Abdul-Hamid, S. N. A., Mansor, M. S., Bharudin, I., Othman, Z., & Jalinas, J. (2022). A Review of Entomopathogenic Nematodes as a Biological Control Agent for Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Insects, 13(3), 245. https://doi.org/10.3390/insects13030245







