Identification and Functional Analysis of Glutathione S-Transferases from Sitophilus zeamais in Olfactory Organ
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects Reared, RNA Extraction and cDNA Synthesis
2.2. SzeaGST Genes Identification and Sequences Analysis
2.3. Bioinformatics Analysis of SzeaGSTd1
2.4. qRT-PCR
2.5. Expression and Purification of Recombinant SzeaGSTd1 Protein
2.6. Enzyme Activity Assay
2.7. Metabolism Assays In Vitro
3. Results
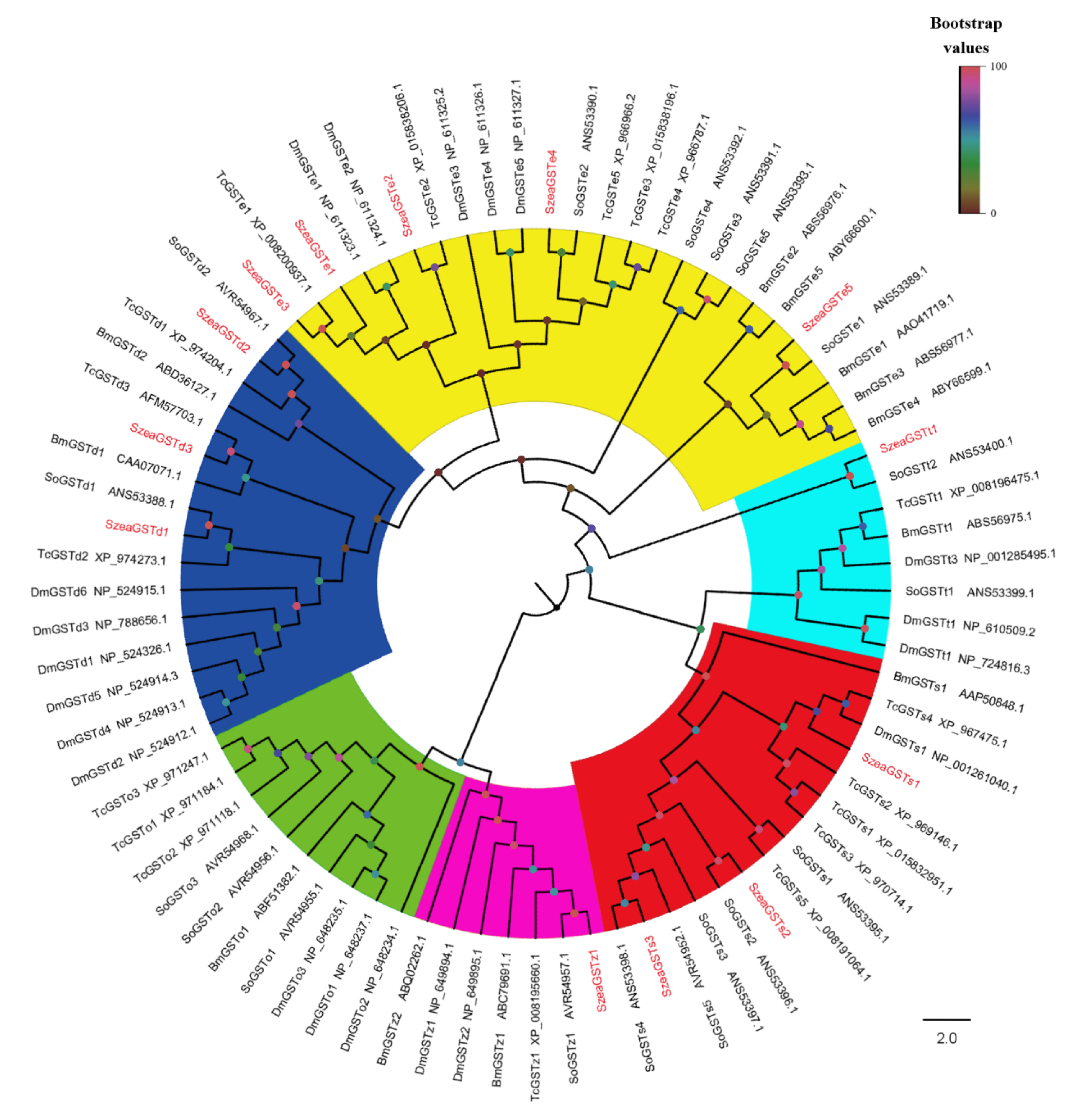
3.1. Identification and Analysis of SzeaGSTs
3.2. The Spatial Expression of SzeaGSTs
3.3. Biochemical Characterization of Recombinant SzeaGSTd1
3.4. Substrate Identification and In Vitro Metabolism with Recombinant SzeaGSTd1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, J.M. Weight loss caused by development of Sitophilus zeamais Motsch. in maize. J. Stored Prod. Res. 1976, 12, 269–272. [Google Scholar] [CrossRef]
- Abass, A.B.; Ndunguru, G.; Mamiro, P.; Alenkhe, B.; Mlingi, N.; Bekunda, M. Post-harvest food losses in a maize-based farming system of semi-arid savannah area of Tanzania. J. Stored Prod. Res. 2014, 57, 49–57. [Google Scholar] [CrossRef]
- Keba, T.; Sori, W. Differential resistance of maize varieties to maize weevil (Sitophilus zeamais Motschulsky) (Coleoptera: Curculionidae) under laboratory conditions. J. Entomol. 2013, 10, 1–12. [Google Scholar] [CrossRef]
- Pimentel, M.A.G.; Faroni, L.R.D.A.; Guedes, R.N.C.; Sousa, A.H.; Tótola, M.R. Phosphine resistance in Brazilian populations of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Stored Prod. Res. 2009, 45, 71–74. [Google Scholar] [CrossRef]
- Khalid, H.; Mendonça, L.P.; Dos Santos, M.F.; Guedes, R.N.C.; Oliveira, E.E. Metabolic and behavioral mechanisms of indoxacarb resistance in Sitophilus zeamais (Coleoptera: Curculionidae). J. Econ. Entomol. 2015, 108, 362–369. [Google Scholar]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in insect olfaction: To smell or not to smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef]
- Steinbrecht, R.A. Odorant-binding proteins: Expression and function. Ann. N. Y. Acad. Sci. 1998, 30, 323–332. [Google Scholar] [CrossRef]
- Vogt, R.G.; Prestwich, G.D.; Lerner, M.R. Odorant-binding-protein subfamilies associate with distinct classes of olfactory receptor neurons in insects. Dev. Neurobiol. 2010, 22, 74–84. [Google Scholar] [CrossRef]
- Suh, E.; Bohbot, J.; Zwiebel, L.J. Peripheral olfactory signaling in insects. Curr. Opin. Insect Sci. 2014, 6, 86–92. [Google Scholar] [CrossRef]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Robertson, H.M.; Warr, C.G.; Carlson, J.R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2003, 100, 14537–14542. [Google Scholar] [CrossRef]
- Mori, K.; Nagao, H.; Yoshihara, Y. The olfactory bulb: Coding and processing of odor molecule information. Science 1999, 286, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Durand, N.; Carot-Sans, G.; Bozzolan, F.; Rosell, G.; Siaussat, D.; Debernard, S.; Chertemps, T.; Maibeche-Coisne, M. Degradation of pheromone and plant volatile components by a same odorant-degradingenzyme in the cotton leafworm, Spodoptera littoralis. PLoS ONE 2011, 6, e29147. [Google Scholar] [CrossRef]
- Ishida, Y.; Leal, W.S. Cloning of putative odorant-degrading enzyme and integumental esterase cDNAs from the wild silkmoth, Antheraea polyphemus. Insect Biochem. Mol. Biol. 2002, 32, 1775–1780. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Su, X.; Fang, J. Identification of biotransformation enzymes in the antennae of codling moth Cydia pomonella. Gene 2016, 580, 73–79. [Google Scholar] [CrossRef]
- Pelletier, J.; Bozzolan, F.; Solvar, M.; Francois, M.C.; Jacquin-Joly, E.; Maibeche-Coisne, M. Identification of candidate aldehyde oxidases from the silkworm Bombyx mori potentially involved in antennal pheromone degradation. Gene 2007, 404, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef]
- Jakobsson, P.J.; Morgenstern, R.; Mancini, J.; Ford-Hutchinson, A.; Persson, B. Common structural features of MAPEG—A widespread superfamily of membrane associated proteins with highly divergent functions in eicosanoid and glutathione metabolism. Prot. Sci. 1999, 8, 689–692. [Google Scholar] [CrossRef]
- Sheehan, D.; Meade, G.; Foley, V.M.; Dowd, C.A. Structure, function and evolution of glutathione transferases: Implications for classification of nonmammalian members of an ancient enzyme superfamily. J. Biochem. 2001, 360, 1–16. [Google Scholar] [CrossRef]
- Shi, H.; Pei, L.; Gu, S.; Zhu, S.; Wang, Y.; Zhang, Y.; Li, B. Glutathione S-transferase (GST) genes in the red flour beetle, Tribolium castaneum, and comparative analysis with five additional insects. Genomics 2012, 100, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.E.; Jani, M.K.; Vogt, R.G. An olfactory-specific glutathione S-transferase in the sphinx moth Manduca sexta. J. Exp. Biol. 1999, 202, 1625–1637. [Google Scholar] [CrossRef]
- Ono, H.; Ozaki, K.; Yoshikawa, H. Identification of cytochrome P450 and glutathione-S-transferase genes preferentially expressed in chemosensory organs of the swallowtail butterfly, Papilio xuthus L. Insect Biochem. Mol. Biol. 2005, 35, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gong, Z.J.; Rao, X.J.; Li, M.Y.; Li, S.G. Identification of putative carboxylesterase and glutathione S-transferase genes from the antennae of the Chilo suppressalis (Lepidoptera: Pyralidae). J. Insect Sci. 2015, 15, 103. [Google Scholar] [CrossRef]
- Tan, X.; Hu, X.M.; Zhong, X.W.; Chen, Q.M.; Xia, Q.Y.; Zhao, P. Antenna-specific glutathione S-transferase in male silkmoth Bombyx mori. Int. J. Mol. Sci. 2014, 15, 7429–7443. [Google Scholar] [CrossRef]
- Huang, X.L.; Fan, D.S.; Liu, L.; Feng, J.N. Identification and characterization of glutathione S-transferase genes in the antennae of codling moth (Lepidoptera: Tortricidae). Ann. Entomol. Soc. Am. 2017, 110, 409–416. [Google Scholar] [CrossRef]
- Dippel, S.; Kollmann, M.; Oberhofer, G.; Montino, A.; Knoll, C.; Krala, M.; Rexer, K.H.; Frank, S.; Kumpf, R.; Schachtner, J.; et al. Morphological and transcriptomic analysis of a beetle chemosensory system reveals a gnathal olfactory center. BMC Biol. 2016, 14, 90. [Google Scholar] [CrossRef]
- Tang, Q.F.; Shen, C.; Zhang, Y.; Yang, Z.P.; Han, R.R.; Wang, J. Antennal transcriptome analysis of the maize weevil Sitophilus zeamais: Identification and tissue expression profiling of candidate odorant-binding protein genes. Arch. Insect Biochem. Physiol. 2019, 101, e25142. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Anderson, I.; Brass, A. Searching DNA databases for similarities to DNA sequences: When is a match significant? Bioinformatics 1998, 14, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione Stransferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Zanan, R.; Khandagale, K.; Hinge, V.; Elangovan, M.; Henry, R.J.; Nadaf, A. Characterization of fragrance in sorghum (Sorghum bicolor (L.) Moench) grain and development of a gene-based marker for selection in breeding. Mol. Breed. 2016, 36, 146. [Google Scholar] [CrossRef]
- Chughtai, M.; Pasha, I.; Anjum, F.M.; Nasir, M.A. Characterization of sorghum and millet with special reference to fatty acid and volatile profile. Turk. J. Agric. Food Sci. Technol. 2015, 3, 515–521. [Google Scholar] [CrossRef][Green Version]
- Sansenya, S.; Hua, Y.; Chumanee, S. The correlation between 2-Acetyl-1-pyrroline content, biological compounds and molecular characterization to the aroma intensities of Thai local rice. J. Oleo Sci. 2018, 67, 893–904. [Google Scholar] [CrossRef]
- Maga, J.A. Cereal volatiles, a review. J. Agric. Food Chem. 1978, 26, 175–178. [Google Scholar] [CrossRef]
- Phillips, T.W.; Jiang, X.L.; Burkholder, W.E.; Phillips, J.K.; Tran, H.Q. Behavioral responses to food volatiles by two species of stored-product coleoptera, Sitophilus oryzae (curculionidae) and Tribolium castaneum (tenebrionidae). J. Chem. Ecol. 1993, 19, 723–734. [Google Scholar] [CrossRef]
- Concepcion, J.C. Quality evaluation, fatty acid analysis and untargeted profiling of volatiles in Cambodian rice. Food Chem. 2018, 240, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, G.G.; Antonio, D.C.; Giuseppe, R. Electrophysiological and behavioral responses of Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae) to Cereal Grain Volatiles. Biomed Res. Int. 2016, 2016, 5460819. [Google Scholar]
- Cho, S.; Kays, S.J. Aroma-active compounds of wild rice (Zizania palustris L.). Food Res. Int. 2013, 54, 1463–1470. [Google Scholar] [CrossRef]
- Toshova, T.B.; Velchev, D.I.; Subchev, M.A.; Toth, M.; Vuts, J.; Pickett, J.A.; Dewhirst, S.Y. Electrophysiological responses and field attraction of the grey corn weevil, Tanymecus (Episomecus) dilaticollis Gyllenhal (Coleoptera: Curculionidae) to synthetic plant volatiles. Chemoecology 2010, 20, 199–206. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Holighaus, G.; Weissbecker, B.; Schutz, S. Electroantennographic responses of red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) to volatile organic compounds. J. Appl. Entomol. 2016, 141, 477–486. [Google Scholar] [CrossRef]
- Wang, S.; Adhikari, K.; Hung, Y.C. Acceptability and preference drivers of freshly roasted peanuts. J. Food Sci. 2016, 82, 174–184. [Google Scholar] [CrossRef]
- Wongsantichon, J.; Robinson, R.; Ketterman, A.J. Structural evidence for conformational changes of Delta class glutathione transferases after ligand binding. Arch. Biochem. Biophys. 2012, 521, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.; Fraichard, S.; Grassein, P.; Delarue, P.; Senet, P.; Nicolaï, A.; Chavanne, E.; Mucher, E.; Artur, Y.; Ferveur, J.F.; et al. Characterization of a Drosophila glutathione transferase involved in isothiocyanate detoxification. Insect Biochem. Mol. 2018, 95, 33–43. [Google Scholar] [CrossRef]
- Xu, Z.B.; Zou, X.P.; Zhang, N.; Feng, Q.L.; Zheng, S.C. Detoxification of insecticides, allechemicals and heavy metals by glutathione S-transferase SlGSTE1 in the gut of Spodoptera litura. Insect Sci. 2015, 22, 503–511. [Google Scholar] [CrossRef]
- Dai, L.L.; Ma, J.N.; Ma, M.Y.; Zhang, H.Q.; Shi, Q.; Zhang, R.R.; Chen, H. Characterisation of GST genes from the Chinese white pine beetle Dendroctonus armandi (Curculionidae: Scolytinae) and their response to host chemical defence. Pest Manag. Sci. 2016, 72, 816–827. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Xu, Y.Y.; Cheng, J.H.; Lin, X.L.; Jing, X.F.; Tian, H.G.; Liu, T.X. Identification of candidate odorant-degrading enzyme genes in the antennal transcriptome of Aphidius gifuensis. Entomol. Res. 2021, 51, 36–54. [Google Scholar] [CrossRef]
- Li, G.W.; Chen, X.L.; Xu, X.L.; Wu, J.X. Degradation of sex pheromone and plant volatile components by an antennal glutathione S-transferase in the oriental fruit moth, Grapholita molesta Busck (Lepidoptera: Tortricidae). Arch. Insect Biochem. Physiol. 2018, 99, e21512. [Google Scholar] [CrossRef] [PubMed]
- Monsoor, M.A.; Proctor, A. Volatile component analysis of commercially milled head and broken rice. J. Food Sci. 2004, 69, 632–636. [Google Scholar] [CrossRef]
- Bryant, R.J.; Mcclung, A.M. Volatile profiles of aromatic and non-aromatic rice cultivars using SPME/GC-MS. Food Chem. 2011, 124, 501–513. [Google Scholar] [CrossRef]




| Gene Name | Length (bp) | GenBank Acc. No. | Mw (kDa) | pI | BLASTX Best Hit | |||
|---|---|---|---|---|---|---|---|---|
| Species | Acc. No. | E-Value | Identity (%) | |||||
| SzeaGSTd1 | 744 | MW390709 | 25.55 | 6.65 | Sitophilus oryzae | XP_030752074.1 | 1 × 10−151 | 98.23 |
| SzeaGSTd2 | 666 | MW390710 | 24.04 | 4.95 | Sitophilus oryzae | XP_030764913.1 | 3 × 10−159 | 98.64 |
| SzeaGSTd3 | 651 | MW390711 | 24.38 | 5.30 | Sitophilus oryzae | XP_030767740.1 | 1 × 10−146 | 99.17 |
| SzeaGSTe1 | 645 | MW390712 | 22.56 | 5.44 | Sitophilus oryzae | XP_030753917.1 | 6 × 10−153 | 98.13 |
| SzeaGSTe2 | 657 | MW390713 | 23.97 | 7.89 | Sitophilus oryzae | XP_030753906.1 | 1 × 10−157 | 99.08 |
| SzeaGSTe3 | 678 | MW390714 | 25.31 | 4.75 | Lissorhoptrus oryzophilus | AVT42199.1 | 2 × 10−138 | 83.56 |
| SzeaGSTe4 | 654 | MW390715 | 24.50 | 5.70 | Sitophilus oryzae | XP_030766193.1 | 2 × 10−153 | 96.77 |
| SzeaGSTe5 | 639 | MW390716 | 24.23 | 5.41 | Sitophilus oryzae | XP_030753847.1 | 2 × 10−149 | 96.70 |
| SzeaGSTs1 | 606 | MW390717 | 21.88 | 5.96 | Sitophilus oryzae | XP_030749148.1 | 3 × 10−145 | 99.00 |
| SzeaGSTs2 | 612 | MW390718 | 13.40 | 8.98 | Sitophilus oryzae | XP_030758386.1 | 2 × 10−142 | 98.03 |
| SzeaGSTs3 | 609 | MW390719 | 23.26 | 7.70 | Sitophilus oryzae | AVR54952.1 | 2 × 10−125 | 85.64 |
| SzeaGSTz1 | 654 | MW390720 | 18.39 | 6.37 | Sitophilus oryzae | AVR54957.1 | 2 × 10−158 | 99.08 |
| SzeaGSTt1 | 720 | MW390721 | 27.80 | 5.41 | Sitophilus oryzae | XP_030760484.1 | 2 × 10−176 | 98.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, D.; Zheng, R.; Huang, J.; Lu, S.; Tang, Q. Identification and Functional Analysis of Glutathione S-Transferases from Sitophilus zeamais in Olfactory Organ. Insects 2022, 13, 259. https://doi.org/10.3390/insects13030259
Xia D, Zheng R, Huang J, Lu S, Tang Q. Identification and Functional Analysis of Glutathione S-Transferases from Sitophilus zeamais in Olfactory Organ. Insects. 2022; 13(3):259. https://doi.org/10.3390/insects13030259
Chicago/Turabian StyleXia, Daosong, Renwen Zheng, Jianhua Huang, Sihan Lu, and Qingfeng Tang. 2022. "Identification and Functional Analysis of Glutathione S-Transferases from Sitophilus zeamais in Olfactory Organ" Insects 13, no. 3: 259. https://doi.org/10.3390/insects13030259
APA StyleXia, D., Zheng, R., Huang, J., Lu, S., & Tang, Q. (2022). Identification and Functional Analysis of Glutathione S-Transferases from Sitophilus zeamais in Olfactory Organ. Insects, 13(3), 259. https://doi.org/10.3390/insects13030259






