Simple Summary
Hoverflies and bees play a key role in plant pollination. The increasing concern about pollinator reduction forces the planning of a sampling monitoring scheme to evaluate the change in the populations of these important insects. The present research provides baseline data about the distribution of hoverflies and bees in the Dolomiti Bellunesi National Park (Northeastern Italy). The hoverfly community shows a unimodal distribution with peak at middle elevation, while bees display a linear reduction in richness and abundance with increasing altitude. Both hoverfly and bee β-diversity at high altitude is dominated by species turnover more than by nestedness.
Abstract
Hoverflies (Diptera: Syrphidae) and bees (Hymenoptera: Anthophila) are two key taxa for plant pollination. In the present research, the altitudinal distribution of these taxa was studied along two gradients (elevation range: 780–2130 m) in the Dolomiti Bellunesi National Park (Northeastern Italy). Pan traps were used as a sampling device to collect both hoverflies and bees. Other than altitude, the effect of landscape complexity and plant diversity were considered as potential predictors of hoverfly and bee richness and abundance along the two gradients. A total of 68 species of hoverflies and 67 of bees were collected during one sampling year, confirming the efficacy of pan traps as a sampling device to study these taxa. Altitude was the main variable affecting both hoverfly and bee distribution. The two taxa show different distribution patterns: hoverflies have a unimodal distribution (richness and abundance) with peak at middle altitude (1500 m), while bees have a monotonic decline (richness and abundance) with increasing altitude. Both hoverfly and bee populations change with the increasing altitude, but the change in hoverflies is more pronounced than in bees. Species turnover dominates the β-diversity both for hoverflies and bees; therefore, the hoverfly and bee communities at higher altitudes are not subsamples of species at lower altitude but are characterized by different species. This poses important conservation consequences. Some rare species, typical of an alpine habitat were recorded; the present research represents important baseline data to plan a monitoring scheme aimed at evaluating the effect of climate change on pollinators in these fragile habitats.
Keywords:
Syrphidae; Anthophila; hoverflies; bees; pan trap; altitudinal distribution; Italian national parks 1. Introduction
Since the mid-1990s the scientific community has become aware of the increasing decline in pollinator biodiversity, commonly referred to as the “pollination crisis” [1,2,3]. Biodiversity loss of bee species, which represent one of the most investigated taxa, have been well-documented in several countries [4,5,6,7,8,9]. Even if less investigated, a similar trend has also been recorded for other pollinator taxa, such as Rhopalocera (Lepidoptera) [10] and Syrphidae (Diptera) [11,12]. The pollination crisis has deeply concerned the whole scientific community not only regarding conservation issues but also because of the great role that the ecosystem service of pollination plays in the world economy: IPBES [13] estimates that 5–8% of world crop production in 2015 is pollinated by animals, which corresponds to an annual value of USD 235–577 billion.
There are several causes that can concur to the “pollination crisis”. An increasing amount of data demonstrates the detrimental impact of pesticides on pollinators [14,15,16,17], which led to restrictions of neonicotinoids and fipronil in the European Union [18,19]. Loss of habitat and fragmentation are additional causes of bee decline due to a reduction in floral and nesting resources, together with a reduction in tolerance thresholds against disease and pesticides (e.g., [15,20,21]). Climate change is considered another major cause of pollinator decline [20,22]. The effect of climate change may be particularly strong in plant–pollinator interactions, where some degree of synchronization is necessary to ensure pollination. Available data on this topic are sometimes conflicting, and to date, it is not fully understood whether climate change can generate negative consequences due to this desynchronization, leading to a reduction in pollination [23,24,25].
Alpine habitats are considered as particularly exposed to climate change [26,27,28]. According to Engler et al. [29], 36–55% of alpine species and 31–51% of subalpine species could lose more than 80% of suitable habitat by 2070 to 2100. The shift towards higher altitudes is one of the main responses of plants and animals to increasing temperatures, but this adaptation may be impossible for alpine species living at the peak of mountains, with the consequence of a greater fragmentation of populations, leading to an increasing risk of extinction [30,31,32].
Moving towards higher altitudes results in changes in the pollinator community. According to Bergmann’s rule, insects should be bigger at higher altitudes because a larger size reduces the ratio between body surface and volume, allowing for a more effective control of heat loss [33]. However, the distribution of taxa along altitudinal gradient did not always confirm this rule [34,35,36,37]. At higher altitudes, the presence of Hymenoptera usually decreases, with a parallel increase in Diptera [38,39,40,41,42]. In particular, moving towards higher elevation, two main consequences, which are often concomitant, can be evinced: a change in physical conditions, including temperature, oxygen partial pressure, and insolation, and changes at landscape levels, such as a reduction in wood coverage [43,44].
In order to better understand how different focal taxa will respond to future climate change in Alpine habitats, it is of fundamental importance to have biodiversity data banks and recordings in order to distinguish the effects of physical conditions from that of landscape shifts. In addition to evaluating the trends of pollinators in the medium and long term, it is necessary to plan monitoring schemes repeated over time. This knowledge is particularly crucial to plan conservation interventions and to understand the effects of climate change on biodiversity, including pollinator species. The availability of data belonging to different geographical areas can allow comparative analyses, thus achieving an understanding of these complex phenomena.
In 2005, the Gran Paradiso National Park developed a biodiversity monitoring project in the Alpine environment that also involved some regional parks of the Western Alps. Since 2013, the initiative has been extended to the other National Parks of the Italian Alps (Val Grande, Stelvio and Dolomiti Bellunesi). The project, called the Biodiversity Project and still active, included a standardized monitoring of different taxa (Aves, Orthoptera, Coleoptera Carabidae and Staphylinidae, Lepidoptera Rhopalocera, Hymenoptera Formicidae and Araneae), along altitudinal transects. In 2020, the Dolomiti Bellunesi National Park also extended the research to Anthophila (Hymenoptera) and Syrphidae (Diptera). The role of bees as pollinators has been largely recognized because they are one of the main taxa in plant pollination [45,46,47]. Although hoverflies receive less attention than bees as pollinator, recently, increasing data have emphasized the importance of these flies in pollination, in some cases being more important than bees (e.g., at higher altitudes and mountain habitats) [48,49,50,51].
The objectives of the present study are the following:
- To evaluate the altitudinal distribution of two important pollinator taxa, i.e., Hymenoptera Anthophila (bees) and Diptera Syrphidae (hoverflies), also including the phenological patterns;
- To compare the effects of landscape and altitude as main factors affecting species distribution.
The present paper is focused on the results of bee and hoverfly sampling, including the influence of environmental and climate factors on diversity of these taxa, paying particular attention to the trends of abundance and species richness monitored at different altitudes.
2. Materials and Methods
2.1. Study Site
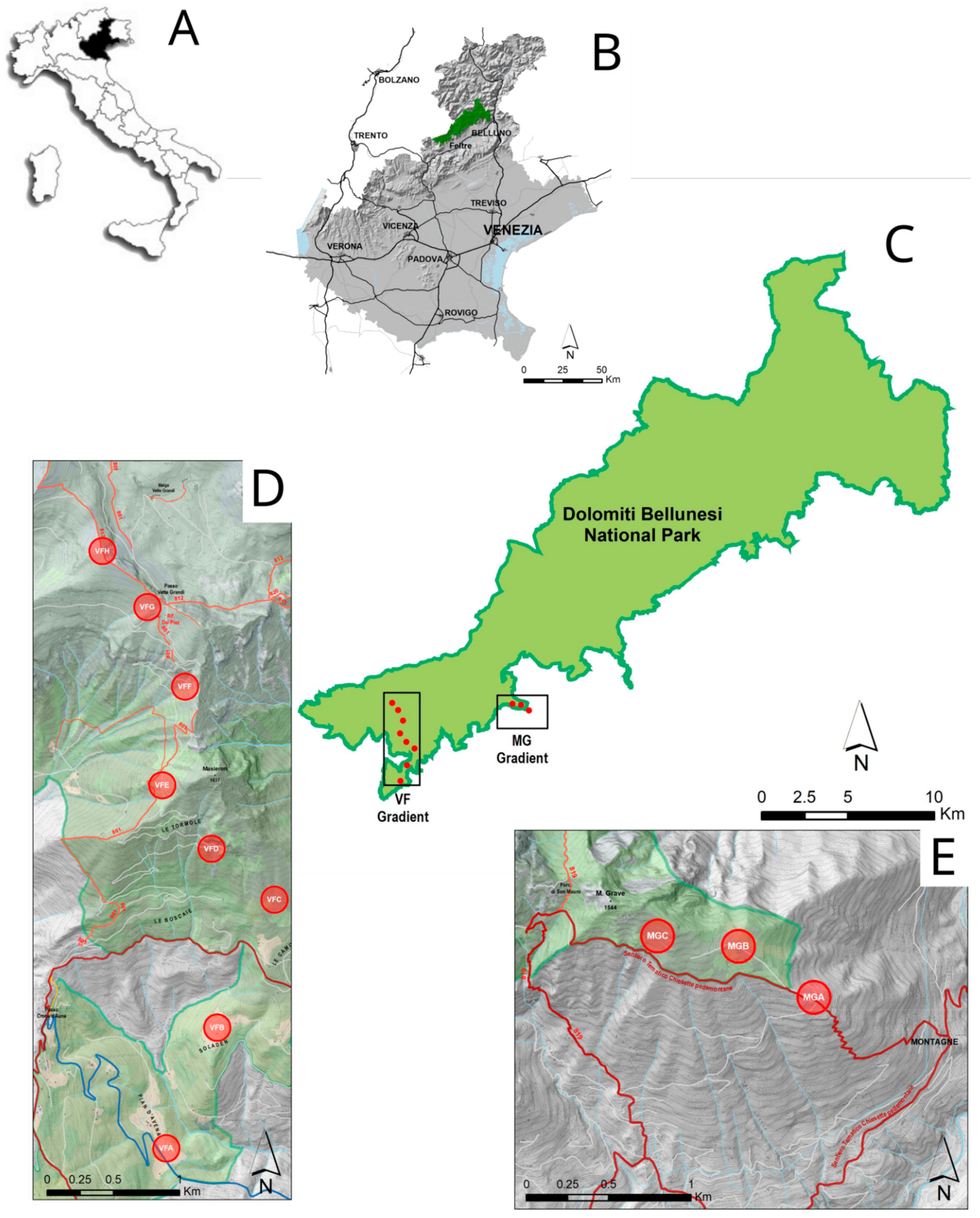
The present research was conducted in 2020 along two altitudinal gradients in the oriental part of Dolomiti Bellunesi National Park in the Veneto Region (Figure 1). The two gradients have been previously selected for the Biodiversity Project monitoring. The altitudinal gradients are as follows:
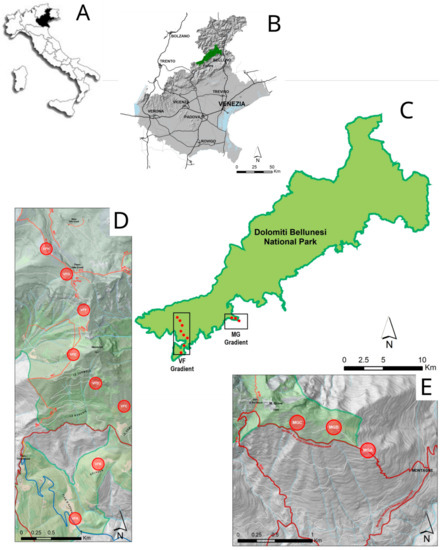
Figure 1.
Maps of the research area: (A): Position of Veneto Region inside Italy; (B): Position of Dolomiti Bellunesi National Park in the Veneto Region; (C): Locations of the two altitudinal gradients in the Dolomiti Bellunesi National Park; (D,E) position of plot along the two altitudinal gradients (respectively, Vette Feltrine—VF—and Mount Grave—MG.
- Vette Feltrine (VF): This gradient starts at 780 until 2130 m a.s.l.; 8 circular plots (100 m radius) were selected at around 200 m altitude difference (Table 1).
 Table 1. Physical and environmental characteristics of the 11 sampling plots along the 2 altitudinal gradients: VF (Vette Feltrine) and MG (Mount Grave). For each sampling point, the geographical position, altitude, slope, and percentage of main habitat are reported.
Table 1. Physical and environmental characteristics of the 11 sampling plots along the 2 altitudinal gradients: VF (Vette Feltrine) and MG (Mount Grave). For each sampling point, the geographical position, altitude, slope, and percentage of main habitat are reported. - Mount Grave (MG): This gradient is shorter than previous one, starting at 1080 m and ending at 1375 m; 3 circular plots were selected at altitude differences of around 200 and 100 m (Table 1).
For each transect, the following predicting variables were measured: altitude (m), temperature (°C), slope (°), landscape complexity, and vegetation diversity. Altitude was measured by GPS and confirmed with the Regional Technical Map. Temperature was recorded using a remote sensor of temperature (Thermochrom i-Button), placed at the centre of each plot. The sensor was placed at a high of 1 m inside a plastic white tube (20 cm long and 10 cm diameter) to protect it from direct solar radiation. The tube had holes to ensure the free circulation of air around the sensor. The temperature was recorded hourly throughout the day. The mean minimum temperature was calculated from the beginning of June to the end of September for each sampling point. During the sampling year (2020), some remote sensors had malfunctions, and for this reason, we have decided to use the average values of the temperatures detected by the sensors in the previous two years. Clinometer (Suunto PM5–1520 PC) was used to measures slope at the centre of each plot. Landscape complexity was evaluated using GIS tools; the map used was the CTR–Regional Technical Map, Veneto Region (https://www.regione.veneto.it/web/ambiente-e-territorio/carta-tecnica-regionale, accessed on 31 January 2022). Each plot consisted of a circular area with a radius of 100 m. The type and extension of vegetation was evaluated in the circular area using ArcMap 9.3 Esri. The percentage of the following habitats were calculated: percentage of woods, percentage of shrubs, percentage of grassland, percentage of scree. The percentage of peat bog was very small (only 7% in only one plot) and not included in the following analysis. The plant species and their coverage were evaluated in 2013 in three points (inside each plot) following the minimum area approach [52]. For the present research, the number of species detected inside the plot (total plant species) and the number of plant species pollinated by insects has been used as plant diversity estimator. Even if the plant diversity was monitored in 2013, the plots along the two gradients were visited each year within the Biodiversity Project, and no consistent change in vegetation was observed between 2013 and 2020.
2.2. Sampling Design
Both hoverflies and bees were sampled by coloured pantraps: white, blue, and yellow, as suggested in current literature [53,54,55]. Pan traps and active netting along transects are considered the most suitable methods to sample bees for their efficiency, repeatability, and standardization [53,56,57,58]. We decided to use pan traps instead of entomological nets in order to avoid a potential sampling bias due to the collector; furthermore, given the high climate variability over time along the gradient, we preferred a sampling method that would reduce the field time. Finally, the steep slope in some sampling points along the gradient would have made sampling by net very laborious and time consuming.
In each plot, 5 sampling points were selected; in each sampling point, a stake was placed, each one supporting 3 white bowls (diameter: 30 cm; height: 5 cm), at about 60 cm from the ground, at similar level than most flowers (Figure 2). The inside of bowls was painted blue, white, or yellow UV-reflecting paints (Sparvar Leuchtfarbe, Spray-Color GmbH, Merzenich; item numbers 3107, 3108, 3104, respectively) [53]. The five sampling points were set at a distance of 25 m along the diameter of the plot. The distance between sampling points was chosen in order to ensure the independence of each group of bowls [59].

Figure 2.
Two sampling points along the Vette Feltrine Gradient ((A): VFA, Pian d’Avena at 780 m; (B): VFH, Vette Grandi at 2130 m); the stick with the pan traps active (C,D).
Pan traps were activated each month from May to September 2020 for a total of five sampling dates; however, due to adverse climate conditions at high altitude, the plots in VF gradient from 1200 to 2100 m were sampled only from June to August. Instead, MG gradient was regularly sampled from May to August. Pan traps contained water and a few drops of surfactant. In each sampling, the traps remained in the field for 2 days in good weather conditions, avoiding sampling on rainy days. The insects collected from the five plots within each transect were pooled together. Bees and hoverflies were identified at species level using the following key:
- -
- Syrphidae: [60,61,62];
- -
- Anthophila: [63] for genera Bombus and Psithyrus; [64] and references included for other genera.
For the identification of five specimens of Bombus (Apidae), for which morphological characters were not informative, molecular identification was carried out, using cytochrome oxidase gene (COI) [65,66]. The details are reported in Table S1.
2.3. Statistical Analysis
The collinearity between predicting variables was evaluated by Spearman linear correlation (p < 0.05). In accordance with this preliminary test, the following pairs of variables were correlated (Table S2):
- Temperature was correlated with altitude;
- Percentage of grassland was inversely correlated with percentage of woods;
- Percentage of scree was correlated with altitude;
- Percentage of scrubs was correlated with percentage of grassland;
- Slope was correlated with plant species pollinated by insects;
- Total plant species was correlated with plant species pollinated by insects.
Temperature and percentages of scree, scrubs, and plant species with entomophilic pollination were excluded. Only the following predicting variables were selected: altitude, slope, type of gradient (VF or MG), total plant species, percentage of wood, or percentage of grassland. The last two variables were alternatively used in multivariate analysis, selecting those with a better predictive power.
In order to detect potential effects of the selected predicting variables on hoverfly and bee community, an ordination analysis was used. A distance-based Redundancy Analysis (db-RDA) was applied to hoverfly and bee matrices of abundance after Hellinger transformation [67,68]. To test the significance of canonical axes, an ANOVA permutation test was applied using the function anova.rda in the vegan package in R. The function was applied to both the full model and, using the setting by = “terms”, to each independent variable [69].
A db-RDA was also applied to ecological categories of hoverflies and bees. For hoverflies, the following categories were used: larval trophic habitus (three categories: zoophagous, saprophagous, phytophagous); larval microhabitat association (five categories: tree foliage, root, herb layer, trunk trees); dimension (three categories: small (6–9 cm), medium (9.5–12 cm), large (>12 cm)). The information about the ecological category of each species was obtained by [12]. For bees, the following categories were selected: diet breadth (three categories: oligolecty, polilecty, social parasite), nest position (three categories: soil, vegetation, holes), dimension (three categories: small (5–10 cm), medium (10.5–15.5 cm), large (>15.5 cm)). For each transect, the total number of species belonging to these categories was pooled.
To investigate the influence of elevation on bee and hoverfly richness and abundance, linear and polynomial quadratic correlations were performed, using elevation as a predictor variable. We applied both models to the three sampling months (June, July, and August) and on the whole period (total). We selected the model with the higher R2. A Durbin–Watson test was performed to evaluate the independence of residuals.
To evaluate the relationship between bee and hoverfly community changes against elevation, two approaches were used. A Mantel test was applied to evaluate whether the diversity in hoverfly and bee community changes according to increasing altitudinal distance [70]. We analysed the association between distance matrices of transect altitudes and Jaccard dissimilarity for hoverfly and bee communities with 10,000 permutations. The test of ANCOVA (Analysis of Covariance) was applied to the two correlations (hoverfly dissimilarities vs. altitudinal distances and bee dissimilarities vs. altitudinal distances) in order to evaluate the presence of differences in slopes and/or intercept. Shapiro and Levene tests were previously performed to evaluate normality and homogeneity of variance, respectively. Finally, the regression lines were compared with the aim to understand the interaction between the two taxa in relation to the altitude variable [71].
Beta-diversity partitioning: The object of this analysis was to separate species turnover (or species replacement) and nestedness (or difference in richness) [72,73]. Therefore, this analysis was used to understand if bee and hoverfly communities at high altitudes can be considered as a subpopulation of low-altitude populations (nestedness dominance) or a population dominated by different species (species turnover dominance). Βeta-diversity partitioning was applied on incidence (presence/absence) matrices of bees and hoverflies [72,74]. The turnover of species (βsim) was calculated using Simpson dissimilarity and nestedness (βnes) using Sørensen dissimilarity index; the sum of these two components is equal to total beta diversity (βsør): βsør = βnes + βsim.
All statistical analyses were employed in R (version R 4.1.0), using the following packages: Hmisc (correlation matrix) [75]; vegan (db-RDA, Mantel test) [69]; betapart (β-diversity partitioning) [76]; all graphs were developed using ggplot2 and ggpubr [77].
3. Results
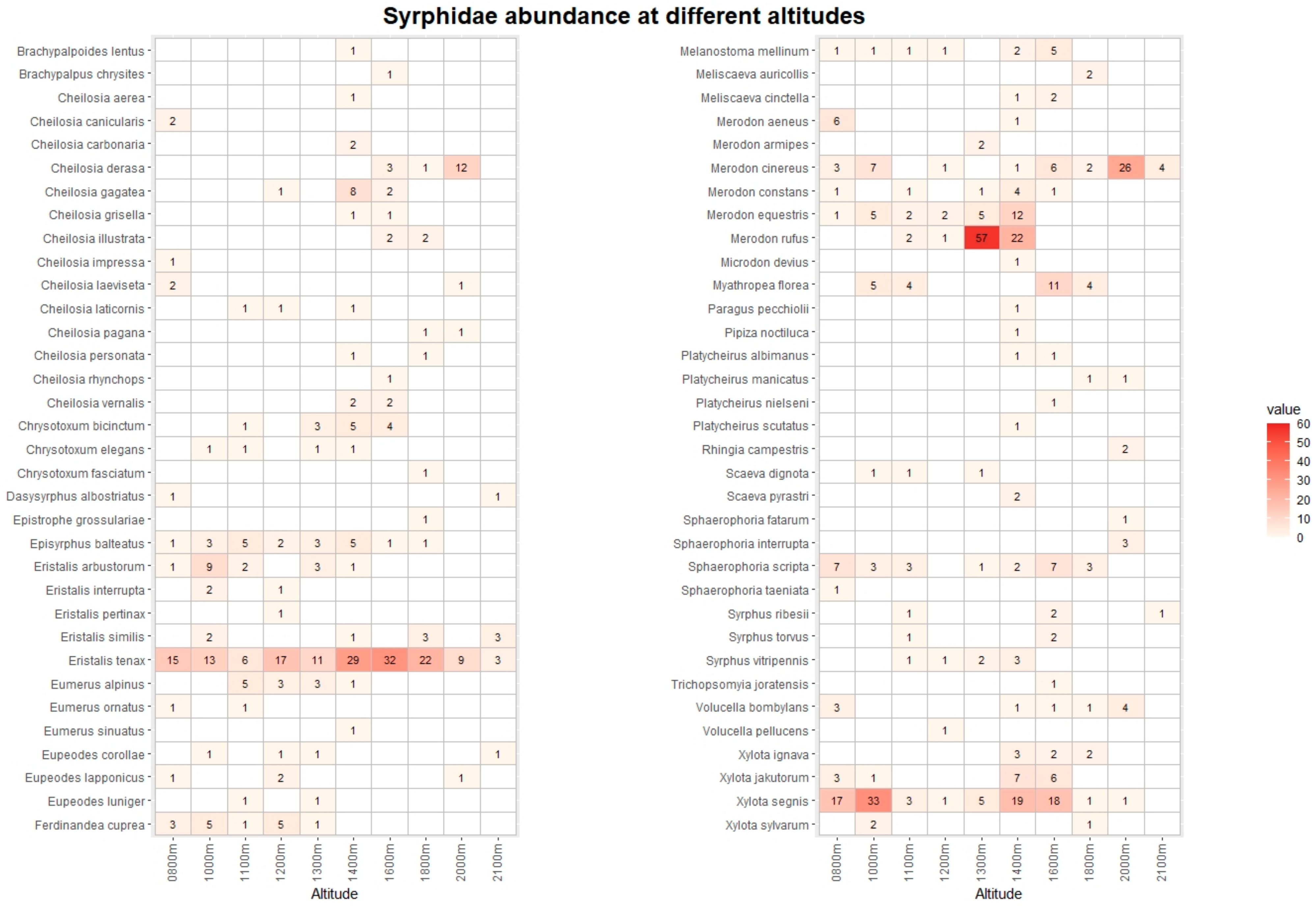
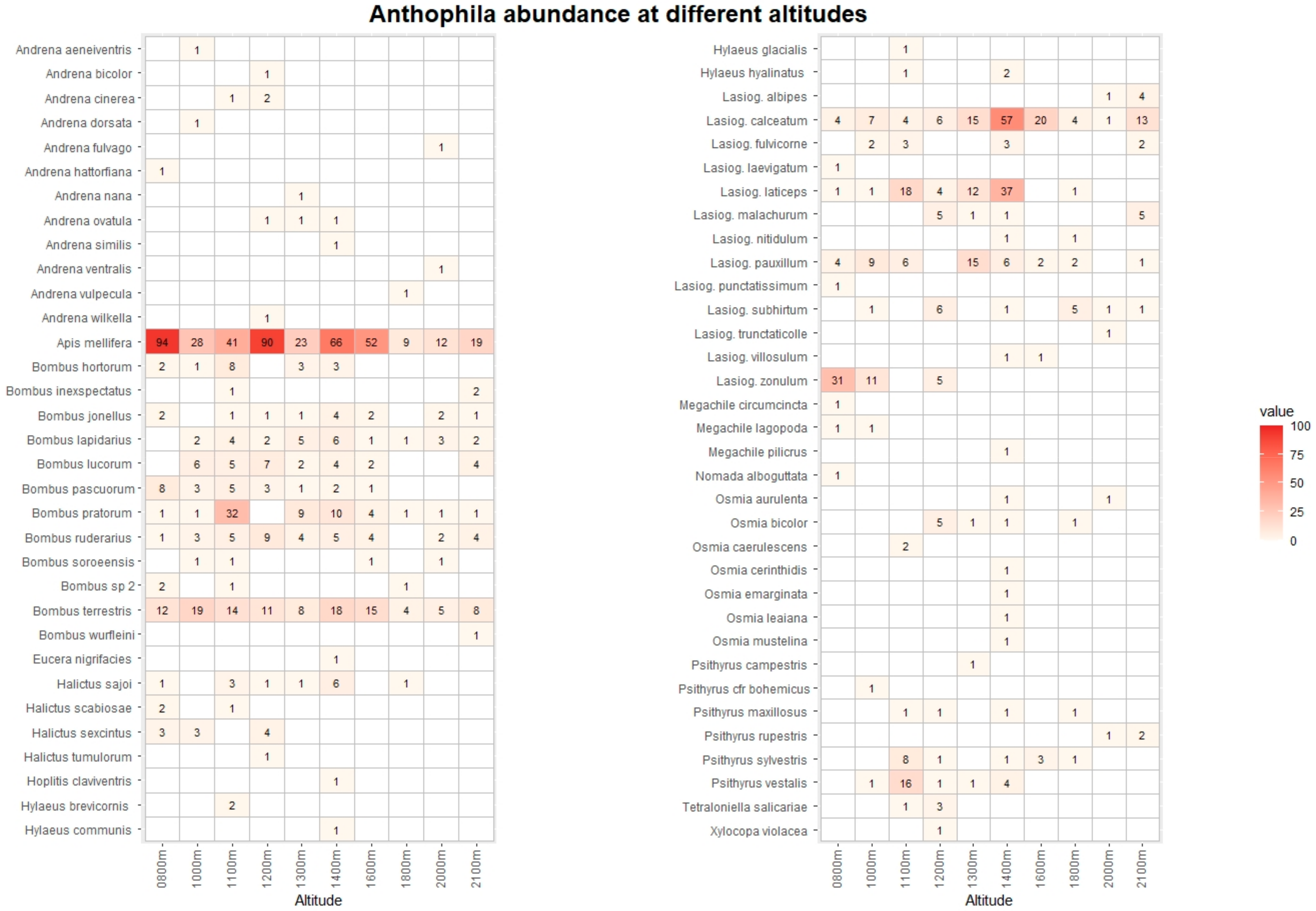
The pan traps collected 768 hoverfly and 1237 bee specimens, belonging to 68 and 67 species, respectively (Tables S3 and S4). Figure 3 and Figure 4 list the species recorded, including absolute abundance at different altitudes. The most common hoverfly species were Eristalis tenax (20.4%), Xylota segnis (13.9%), and Merodon rufus (10.7%). The more abundant bee species were Apis mellifera (35.1%), Lasioglossum calceatum (10.6%), and Bombus terrestris (9.2%). A total of 29 (3.9% of total) hoverfly and 68 (5.5%) bee specimens were collected in May and September only at low-altitude sites, and for this reason, they were excluded from the following analysis.
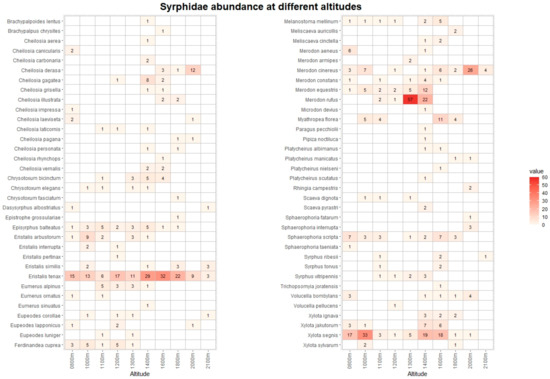
Figure 3.
Heatmap with list of Syrphidae species and their abundance at different altitudes. The data from Vette Feltrine and Mount Grave are pooled. The intensity of color is proportional to specimen abundance.
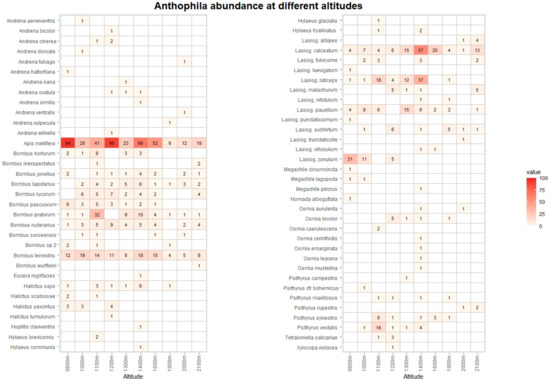
Figure 4.
Heatmap with list of Anthophila species and their abunance at different altitudes. The data from Vette Feltrine and Mount Grave are pooled. The intensity of color is proportional to specimen abundance.
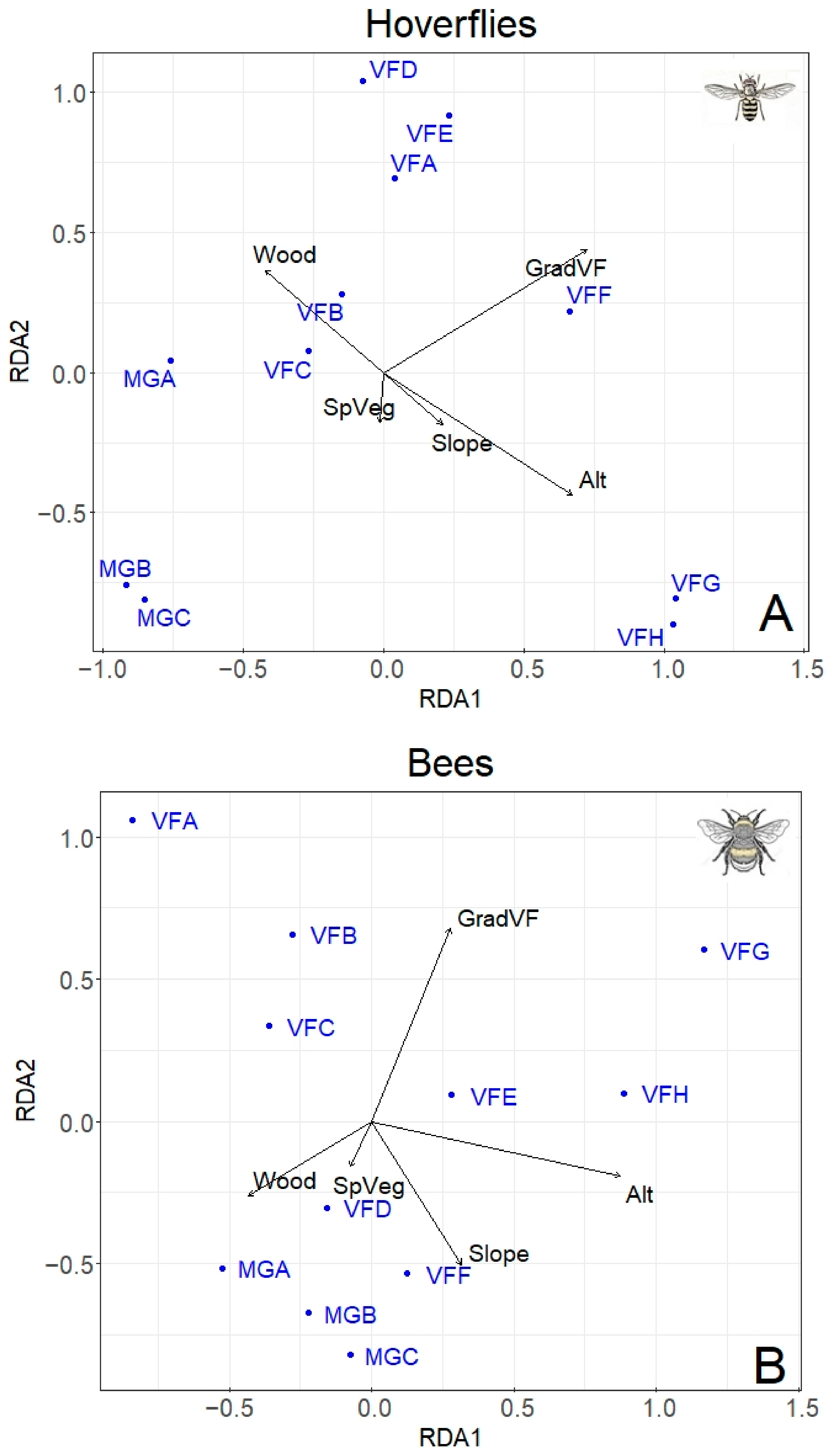
db-RDA was performed separately using hoverfly (68 species) and bee (67 species) matrices of abundances, including five environmental variables (Figure 5). For both hoverflies and bees, the best model was the one including the altitude, the percentage of wood, the slope, the type of gradient, and the total plant species. This model explained 66.7% of the total variability for hoverflies and 66.5% for bees. The hoverfly family was significantly influenced by both the type of gradient (F = 3.53, p < 0.001) and the altitude (F = 3.12, p = 0.011). No effect of the percentage of wood, slope, and total plant species was detected on hoverfly population. Similar results were found for bees: A significant effect of the type of gradient (F = 3.57, p < 0.001) and altitude (F = 2.3, p = 0.006) was recorded; in addition, the slope also showed a significant effect (F = 1.93, p = 0.017), while no influence of the percentage of wood and the total plant species was observed.
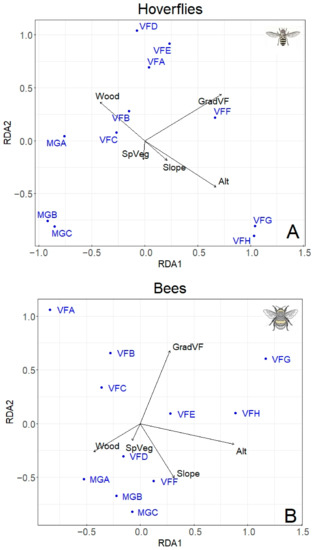
Figure 5.
Redundancy Analysis (db-RDA) showing the effect of main predictors (altitude, type of gradient, slope, percentage of wood, total number of plant species) on hoverfly (A) and bee (B) populations at different altitudes. The sampling plots are in blues (for the abbreviations see Table 1). Proportion of variability explained by the two axes: Hoverfly (A) RDA1 = 27%; RDA2 = 19.38%; Bee (B) RDA1 = 28.46%; RDA2 = 18.81%.
No significant model was obtained when db-RDA was applied to the ecological categories of hoverflies and bees. The altitude, type of gradient, slope, percentage of wood, and total number of plants had no effect on hoverfly and bee categories; in particular, no trend of increasing size was correlated with altitude, either for hoverflies or bees.
Since the different types of gradient (Mount Grave and Vette Feltrine) had a significant effect on both hoverfly and bee population, in the subsequent analysis, only data from Vette Feltrine were considered, removing Mount Grave. Indeed, the Vette Feltrine plots showed a higher association with the “altitude” vector (Figure 5).
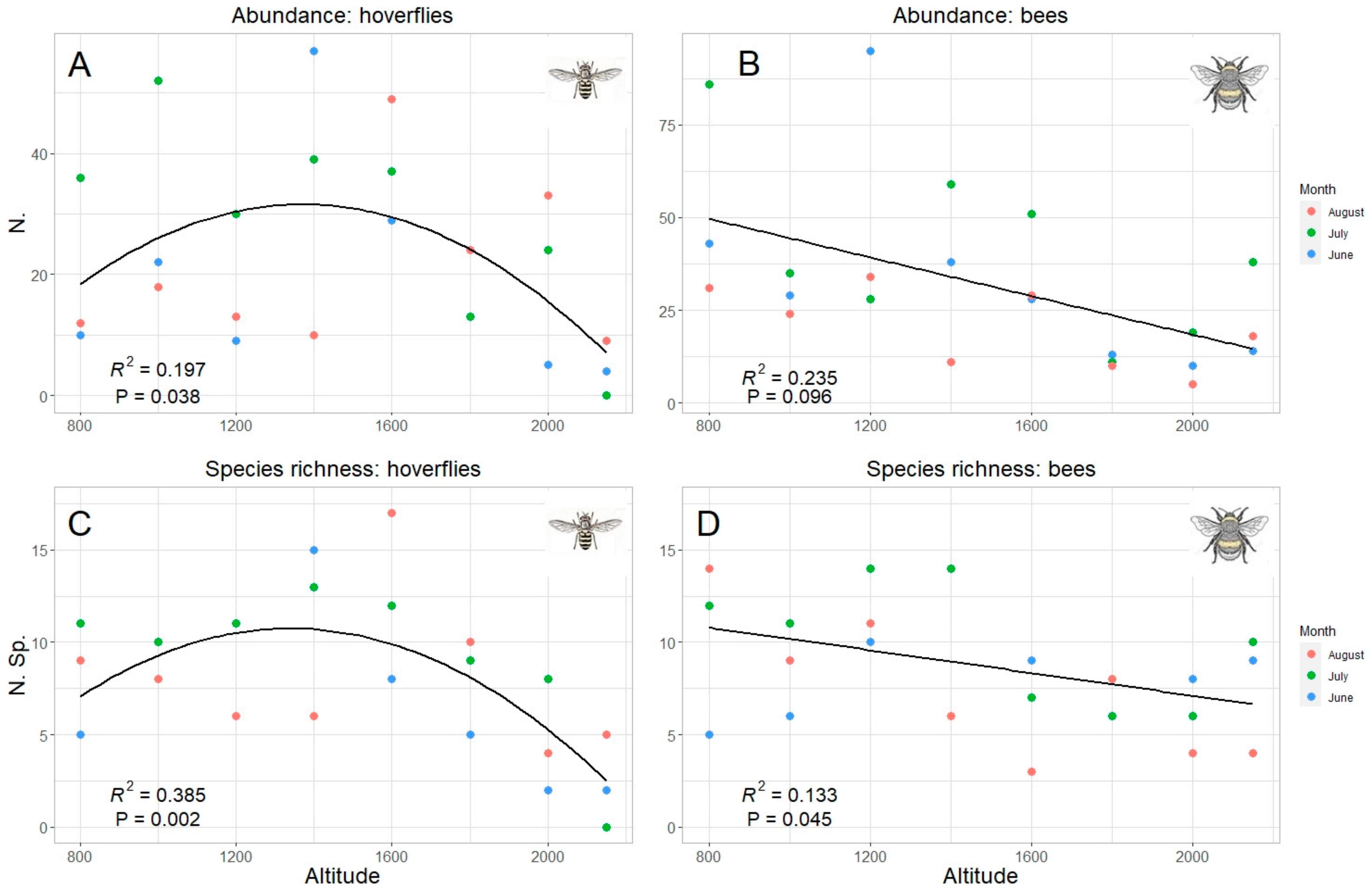
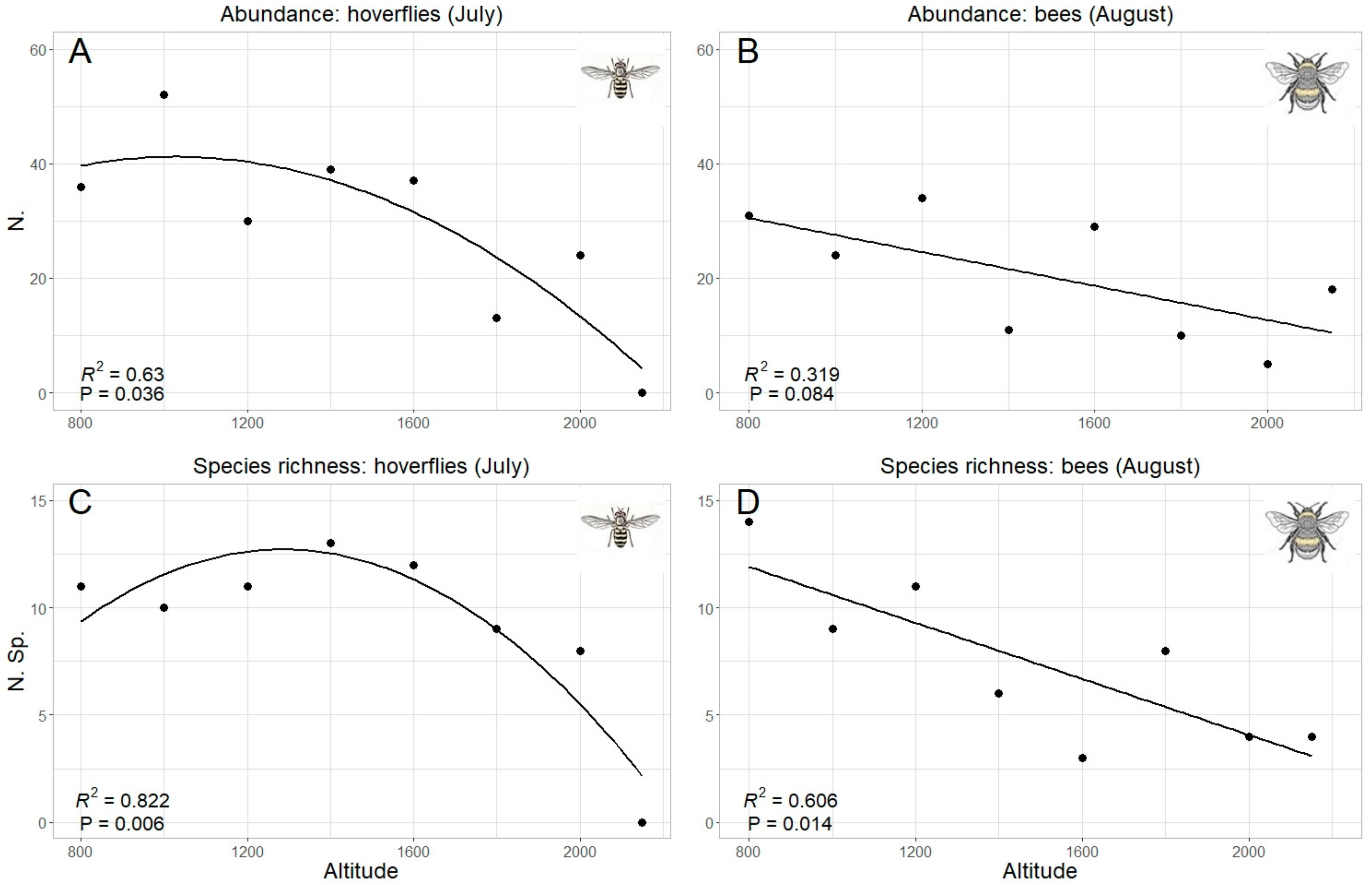
Since “altitude” was the predicting variable which mostly explained data pattern, a specific analysis was performed for this factor. Both total abundance and species richness of hoverflies and bees were significantly correlated with the altitude (Table 2). However, while the correlation was linear (negative) in bees, hoverfly showed a curvilinear polynomial correlation of quadratic type (Figure 6). The same trends were also obtained considering the data collected in each sampling month, even if a significant correlation was detected only in one month both in hoverflies (July both for species richness and abundance) and bees (August only for species richness) (Table 2, Figure 7). Therefore, the highest abundance and richness in the two taxa were recorded at different altitudes, namely, at medium altitudes for hoverflies (around 1500 m) and at lower ones (around 800 m) for bees.

Table 2.
Results of best model (linear or quadratic) testing the effect of altitude on abundance and species richness of hoverflies and bees. The model has been tested both for all data pooled and for three sampling months separately. In bold, the model is statistically significant (p < 0.05). significance levels: 0.05–0.1; * 0.01–0.05; ** 0–0.01.
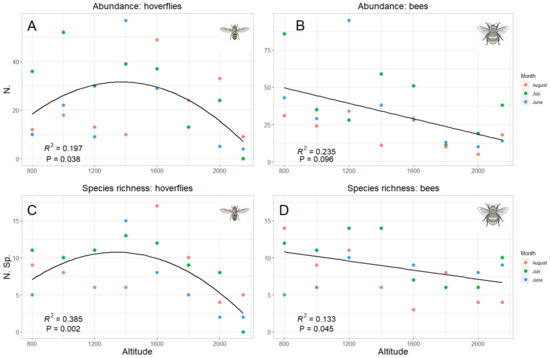
Figure 6.
Relation between hoverfly (A,C) and bee (B,D) abundance (A,B) and species richness (C,D) and altitude; data for all months are pooled. The best model (linear or quadratic) has been selected in accordance with the results in Table 2.
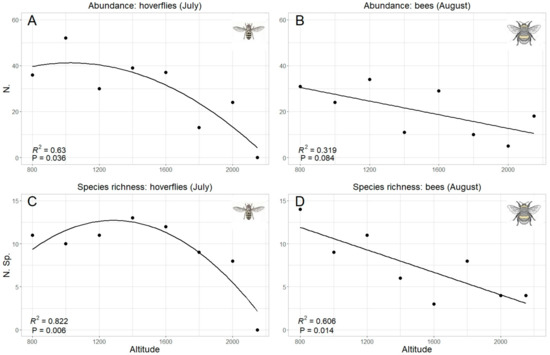
Figure 7.
Relation between hoverfly (A,C) and bee (B,D) abundance (A,B) and species richness (C,D) and altitude; only the months with the best fit have been selected, in accordance with the results in Table 2.
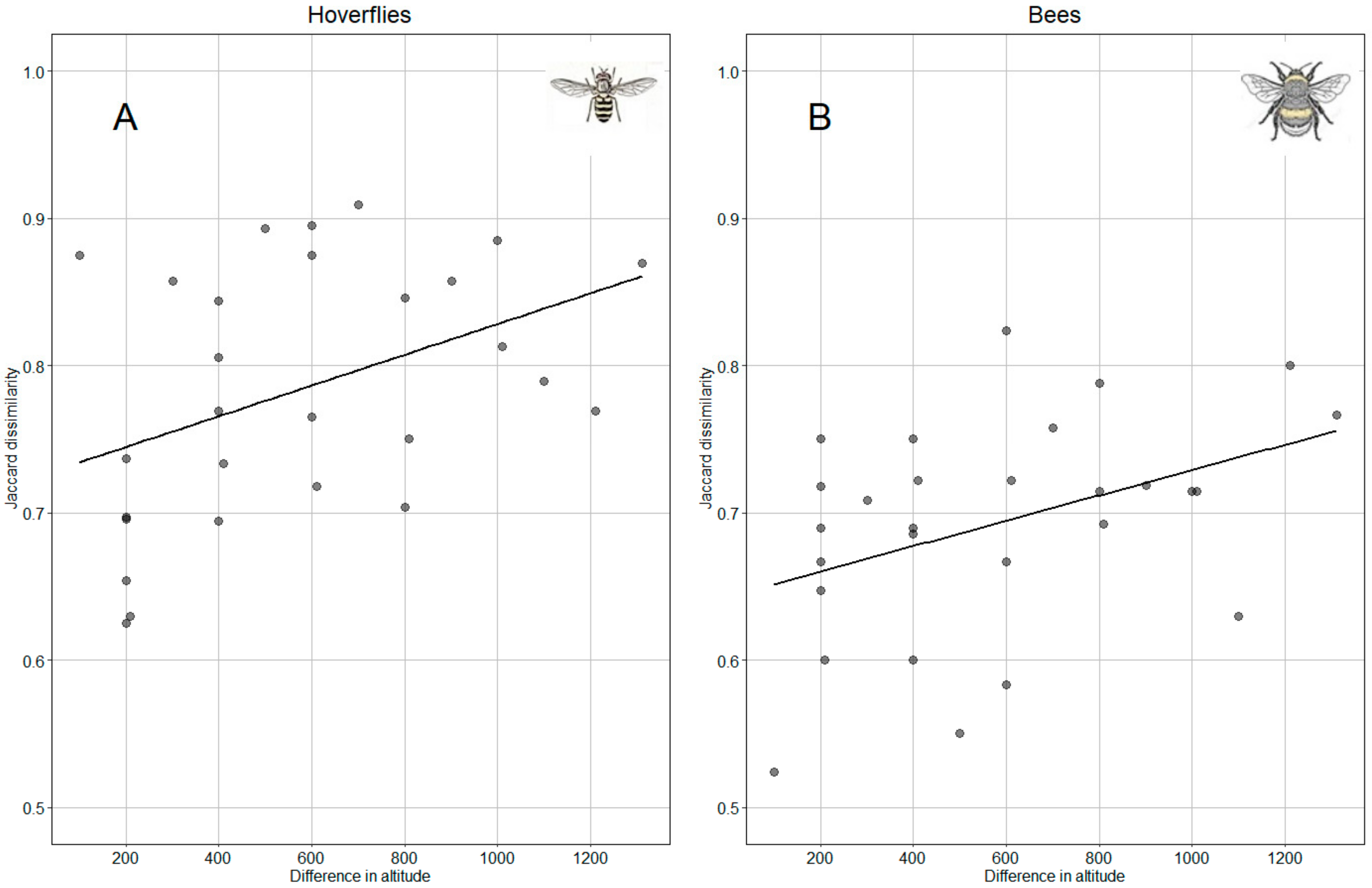
For both hoverflies and bees, the Mantel test revealed a significant correlation between community dissimilarity against the Euclidean distance of altitude among sites (hoverflies: Mantel r = 0.48, p = 0.004; bees: Mantel r = 0.47, p = 0.016). This means that as the altitudinal distance between the sites increases, the dissimilarity between the communities of the taxa also enhances (Figure 8).
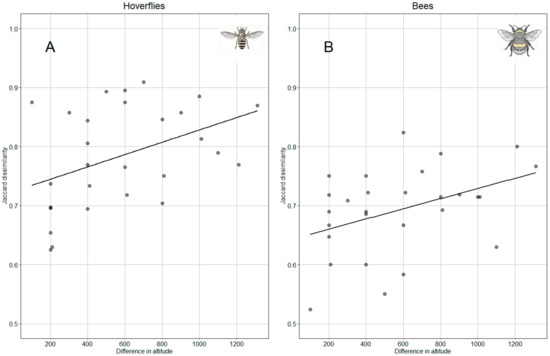
Figure 8.
Relation of Jaccard dissimilarity index of hoverfly (A) and bee (B) with altitude.
According to Shapiro and Levene tests, data assumptions are satisfied for the application of ANCOVA. The model performed with altitude and taxa (hoverflies and bees) was significant for both variables (F(1,53) = 11,254, p = 0.001 and F(1,53) = 21,846, p < 0.0001, respectively), but the interaction between altitude and taxa was not significant. Parallelism test showed no difference between correlation slopes (Figure 8) while elevations of the lines were significantly different (p < 0.0001); in other words, the change in hoverfly population along increasing altitude is more pronounced than that recorded for bees.
β-diversity partitioning showed that differences between transects were mainly due to species turnover, which accounted for 89% of total variation (compared to 11% for nestedness) for hoverflies and 91.2% of turnover (8.8% of nestedness) for bees.
4. Discussion
The number of species recorded was 68 for hoverflies and 67 for bees, making up 13% and 5.6% of total Italian fauna, respectively. Eristalis tenax was the dominant hoverfly species, a taxon that belongs to a genus considered among the most efficient pollinators for its peculiar biological and morphological traits (high mobility, low ecological requirements, dense hairiness) [50]. Some species are of conservation interest because they are considered rare. Among them, Brachypalpus chrysites (Syrphidae) is a saproxylic species, and this is the second recorded in North Eastern Italy (Sommaggio, unpublished data); Trychopsomyia joratensis has predator larvae, which probably develop in humid forests, their record is the second one in Italy [78]. Cheilosia laeviseta is a species associated with mountain grasslands, found only in the Pyrenees, Alps, and Balkans [12]: in the present study, it was recorded only at high altitudes. Three bee species, Andrena hattorfiana, A. ovatula, and Lasioglossum laticeps, are considered as Near Threatened in [9], while Bombus inexpectatus is included in bee red list as Endangered. Bombus inexpectatus is associated with high mountain habitats of the Alps and Pyrenees; its highly fragmented distribution exposes this species to the risk of local extinction.
Several investigations compared the efficiency of different methods in sampling hoverflies and especially bees [53,56,57,58]. Pan traps and entomological nets along transects proved to be the most efficient methods, though not fully exhaustive. Malaise traps have been suggested as the standard sampling device to study hoverfly population [79], although pan traps are also considered a proper sampling method where a large number of replicas are carried out [80]. However, some criticisms have been expressed towards the use of pan traps [81]. Pan trap and active netting collections reported different assemblages of species; in addition, pan traps seem to be more efficient in the absence of floral resources, in opposition to active netting [82]. Also in the present study, some bias in hoverfly and bee sampling due to the use of pan traps may be likely. For this reason, the list of species resulting from this study may not be complete. The integration of pan traps with active netting could be a solution for a more exhaustive sampling of bee and hoverfly populations, but active netting along a transect of over 1300 m in altitude is almost impossible for a single sampler to perform, ensuring the same climatic conditions. Active netting would have determined additional bias by the sampler, sampling day, and time of day, seriously affecting the sampling.
The effect of altitude on species distribution has been studied for a long time. Two patterns of animal distribution along an altitudinal gradient have been more frequently detected: (1) a monotonic decline in species richness with increasing altitude and (2) a unimodal peak at middle elevations [83,84,85,86]. Little research has focused on Diptera and Hymenoptera, however, both distribution patterns were recorded in families belonging to these taxa: A unimodal peak at middle elevation was recorded in Anthophila [87], Tabanidae [88], Simuliidae [89], and Empididae [90]; instead, a monotonic decline was observed in Anthophila [91], Vespidae [92], and Tephritidae [93].
The altitudinal range, geographic area, and sampling device are variables affecting the distribution of taxa on an altitudinal gradient [94,95]. In the Swiss Alps, Beck et al. [96] found diverse patterns in moths in different seasons: a monotonic decline in spring and autumn and a unimodal peak at middle elevation in summer. At specific conditions, additional patterns have been found; for example, at low altitude gradients (400–700 m), Baumann et al. [97] found richness and abundance of wild bees and abundance (but not richness) of hoverflies increasing with altitude. The same pattern (increasing diversity following altitude increase) has been detected also in Formicidae in arid conditions [98].
Peters et al. [86] analysed the altitudinal distribution of 25 plant and animal taxa along a 3.7 km gradient in Kilimangiaro Mountain: A monotonic decrease with altitude was detected in six invertebrate taxa (including wild bees), while a unimodal peak at middle elevation was observed in six taxa (including hoverflies). Data collected in Dolomiti Bellunesi National Park confirm that different taxa may exhibit different distribution patterns along an altitudinal gradient; in particular, hoverflies have a unimodal distribution with a peak around 1400–1500 m (at least in the studied period), while wild bees display a monotonic decrease with increasing altitude.
Despite the large amount of data available on organism distribution along altitudinal gradients, the drivers that determine these patterns are still far from being understood [99,100,101]. Several hypotheses have been advanced; traditionally, abiotic drivers (mainly temperature and water availability) have been largely evaluated as main factors affecting the biodiversity on mountains [86,101]. The “productivity hypothesis” focuses mainly on the different distribution of resources with altitude [102,103,104]. Plant richness and differences in habitats were the biotic drivers widely suggested to explain biodiversity distribution [105]. The “area hypothesis” stated that spatial constraints reduce biodiversity with increasing altitude [106]. Finally, the Mid Domain Effects suggests that stochastic geometric phenomena can be responsible for different distribution of species; this theory has been largely used to explain the unimodal distribution [85,107,108,109].
In the present study, abiotic factors (in particular, temperature, strongly correlated with altitude) seem to be more important in affecting hoverfly and bee populations along the altitudinal gradient. The biotic parameters (in particular, the plant diversity and landscape composition) seem to have no effect, or a marginal one, on hoverfly and bee populations in the altitudinal gradient examined in the present study. Viterbi et al. [110] found that invertebrate (spiders, ground and rove beetles, and butterflies) distribution along an altitudinal gradient was affected by temperature, while bird community was influenced mainly by habitat structure. Peters et al. [86] found different potential drivers in various taxa, but temperature proved to be the main one at total biodiversity level. The different pattern observed in hoverflies and bees in our study may be due to a stronger effect of temperature on bees than in hoverflies or a Mid Domain Effect on hoverflies. Bee populations at high altitudes were dominated by Bombus species (50% of all species at high altitude belonged to this genus); instead, more hoverfly genera were recorded at high altitude (e.g., Cheilosia, Merodon, Eristalis, Volucella). Further studies are necessary to better understand and explain the differences observed between these two taxa.
Despite the fact that hoverflies and wild bees showed different altitudinal patterns, in both cases the change in population structure was dominated by diversity turnover more than nestedness [72,110,111,112]. This mean that for both taxa, population at high altitude is not a subset of that of lower altitude, but different species are present at different altitudes. In the present study, Cheilosia derasa, C. grisella, C. laeviseta, Merodon aeneus, Platycheirus manicatus, and Sphaerophoria fatarum are species found only at high altitudes; in the Alps, these species are typical of montane grasslands [12]. The dominance of species turnover moving with altitude has been confirmed for other taxa (e.g., [110,111,112]).
The dissimilarity of hoverfly and bee populations increased with growing altitudinal distance. Similar results were detected for bees and wasps by Perillo et al. [91]. However, in our study, the change was more pronounced in hoverflies than bees: At the same distance along the gradient, the hoverflies community is more differentiated than that of bees. This can be explained by a difference in mobility and/or a greater specialization of hoverflies species with consequently narrower ranges of altitudinal distribution.
The strong differentiation of hoverfly and wild bee populations at different altitudes entails important conservation problems. The presence of well-differentiated populations of important pollinators at different altitudinal ranges highlights the need to plan conservation and monitoring interventions along the entire altitude gradient. Although at higher altitudes, at least in temperate areas, the anthropic impact is lesser, large-scale negative stressors should not be overlooked, in primis climate change. The list of species collected in the present research, together with their altitudinal distribution, are crucial to establish baselines for future research aimed at monitoring the spatial-temporal trend of these important pollinator taxa in order to understand the effects of climate change on biodiversity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13030293/s1, Table S1: list of Bombus specimens identified using barcoding. Table S2: Correlation matrix between predicting variables; Table S3: List of collected Syrphidae with their ecological characteristics; Table S4. List of collected Anthophila with their ecological characteristics.
Author Contributions
Conception and design: D.S., E.V., P.F. and G.B.; fata recording: D.S., E.V. and P.F.; species identification: D.S. and L.Z.; analysis: D.S.; interpretation: D.S., E.V., P.F., L.Z. and G.B.; preparation of manuscript and review: D.S. and G.B.; funding acquisition: E.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministero dell’Ambiente e della Tutela del Territorio e del Mare–Progetto “Conoscere e proteggere gli impollinatori del Parco Nazionale Dolomiti Bellunesi”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We are thankful to Giovanni Timossi (EntomoReasearch) for his fieldwork with pan traps and Reparto Carabinieri of Dolomiti Bellunesi National Park for their support during sampling activities. Luciano Filippi (Cecina) helped us with the identification of bees while Valeria Malagnini and Federico Pedrazzoli (Edmund Mach Foundation) made the DNA barcoding. Monica Mezzomo (Dolomiti Bellunesi National Park) supported us with cartography; Alberto Scariot and Michele Cassol realized the vegetation monitoring.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghazoul, J. Qualifying pollinator decline evidence. Science 2015, 348, 981–982. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Tandon, R.; Koul, M. ‘Global Pollinator Crisis’ and its impact on crop productivity and sustenance of plant diversity. In Reproductive Ecology of Flowering Plants: Patterns and Processes; Tandon, R., Shivanna, K.R., Koul, M., Eds.; Springer: Singapore, 2020; pp. 395–413. [Google Scholar]
- Ollerton, J. Pollinators & Pollination: Nature and Society; Pelagic Publishing: Exeter, UK, 2021; 286p. [Google Scholar]
- Kosior, A.; Celary, W.; Olejniczak, P.; Fijał, J.; Król, W.; Solarz, W.; Płonka, P. The decline of the bumble bees and cuckoo bees (Hymenoptera: Apidae: Bombini) of Western and Central Europe. Oryx 2007, 41, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Grixti, J.C.; Wong, L.T.; Cameron, S.A.; Favret, C. Decline of bumble bees (Bombus) in the North American Midwest. Biol. Conserv. 2009, 142, 75–84. [Google Scholar] [CrossRef]
- Williams, P.H.; Osborne, J.L. Bumblebee vulnerability and conservation world-wide. Apidologie 2009, 40, 367–387. [Google Scholar] [CrossRef] [Green Version]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Bartomeus, I.; Ascher, J.S.; Gibbs, J.; Danforth, B.N.; Wagner, D.L.; Hedtke, S.M.; Winfree, R. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Natl. Acad. Sci. USA 2013, 110, 4656–4660. [Google Scholar] [CrossRef] [Green Version]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; Criado, M.G.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014; 84p. [Google Scholar]
- Van Swaay, C.A.M.; Cuttelod, A.; Collins, S.; Maes, D.; Munguira, M.L.; Settele, J.; Verovnik, R.; Verstrael, T.; Warren, M.S.; Wiemers, M.; et al. European Red List of Butterflies; Publications Office of the European Union: Luxembourg, 2010; 47p. [Google Scholar]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Speight, M.C.D. Species accounts of European Syrphidae, 2020. In Syrph the Net, the Database of European Syrphidae (Diptera); Syrph the Net Publications: Dublin, Ireland, 2020; Volume 104, 314p. [Google Scholar]
- IPBES. The assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on pollinators, pollination and food production. In Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Potts, S.G., Imperatriz-Fonseca, V.L., Ngo, H.T., Eds.; NERC Open Research Archive: Bonn, Germany, 2016. [Google Scholar]
- Godfray, H.C.J.; Blacquière, T.; Field, L.; Hails, R.S.; Potts, S.G.; Raine, N.E.; Vanbergen, A.; McLean, A.R. A restatement of recent advances in the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B Boil. Sci. 2015, 282, 20151821. [Google Scholar] [CrossRef] [Green Version]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Wright, G.A.; Softley, S.; Earnshaw, H. Low doses of neonicotinoid pesticides in food rewards impair short-term olfactory memory in foraging-age honeybees. Sci. Rep. 2015, 5, 15322. [Google Scholar] [CrossRef] [Green Version]
- Stanley, D.A.; Garratt, M.P.D.; Wickens, J.; Wickens, V.; Potts, S.G.; Raine, N. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 2015, 528, 548–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatt, B.K.; Rundlöf, M.; Smith, H.G. Maintaining the Restriction on neonicotinoids in the European union–benefits and risks to bees and pollination services. Front. Ecol. Evol. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Kathage, J.; Castañera, P.; Alonso-Prados, J.L.; Gómez-Barbero, M.; Rodríguez-Cerezo, E. The impact of restrictions on neonicotinoid and fipronil insecticides on pest management in maize, oilseed rape and sunflower in eight European Union regions. Pest. Manag. Sci. 2018, 74, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [Green Version]
- Winfree, R. The conservation and restoration of wild bees. Ann. N. Y. Acad. Sci. 2010, 1195, 169–197. [Google Scholar] [CrossRef]
- Willmer, P. Ecology: Pollinator–Plant synchrony tested by climate change. Curr. Biol. 2012, 22, R131–R132. [Google Scholar] [CrossRef] [Green Version]
- Iler, A.M.; Inouye, D.W.; Høye, T.T.; Miller-Rushing, A.J.; Burkle, L.A.; Johnston, E.B. Maintenance of temporal synchrony between syrphid flies and floral resources despite differential phenological responses to climate. Glob. Chang. Biol. 2013, 19, 2348–2359. [Google Scholar] [CrossRef]
- Benadi, G.; Hovestadt, T.; Poethke, H.-J.; Blüthgen, N. Specialization and phenological synchrony of plant-pollinator interactions along an altitudinal gradient. J. Anim. Ecol. 2013, 83, 639–650. [Google Scholar] [CrossRef]
- Rixen, C.; Wipf, S.; Frei, E.R.; Stöckli, V. Faster, higher, more? Past, present and future dynamics of alpine and arctic flora under climate change. Alp. Bot. 2014, 124, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Elsen, P.; Tingley, M. Global mountain topography and the fate of montane species under climate change. Nat. Clim. Chang. 2015, 5, 772–776. [Google Scholar] [CrossRef]
- Inouye, D.W. Effects of climate change on alpine plants and their pollinators. Ann. N. Y. Acad. Sci. 2020, 1469, 26–37. [Google Scholar] [CrossRef]
- Engler, R.; Randin, C.F.; Thuiller, W.; Dullinger, S.; Zimmermann, N.E.; Araújo, M.B.; Pearman, P.B.; Le Lay, G.; Piedallu, C.; Albert, C.H.; et al. 21st century climate change threatens mountain flora unequally across Europe. Glob. Chang. Biol. 2011, 17, 2330–2341. [Google Scholar] [CrossRef]
- Pyke, G.H.; Thomson, J.D.; Inouye, D.W.; Miller, T.J. Effects of climate change on phenologies and distributions of bumble bees and the plants they visit. Ecosphere 2016, 7, e01267. [Google Scholar] [CrossRef] [Green Version]
- Biella, P.; Bogliani, G.; Cornalba, M.; Manino, A.; Neumayer, J.; Porporato, M.; Rasmont, P.; Milanesi, P. Distribution patterns of the cold adapted bumblebee Bombus alpinus in the Alps and hints of an uphill shift (Insecta: Hymenoptera: Apidae). J. Insect Conserv. 2017, 21, 357–366. [Google Scholar] [CrossRef]
- Dullinger, S.; Gattringer, A.; Thuiller, W.; Moser, D.; Zimmermann, N.; Guisan, A.; Willner, W.; Plutzar, C.; Leitner, M.; Mang, T.; et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Chang. 2012, 2, 619–622. [Google Scholar] [CrossRef]
- Brown, J.H.; Lomolino, M.V. Biogeography; Sinauer Associates, Inc. Publishers: Sunderland, MA, USA, 1998. [Google Scholar]
- Cushman, J.H.; Lawton, J.H.; Manly, B.F.J. Latitudinal patterns in European ant assemblages: Variation in species richness and body size. Oecologia 1993, 95, 30–37. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Lawton, J.H. Latitudinal gradients in butterfly body sizes: Is there a general pattern? Oecologia 1995, 102, 31–36. [Google Scholar] [CrossRef]
- Hawkins, B.A.; De Vries, P.J. Altitudinal gradients in the body sizes of Costa Rican Butterflies. Acta Oecol. 1996, 17, 185–194. [Google Scholar]
- Malo, J.E.; Baonza, J. Are there predictable clines in plant-pollinator interactions along altitudinal gradients? The example of Cytisus scoparius (L.) Link in the Sierra de Guadarrama (Central Spain). Divers. Distrib. 2002, 8, 365–371. [Google Scholar] [CrossRef]
- Arroyo, M.T.K.; Primack, R.; Armesto, J. Community studies in pollination ecology in the high temperate Andes of central Chile. I. pollination mechanisms and altitudinal variation. Am. J. Bot. 1982, 69, 82–97. [Google Scholar] [CrossRef]
- Inouye, D.W.; Pyke, G.H. Pollination biology in the Snowy Mountains of Australia: Comparisons with montane Colorado, USA. Austral. Ecol. 1988, 13, 191–205. [Google Scholar] [CrossRef]
- Warren, S.D.; Booth, G.M.; Harper, K.T. Elevational Distribution of Insect Pollinators. Am. Midl. Nat. 1988, 120, 325–330. [Google Scholar] [CrossRef]
- McCall, C.; Primack, R.B. Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. Am. J. Bot. 1992, 79, 434–442. [Google Scholar] [CrossRef]
- Kearns, C.A. Anthophilous fly distribution across an elevation gradient. Am. Midl. Nat. 1992, 127, 172–182. [Google Scholar] [CrossRef]
- Forister, M.L.; McCall, A.C.; Sanders, N.J.; Fordyce, J.A.; Thorne, J.H.; O’Brien, J.; Waetjen, D.P.; Shapiro, A.M. Compounded effects of climate change and habitat alteration shift patterns of butterfly diversity. Proc. Natl. Acad. Sci. USA 2010, 107, 2088–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Burgett, M.; Rucker, R.R.; Thurman, W.N. Economics and Honey Bee Pollination Markets. Am. Bee J. 2004, 144, 269–271. [Google Scholar]
- Calderone, N.W. Insect pollinated crops, insect pollinators and US agriculture: Trend analysis of aggregate data for the period 1992–2009. PLoS ONE 2012, 7, e37235. [Google Scholar] [CrossRef] [Green Version]
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. BioScience 2006, 56, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Galliot, J.-N.; Brunel, D.; Bérard, A.; Chauveau, A.; Blanchetête, A.; Lanore, L.; Farruggia, A. Investigating a flower-insect forager network in a mountain grassland community using pollen DNA barcoding. J. Insect Conserv. 2017, 21, 827–837. [Google Scholar] [CrossRef]
- Lucas, A.; Bodger, O.; Brosi, B.; Ford, C.R.; Forman, D.W.; Greig, C.; Hegarty, M.; Neyland, P.J.; de Vere, N. Generalisation and specialisation in hoverfly (Syrphidae) grassland pollen transport networks revealed by DNA metabarcoding. J. Anim. Ecol. 2018, 87, 1008–1021. [Google Scholar] [CrossRef] [Green Version]
- Lucas, A.; Bodger, O.; Brosi, B.J.; Ford, C.R.; Forman, D.W.; Greig, C.; Hegarty, M.; Jones, L.; Neyland, P.J.; de Vere, N. Floral resource partitioning by individuals within generalised hoverfly pollination networks revealed by DNA metabarcoding. Sci. Rep. 2018, 8, 5133. [Google Scholar] [CrossRef] [Green Version]
- Dunn, L.; Lequerica, M.; Reid, C.R.; Latty, T. Dual ecosystem services of syrphid flies (Diptera: Syrphidae): Pollinators and biological control agents. Pest. Manag. Sci. 2020, 76, 1973–1979. [Google Scholar] [CrossRef]
- Westhoff, V.; Van Der Maarel, E. The Braun-Blanquet Approach. In Classification of Plant Communities, 2nd ed.; Whittaker, R.H., Ed.; Springer: Dordrecht, The Netherlands, 1978; pp. 287–399. [Google Scholar]
- Westphal, C.; Bommarco, R.; Carré, G.; Lamborn, E.; Morison, N.; Petanidou, T.; Potts, S.G.; Roberts, S.P.M.; Szentgyörgyi, H.; Tscheulin, T.; et al. Measuring bee diversity in different European habitats and biogeographical regions. Ecol. Monogr. 2008, 78, 653–671. [Google Scholar] [CrossRef] [Green Version]
- Moreira, E.F.; Santos, R.L.D.S.; Penna, U.L.; Angel-Coca, C.; de Oliveira, F.F.; Viana, B.F. Are pan traps colors complementary to sample community of potential pollinator insects? J. Insect Conserv. 2016, 20, 583–596. [Google Scholar] [CrossRef]
- O’Connor, R.S.; Kunin, W.E.; Garratt, M.P.D.; Potts, S.G.; Roy, H.E.; Andrews, C.; Jones, C.M.; Peyton, J.M.; Savage, J.; Harvey, M.C.; et al. Monitoring insect pollinators and flower visitation: The effectiveness and feasibility of different survey methods. Methods Ecol. Evol. 2019, 10, 2129–2140. [Google Scholar] [CrossRef]
- Hutchinson, L.A.; Oliver, T.H.; Breeze, T.D.; O’Connor, R.S.; Potts, S.G.; Roberts, S.P.M.; Garratt, M.P.D. Inventorying and monitoring crop pollinating bees: Evaluating the effectiveness of common sampling methods. Insect Conserv. Divers. 2021, 2021, 12557. [Google Scholar] [CrossRef]
- Krahner, A.; Schmidt, J.; Maixner, M.; Porten, M.; Schmitt, T. Evaluation of four different methods for assessing bee diversity as ecological indicators of agro-ecosystems. Ecol. Indic. 2021, 125, 107573. [Google Scholar] [CrossRef]
- Prendergast, K.S.; Menz, M.H.M.; Dixon, K.W.; Bateman, P.W. The relative performance of sampling methods for native bees: An empirical test and review of the literature. Ecosphere 2020, 11, 03076. [Google Scholar] [CrossRef]
- Droege, S.; Tepedino, V.J.; Lebuhn, G.; Link, W.; Minckley, R.L.; Chen, Q.; Conrad, C. Spatial patterns of bee captures in North American bowl trapping surveys. Insect Conserv. Divers. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Barkalov, A.V.; Ståhls, G. Revision of the Palaearctic bare-eyed and black-legged species of the genus Cheilosia Meigen (Diptera, Syrphidae). Acta Zool. Fenn. 1997, 208, 1–74. [Google Scholar]
- Van Veen, M. Hoverflies of Northwest Europe: Identification Keys to the Syrphidae; KNNV Publishing: Utrecht, The Netherlands, 2010; 256p. [Google Scholar]
- Speight, M.C.D.; Sarthou, J.-P. StN keys for the identification of the European species of various genera of Syrphidae. In Syrph the Net, the Database of European Syrphidae (Diptera); Syrph the Net Publications: Dublin, Ireland, 2017; Volume 99, 139p. [Google Scholar]
- Intoppa, F.; Piazza, M.G.; Bolchi Serini, G.; Cornalba, M. I bombi. Guida al riconoscimento delle specie italiane. In CRA-Unità di Ricerca di Apicoltura e Bachicoltura; CSR: Roma, Italy, 2009; Volume 174. [Google Scholar]
- Michez, D.; Rasmont, P.; Terzo, M.; Vereecken, N.J. Bees of Europe. In Hymenoptera of Europe—1; N.A.P.: Verrières-le-Buisson, France, 2019; p. 545. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplifcation of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Villalta, I.; Ledet, R.; Baude, M.; Genoud, D.; Bouget, C.; Cornillon, M.; Moreau, S.; Courtial, B.; Lopez-Vaamonde, C. A DNA barcode-based survey of wild urban bees in the Loire Valley, France. Sci. Rep. 2021, 11, 4770. [Google Scholar] [CrossRef]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package “Vegan”. 2020. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 31 January 2022).
- Nekola, J.C.; White, P.S. The distance decay of similarity in biogeography and ecology. J. Biogeogr. 1999, 26, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Andrade, J.; Estévez-Pérez, M. Statistical comparison of the slopes of two regression lines: A tutorial. Anal. Chim. Acta 2014, 838, 1–12. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Soininen, J.; Heino, J.; Wang, J. A meta-analysis of nestedness and turnover components of beta diversity across organisms and ecosystems. Glob. Ecol. Biogeogr. 2018, 27, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Baselga, A.; Orme, C.D.L. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Harrell, F.E.; Dupont, C. Package “Hmisc”. 2021. Available online: https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf (accessed on 31 January 2022).
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Logez, M.; Henriques-Silva, R. Package “Betapart”. 2021. Available online: https://cran.r-project.org/web/packages/betapart/betapart.pdf (accessed on 15 January 2022).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA; Available online: http://ggplot2.tidyverse.org (accessed on 31 January 2022).
- Romig, T. Syrphidae—Part 2. In Diptera Stelviana: A Dipterological Perspective on a Changing Alpine Landscape; Ziegler, J., Ed.; Studia Dipterologica Supplement 21; Ampyx-Verlag: Halle/Salle, Germany, 2016; Volume 2. [Google Scholar]
- Speight, M.C.D.; Castella, E.; Obrdlik, P. Use of the Syrph the Net database 2000. In Syrph the Net, the Database of European Syrphidae; Syrph the Net Publications: Dublin, Ireland, 2000; Volume 25. [Google Scholar]
- Ssymank, A. Habitat use by Syrphidae (Diptera) in the valley of the river Strom—Part 2: Comparison of wet grassland and its succession stages. Volucella 2007, 8, 165–184. [Google Scholar]
- Hickman, J.M.; Wratten, S.D.; Jepson, P.C.; Frampton, C.M. Effect of hunger on yellow water trap catches of hoverfly (Diptera: Syrphidae) adults. Agric. For. Entomol. 2001, 3, 35–40. [Google Scholar] [CrossRef]
- Pei, C.K.; Hovick, T.J.; Duquette, C.A.; Limb, R.F.; Harmon, J.P.; Geaumont, B.A. Two common bee-sampling methods reflect different assemblages of the bee (Hymenoptera: Apoidea) community in mixed-grass prairie systems and are dependent on surrounding floral resource availability. J. Insect Conserv. 2022, 26, 69–83. [Google Scholar] [CrossRef]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Colwell, R.K.; Gotelli, N.J.; Ashton, L.; Beck, J.; Brehm, G.; Fayle, T.M.; Fiedler, K.; Forister, M.L.; Kessler, M.; Kitching, R.L.; et al. Midpoint attractors and species richness: Modelling the interaction between environmental drivers and geometric constraints. Ecol. Lett. 2016, 19, 1009–1022. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.; Hemp, A.; Appelhans, T.; Behler, C.; Classen, A.; Detsch, F.; Ensslin, A.; Ferger, S.W.; Frederiksen, S.B.; Gebert, F.; et al. Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun. 2016, 7, 13736. [Google Scholar] [CrossRef] [Green Version]
- Widhiono, I.; Sudiana, E.; Darsono, D. Diversity of wild bees along elevational gradient in an agricultural area in central Java, Indonesia. Psyche A J. Entomol. 2017, 2017, 2968414. [Google Scholar] [CrossRef] [Green Version]
- Hackenberger, D.; Jarić, D.; Krcmar, S. Distribution of tabanids (Diptera: Tabanidae) along a two-sided altitudinal transect. Environ. Entomol. 2009, 38, 1600–1607. [Google Scholar] [CrossRef]
- Srisuka, W.; Sulin, C.; Aupalee, K.; Phankaen, T.; Taai, K.; Thongsahuan, S.; Saeung, A.; Takaoka, H. Community structure, biodiversity and spatiotemporal distribution of the black flies (Diptera: Simuliidae) using malaise traps on the highest mountain in Thailand. Insects 2021, 12, 504. [Google Scholar] [CrossRef]
- Słowińska, I.; Jaskuła, R. Distributional Patterns of Aquatic Empididae (Diptera) along an Elevational Diversity Gradient in a Low Mountain Range: An Example from Central Europe. Insects 2021, 12, 165. [Google Scholar] [CrossRef]
- Perillo, L.N.; Neves, F.; Antonini, Y.; Martins, R.P. Compositional changes in bee and wasp communities along Neotropical mountain altitudinal gradient. PLoS ONE 2017, 12, e0182054. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Longino, J.T.; Colwell, R.K.; O’Donnell, S. Elevational Patterns of Diversity and Abundance of Eusocial Paper Wasps (Vespidae) in Costa Rica. Biotropica 2009, 41, 338–346. [Google Scholar] [CrossRef]
- Finnie, S.; Sam, K.; Leponce, M.; Basset, Y.; Drew, D.; Schutze, M.K.; Dahl, C.; Damag, M.; Dilu, M.; Gewa, B.; et al. Assemblages of fruit flies (Diptera: Tephritidae) along an elevational gradient in the rainforests of Papua New Guinea. Insect Conserv. Divers. 2021, 14, 348–355. [Google Scholar] [CrossRef]
- Wolda, H. Altitude, habitat and tropical insect diversity. Biol. J. Linn. Soc. 1987, 30, 313–323. [Google Scholar] [CrossRef]
- McCoy, E.D. The Distribution of Insects along Elevational Gradients. Oikos 1990, 58, 313–322. [Google Scholar] [CrossRef]
- Beck, J.; Altermatt, F.; Hagmann, R.; Lang, S. Seasonality in the altitude–diversity pattern of Alpine moths. Basic Appl. Ecol. 2010, 11, 714–722. [Google Scholar] [CrossRef]
- Baumann, K.; Keune, J.; Wolters, V.; Jauker, F. Distribution and pollination services of wild bees and hoverflies along an altitudinal gradient in mountain hay meadows. Ecol. Evol. 2021, 11, 11345–11351. [Google Scholar] [CrossRef]
- Sanders, N.; Moss, J.; Wagner, D. Patterns of ant species richness along elevational gradients in an arid ecosystem. Glob. Ecol. Biogeogr. 2003, 12, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. 2005, 80, 489–513. [Google Scholar] [CrossRef] [Green Version]
- Rohde, K. Latitudinal gradients in species diversity: The search for the primary cause. Oikos 1992, 65, 514–527. [Google Scholar] [CrossRef] [Green Version]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Field, R.; Cornell, H.V.; Currie, D.J.; Guégan, J.-F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; O’Brien, E.M.; et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology 2003, 84, 3105–3117. [Google Scholar] [CrossRef] [Green Version]
- Hurlbert, A.H.; Stegen, J. When should species richness be energy limited, and how would we know? Ecol. Lett. 2014, 17, 401–413. [Google Scholar] [CrossRef]
- Belmaker, J.; Jetz, W. Relative roles of ecological and energetic constraints, diversification rates and region history on global species richness gradients. Ecol. Lett. 2015, 18, 563–571. [Google Scholar] [CrossRef]
- Novotny, V.; Drozd, P.; Miller, S.E.; Kulfan, M.; Janda, M.; Basset, Y.; Weiblen, G.D. Why are there so many species of herbivorous insects in tropical rainforests? Science 2006, 313, 1115–1118. [Google Scholar] [CrossRef] [Green Version]
- Romdal, T.S.; Grytnes, J.-A. An indirect area effect on elevational species richness patterns. Ecography 2007, 30, 440–448. [Google Scholar] [CrossRef]
- Brehm, G.; Colwell, R.K.; Kluge, J. The role of environment and mid-domain effect on moth species richness along a tropical elevational gradient. Glob. Ecol. Biogeogr. 2007, 16, 205–219. [Google Scholar] [CrossRef]
- Colwell, R.K.; Rahbek, C.; Gotelli, N.J. The mid-domain effect and species richness patterns: What have we learned so far? Am. Nat. 2004, 163, E1–E23. [Google Scholar] [CrossRef] [Green Version]
- Zapata, F.A.; Gaston, K.J.; Chown, S.L. Mid-domain models of species richness gradients: Assumptions, methods and evidence. J. Anim. Ecol. 2003, 72, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Viterbi, R.; Cerrato, C.; Bassano, B.; Bionda, R.; Von Hardenberg, A.; Provenzale, A.; Bogliani, G. Patterns of biodiversity in the northwestern Italian Alps: A multi-taxa approach. Community Ecol. 2013, 14, 18–30. [Google Scholar] [CrossRef]
- Bishop, T.; Robertson, M.P.; van Rensburg, B.; Parr, C. Contrasting species and functional beta diversity in montane ant assemblages. J. Biogeogr. 2015, 42, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Fontana, V.; Guariento, E.; Hilpold, A.; Niedrist, G.; Steinwandter, M.; Spitale, D.; Nascimbene, J.; Tappeiner, U.; Seeber, J. Species richness and beta diversity patterns of multiple taxa along an elevational gradient in pastured grasslands in the European Alps. Sci. Rep. 2020, 10, 12516. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).